

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50386178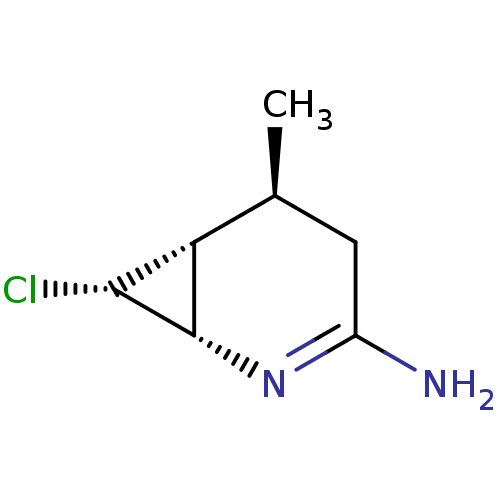 (CHEMBL1800346 | ONO-1714) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH Curated by ChEMBL | Assay Description Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting | Bioorg Med Chem Lett 21: 4228-32 (2011) Article DOI: 10.1016/j.bmcl.2011.05.073 BindingDB Entry DOI: 10.7270/Q2H996GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50386178 (CHEMBL1800346 | ONO-1714) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH Curated by ChEMBL | Assay Description Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting | Bioorg Med Chem Lett 21: 4228-32 (2011) Article DOI: 10.1016/j.bmcl.2011.05.073 BindingDB Entry DOI: 10.7270/Q2H996GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50124535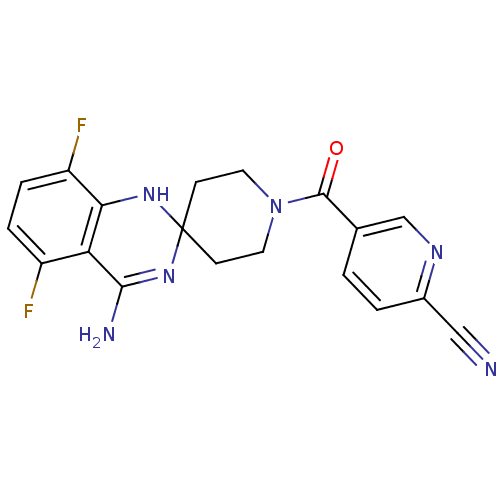 (1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 37.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH Curated by ChEMBL | Assay Description Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting | Bioorg Med Chem Lett 21: 4228-32 (2011) Article DOI: 10.1016/j.bmcl.2011.05.073 BindingDB Entry DOI: 