 Found 805 hits with Last Name = 'hickey' and Initial = 'm'
Found 805 hits with Last Name = 'hickey' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384817
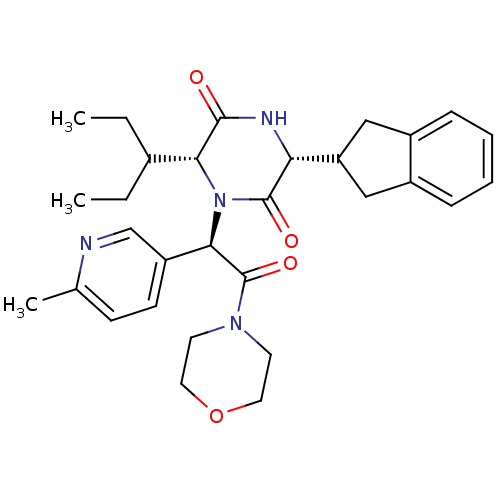
(CHEMBL2037514)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-4-20(5-2)26-28(35)32-25(24-16-21-8-6-7-9-22(21)17-24)29(36)34(26)27(23-11-10-19(3)31-18-23)30(37)33-12-14-38-15-13-33/h6-11,18,20,24-27H,4-5,12-17H2,1-3H3,(H,32,35)/t25-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117772
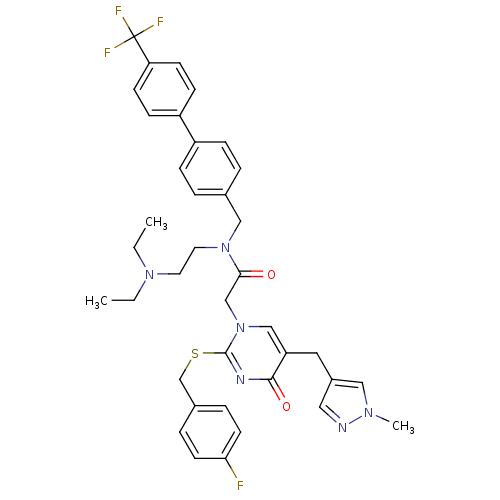
(CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C38H40F4N6O2S/c1-4-46(5-2)18-19-47(23-27-6-10-30(11-7-27)31-12-14-33(15-13-31)38(40,41)42)35(49)25-48-24-32(20-29-21-43-45(3)22-29)36(50)44-37(48)51-26-28-8-16-34(39)17-9-28/h6-17,21-22,24H,4-5,18-20,23,25-26H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Steady state and transient kinetics to a freely reversible, non-covalently bound, human recombinant Phospholipase A2 (rhLp-PLA2) was determined |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384816

(CHEMBL2037516)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H38N4O3/c1-7-19(8-2)25-27(34)31-24(22-15-20-11-9-10-12-21(20)16-22)28(35)33(25)26(29(36)32(5)6)23-14-13-17(3)30-18(23)4/h9-14,19,22,24-26H,7-8,15-16H2,1-6H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384800
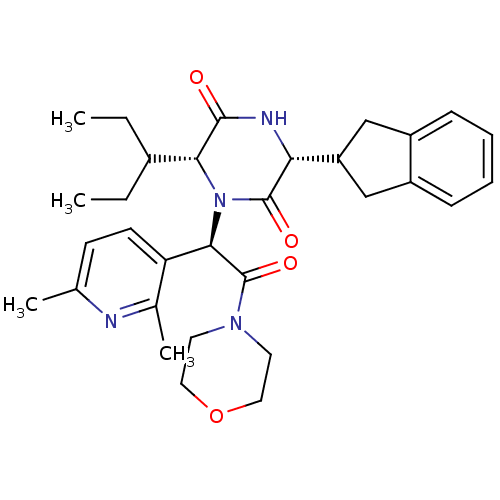
(CHEMBL2037517)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C31H40N4O4/c1-5-21(6-2)27-29(36)33-26(24-17-22-9-7-8-10-23(22)18-24)30(37)35(27)28(25-12-11-19(3)32-20(25)4)31(38)34-13-15-39-16-14-34/h7-12,21,24,26-28H,5-6,13-18H2,1-4H3,(H,33,36)/t26-,27-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384823
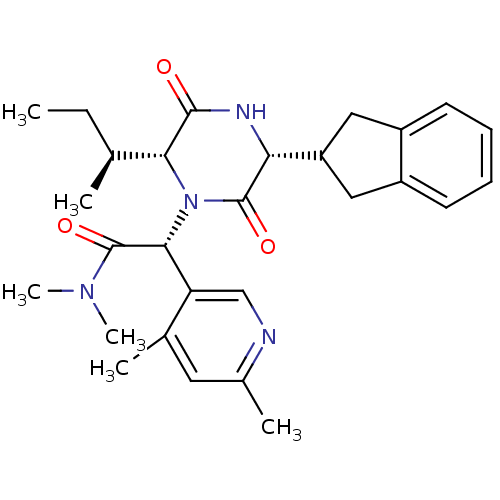
(CHEMBL2037507)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-7-16(2)24-26(33)30-23(21-13-19-10-8-9-11-20(19)14-21)27(34)32(24)25(28(35)31(5)6)22-15-29-18(4)12-17(22)3/h8-12,15-16,21,23-25H,7,13-14H2,1-6H3,(H,30,33)/t16-,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384822