10.7270/Q2H996GJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50418856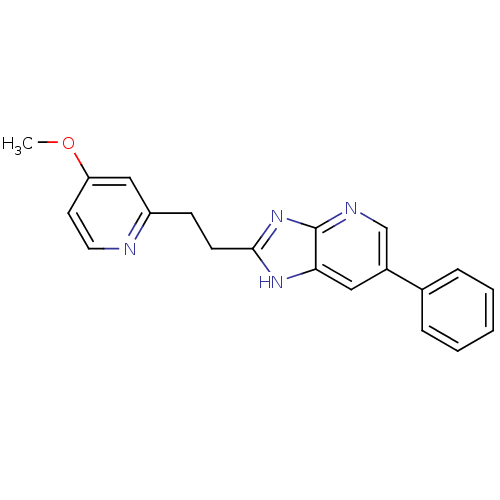 (CHEMBL1800534) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH Curated by ChEMBL | Assay Description Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting | Bioorg Med Chem Lett 21: 4228-32 (2011) Article DOI: 10.1016/j.bmcl.2011.05.073 BindingDB Entry DOI: 10.7270/Q2H996GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091805 (2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 44.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH Curated by ChEMBL | Assay Description Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting | Bioorg Med Chem Lett 21: 4228-32 (2011) Article DOI: 10.1016/j.bmcl.2011.05.073 BindingDB Entry DOI: 10.7270/Q2H996GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50418855 (CHEMBL1800533) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH Curated by ChEMBL | Assay Description Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting | Bioorg Med Chem Lett 21: 4228-32 (2011) Article DOI: 10.1016/j.bmcl.2011.05.073 BindingDB Entry DOI: 10.7270/Q2H996GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50418844 (CHEMBL1800347) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH Curated by ChEMBL | Assay Description Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting | Bioorg Med Chem Lett 21: 4228-32 (2011) Article DOI: 10.1016/j.bmcl.2011.05.073 BindingDB Entry DOI: 10.7270/Q2H996GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50418849 (CHEMBL1738840) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH Curated by ChEMBL | Assay Description Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting | Bioorg Med Chem Lett 21: 4228-32 (2011) Article DOI: 10.1016/j.bmcl.2011.05.073 BindingDB Entry DOI: 10.7270/Q2H996GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332053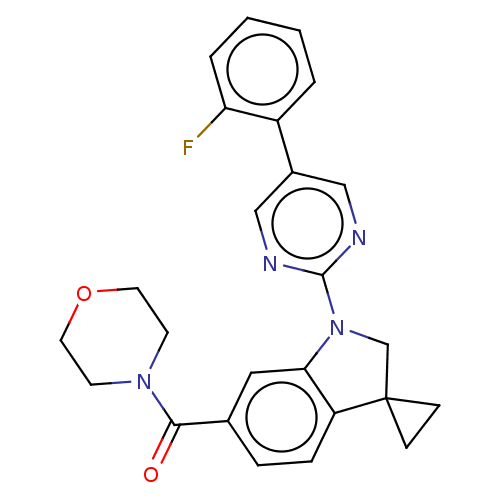 (US10189854, Compound 1 | [1-[5-(2-Fluorophenyl)-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332054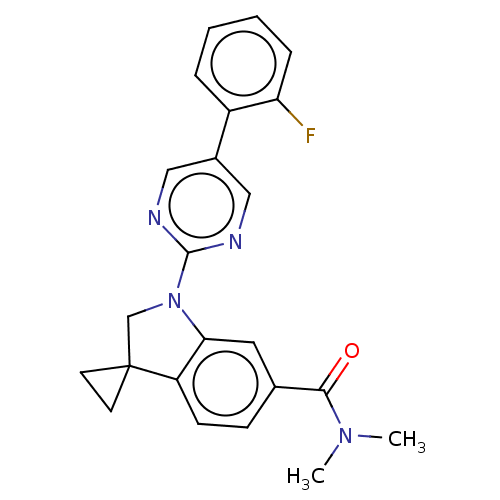 (1-[5-(2-Fluorophenyl)-pyrimidin-2-yl]-N,N-dimethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332055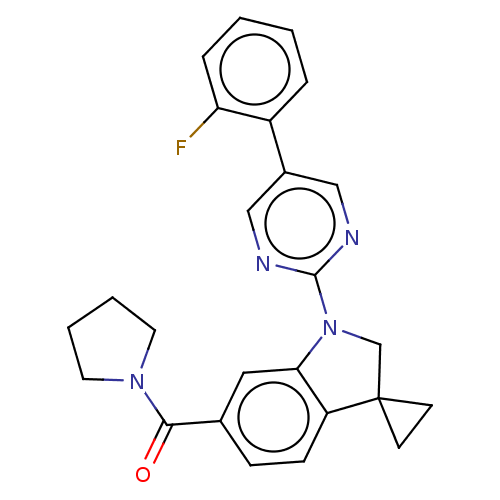 (US10189854, Compound 3 | [1-[5-(1-Fluorophenyl)-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332056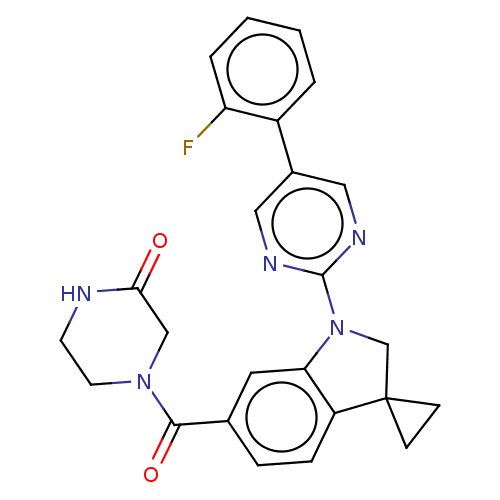 (4-[1-[5-(2-Fluorophenyl)-pyrimidin-2-yl]-spiro[1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332057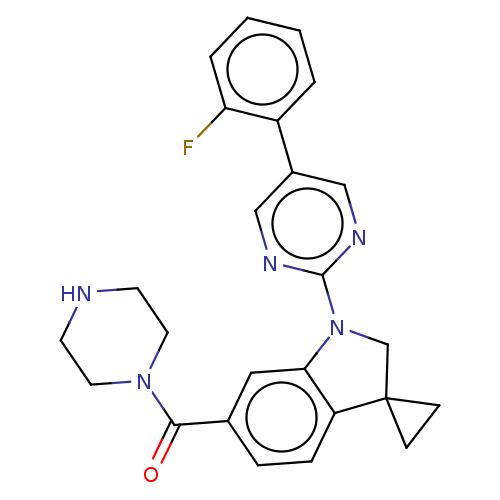 (US10189854, Compound 5 | [1-[5-(2-Fluorophenyl)-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332059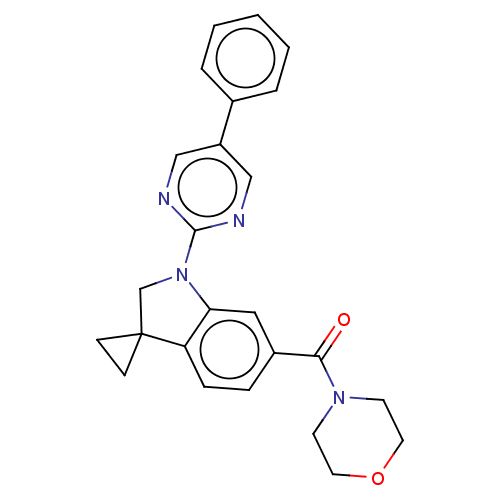 (Morpholin-4-yl-[1-(5-phenyl-pyrimidin-2-yl)-spiro[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332060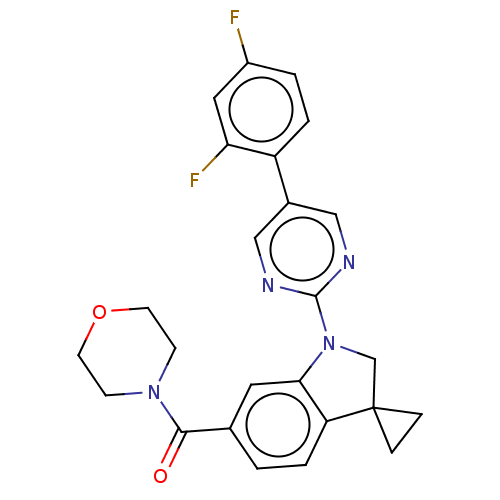 (US10189854, Compound 8 | [1-[5-(2,4-Difluoro-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332062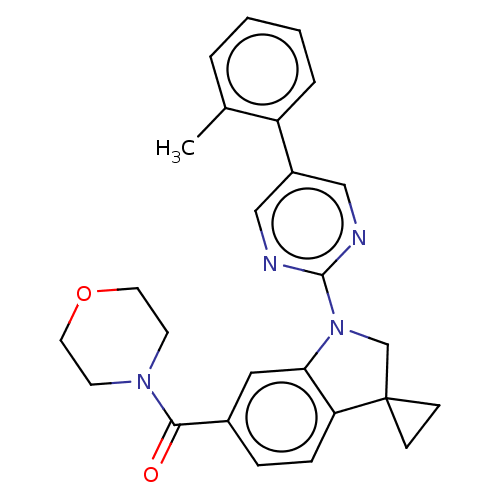 (Morpholin-4-yl-[1-(5-o-tolyl-pyrimidin-2-yl)-spiro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332063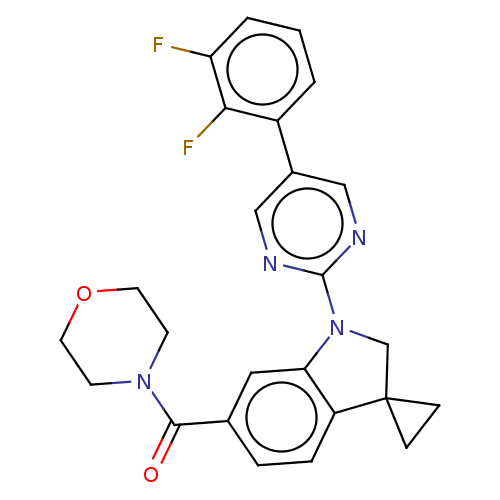 (US10189854, Compound 15 | [1-[5-(2,3-Difluoro-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332064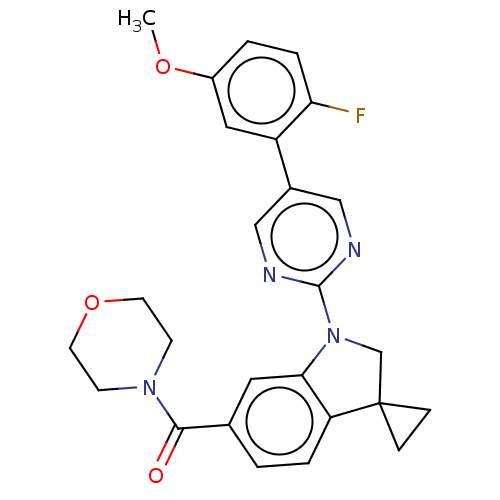 (US10189854, Compound 16 | [1-[5-(2-Fluoro-5-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332065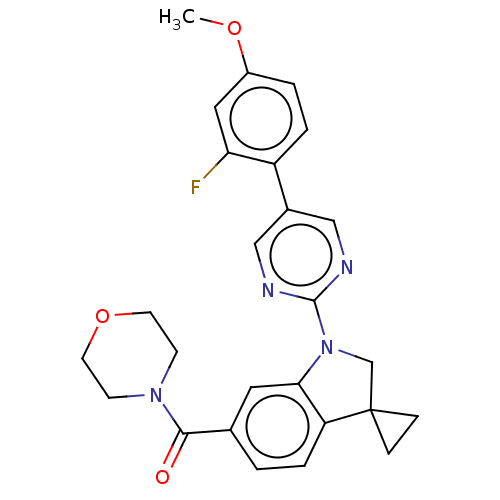 (US10189854, Compound 17 | [1-[5-(2-Fluoro-4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332070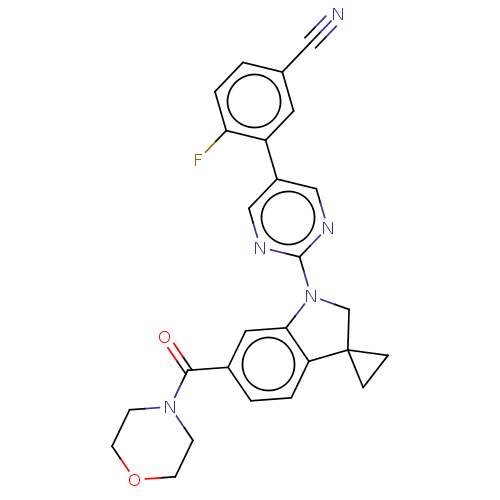 (4-Fluoro-3-[2-[6-(morpholine-4-carbonyl)-spiro[1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332071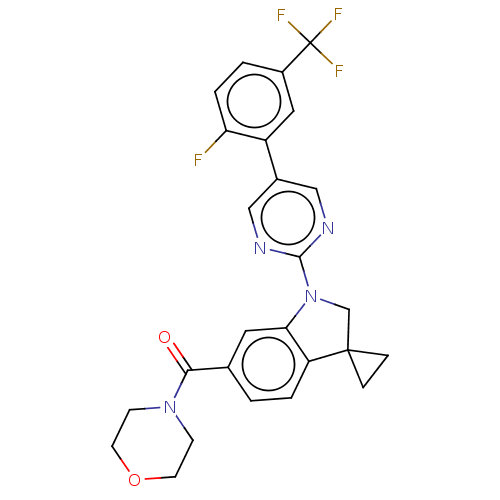 (US10189854, Compound 23 | [1-[5-[2-Fluoro-5-(trifl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332074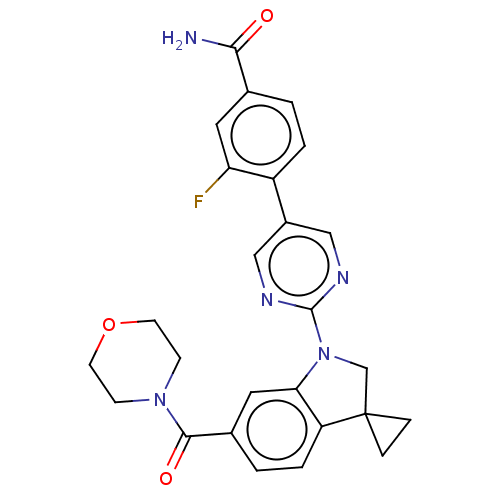 (3-Fluoro-4-[2-[6-(morpholine-4-carbonyl)-spiro[1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332075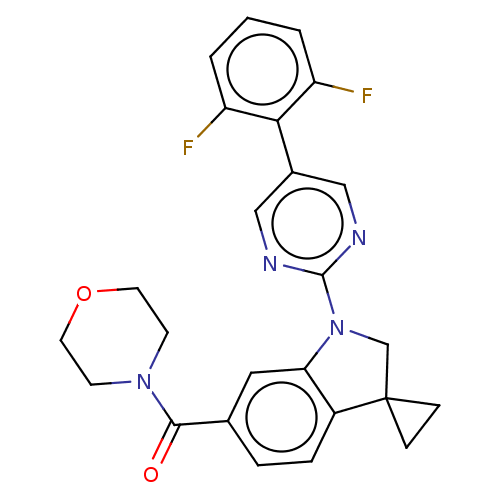 (US10189854, Compound 27 | [1-[5-(2,6-Difluoro-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332077 (US10189854, Compound 29 | [1-[5-(2-Fluoro-4-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332079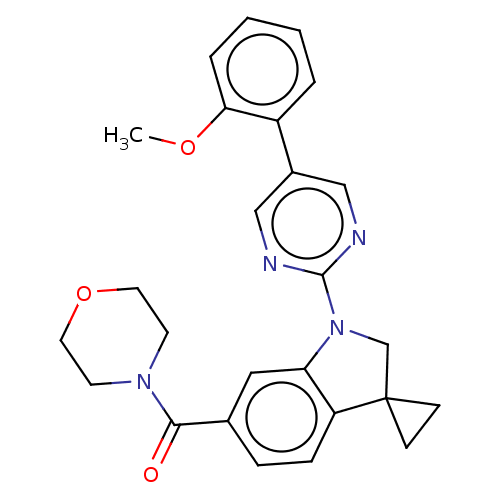 (US10189854, Compound 31 | [1-[5-(2-Methoxyphenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332081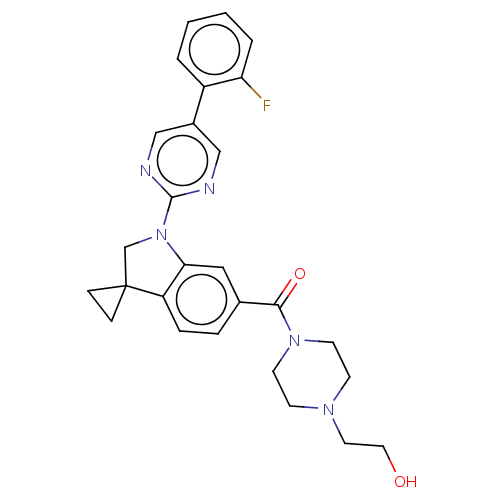 (US10189854, Compound 34 | US10189854, Compound 94 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332082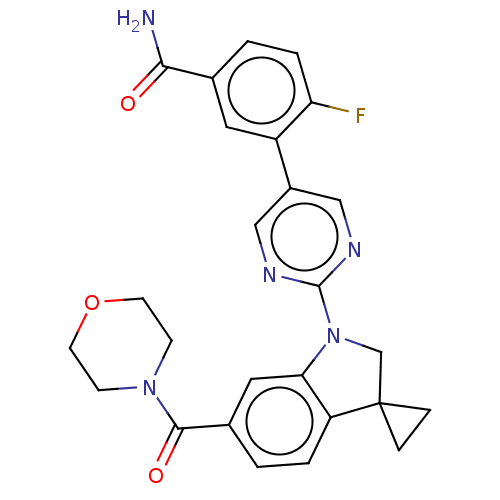 (4-Fluoro-3-[2-[6-(morpholine-4-carbonyl)-spiro[1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332084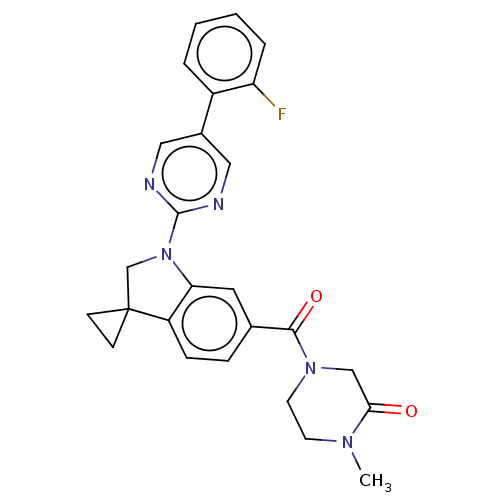 (4-[1-[5-(2-Fluorophenyl)-pyrimidin-2-yl]spiro[1,2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332086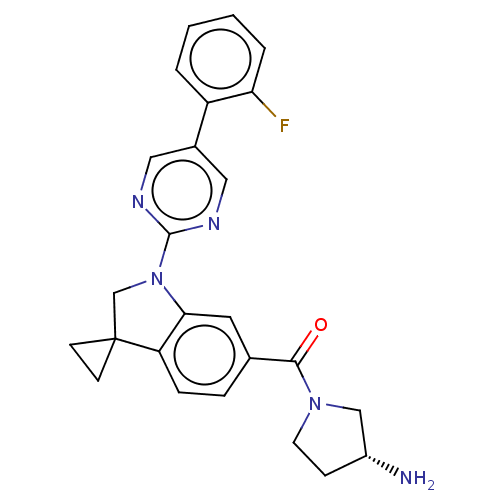 (US10189854, Compound 40 | [(3R)-3-Amino-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332087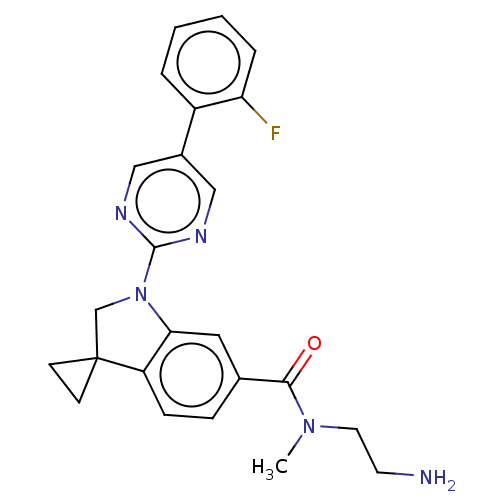 (N-(2-Amino-ethyl)-1-[5-(2-fluorophenyl)-pyrimidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332088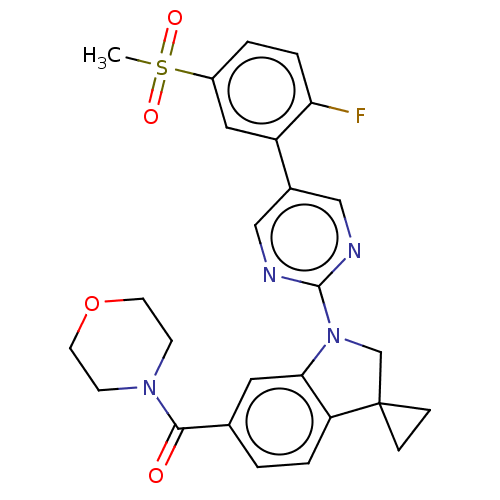 (US10189854, Compound 42 | [1-[5-(2-Fluoro-5-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332089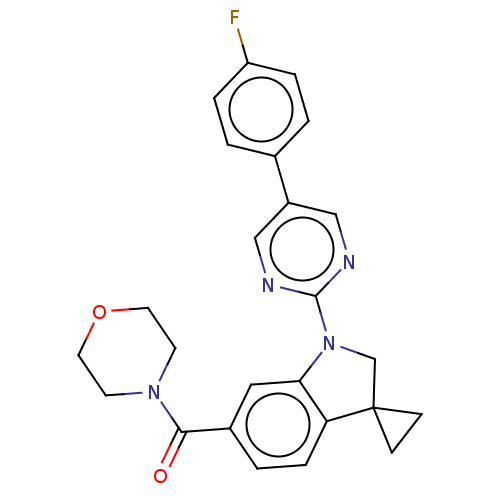 (US10189854, Compound 43 | [1-[5-(4-Fluorophenyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332090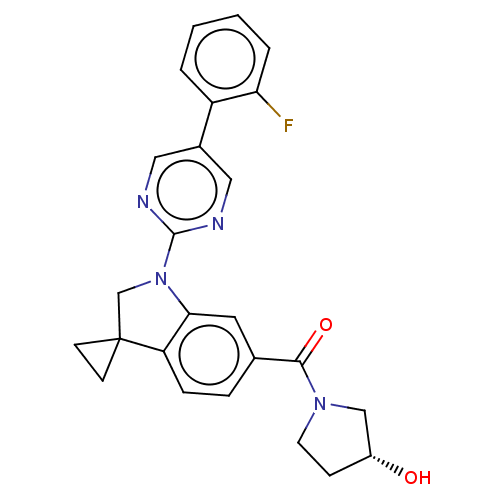 (US10189854, Compound 44 | [1-[5-(2-Fluorophenyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332094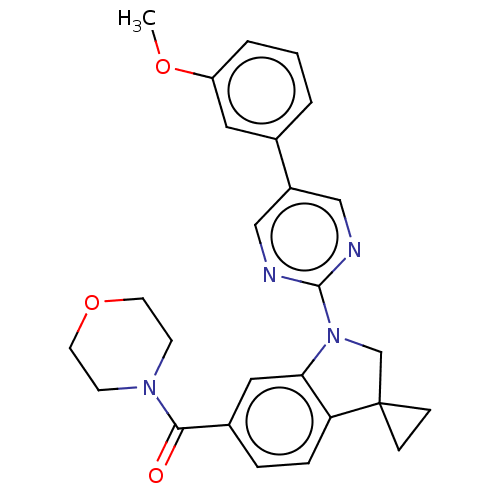 (US10189854, Compound 48 | [1-[5-(3-Methoxyphenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332095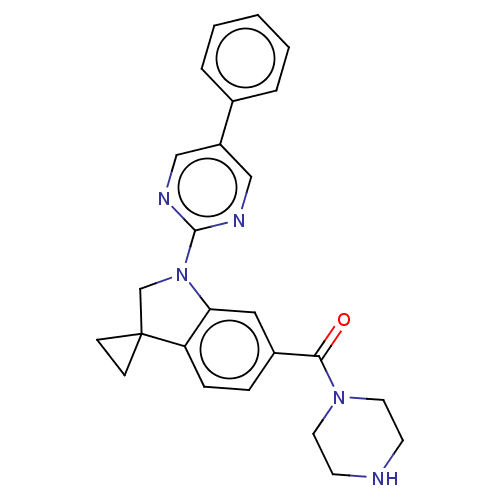 (US10189854, Compound 49 | [1-(5-Phenyl-pyrimidin-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332096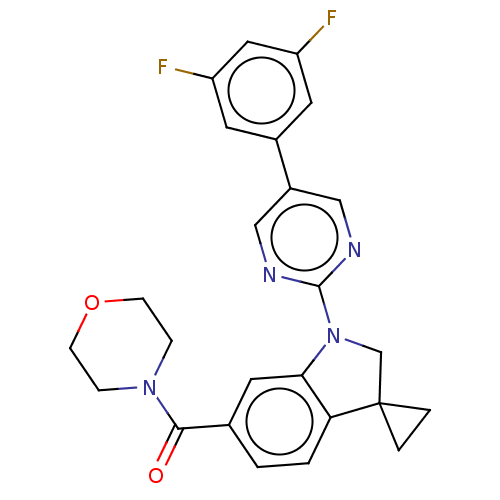 (US10189854, Compound 50 | [1-[5-(3,5-Difluoro-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332098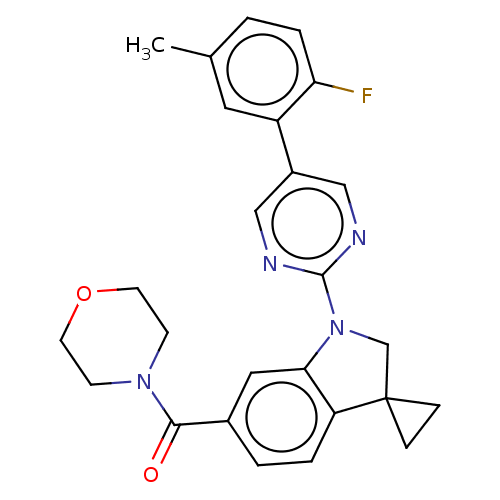 (US10189854, Compound 52 | [1-[5-(2-Fluoro-5-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332100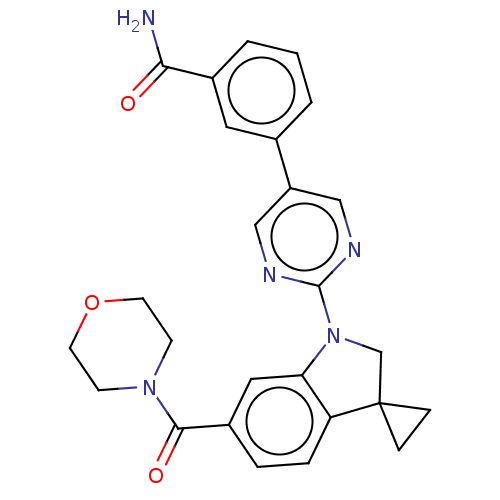 (3-[2-[6-(Morpholine-4-carbonyl)-spiro[1,2-dihydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332101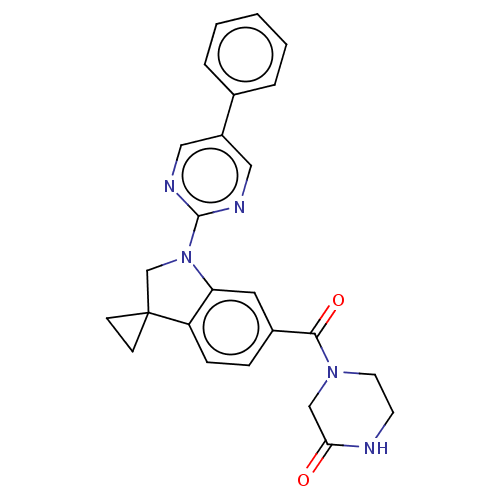 (4-[1-(5-Phenyl-pyrimidin-2-yl)-spiro[1,2-dihydro-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332102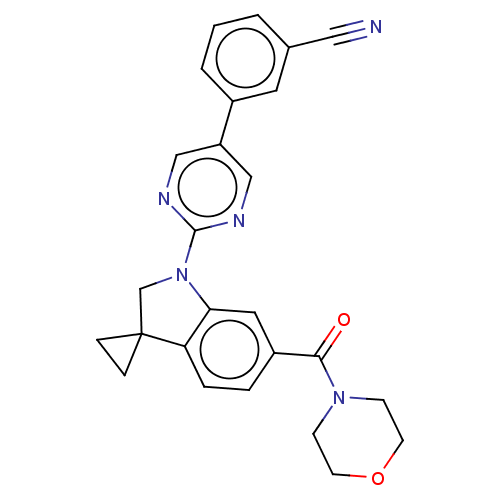 (3-[2-[6-(Morpholine-4-carbonyl)-spiro[1,2-dihydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332103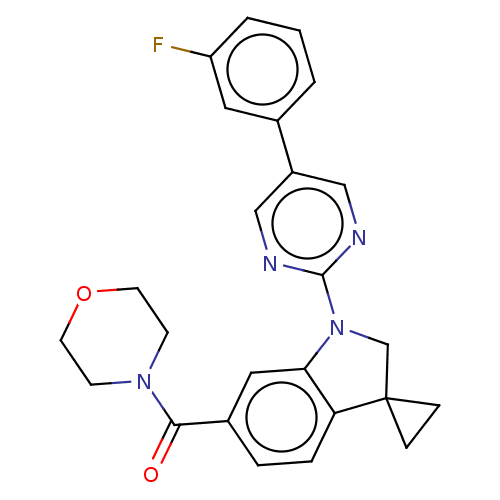 (US10189854, Compound 57 | [1-[5-(3-Fluorophenyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332104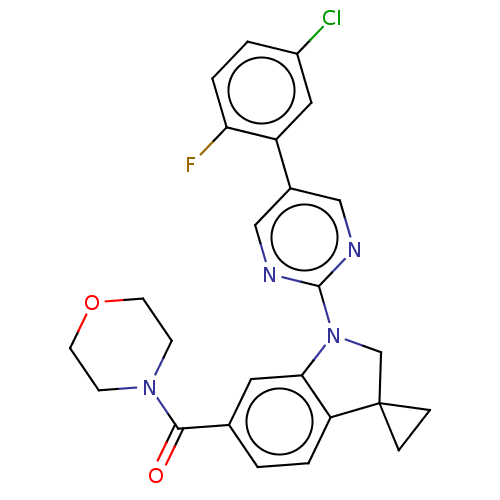 (US10189854, Compound 58 | [1-[5-(5-Chloro-2-fluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332105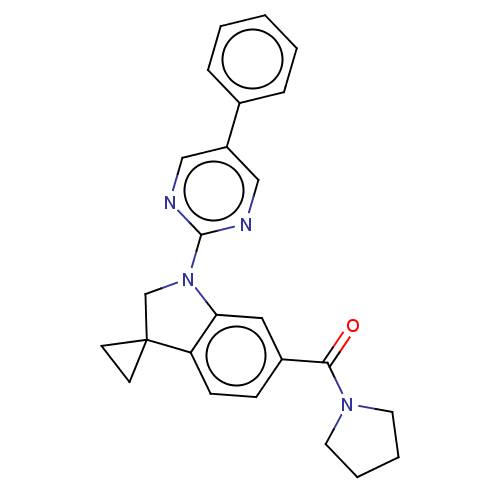 (US10189854, Compound 59 | [1-(5-Phenyl-pyrimidin-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332106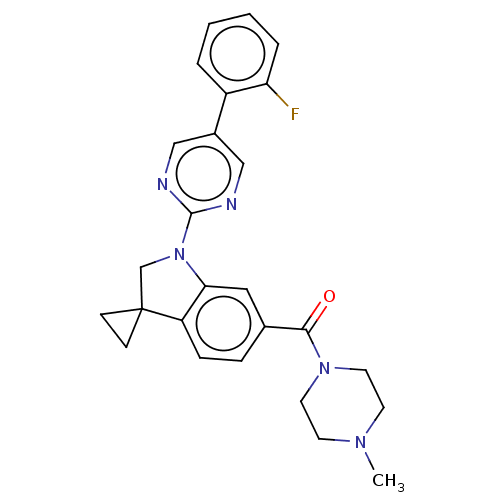 (US10189854, Compound 60 | [1-[5-(2-Fluorophenyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332109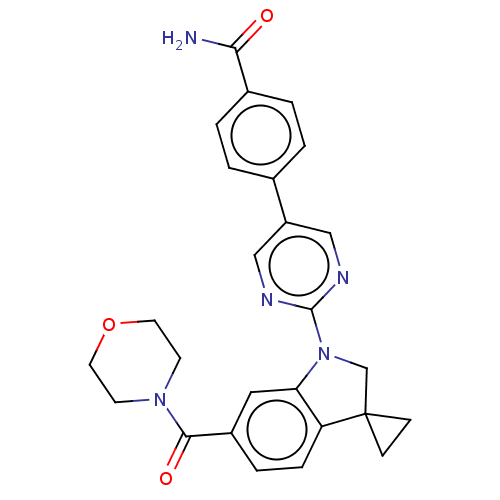 (4-[2-[6-(Morpholine-4-carbonyl)-spiro[1,2-dihydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332111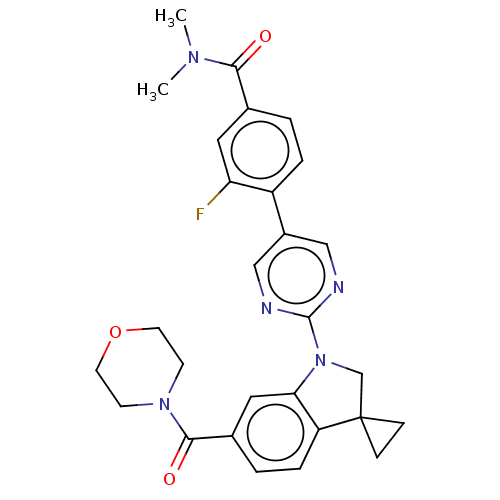 (3-Fluoro-N,N-dimethyl-4-[2-[6-(morpholine-4-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332118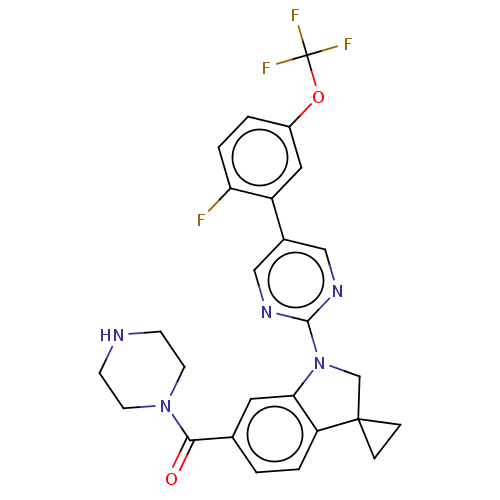 (US10189854, Compound 72 | [1-[5-[2-Fluoro-5-(trifl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332119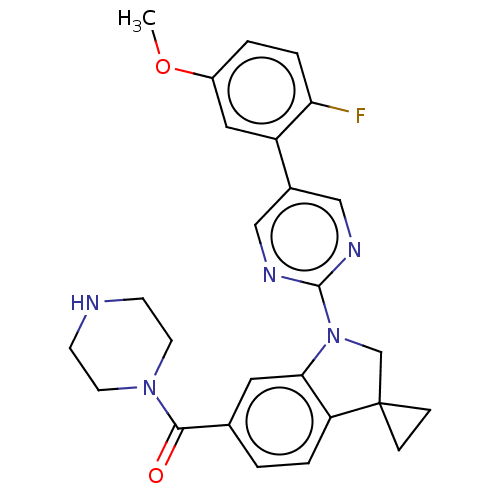 (US10189854, Compound 73 | [1-[5-(2-Fluoro-5-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332128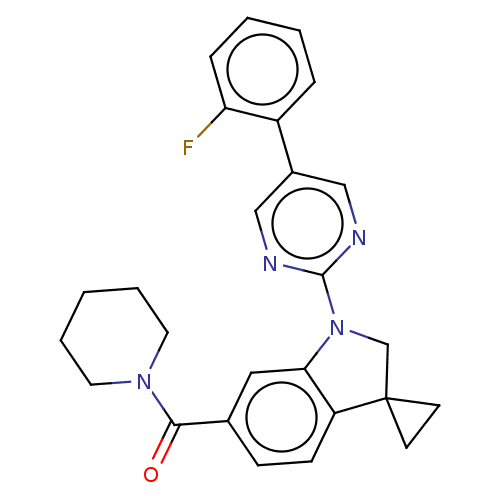 (US10189854, Compound 84 | [1-[5-(2-Fluorophenyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM332129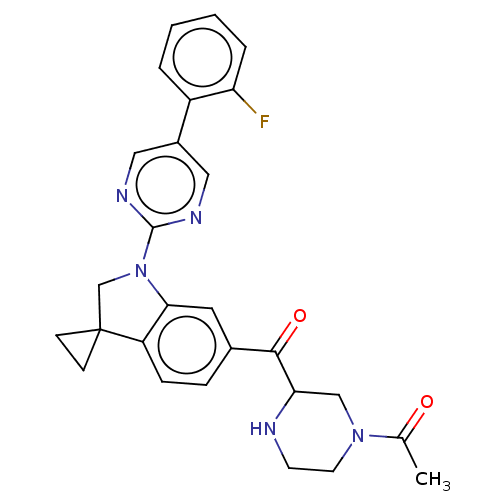 (1-[5-[1-[5-(2-Fluorophenyl)-pyrimidin-2-yl]-spiro[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The inhibiting effect of the compounds on the enzyme activity of human PDE4B1 was measured by the quantification of 5′-adenosine monophosphate ... | US Patent US10189854 (2019) BindingDB Entry DOI: 10.7270/Q2N87CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 446 total ) | Next | Last >> |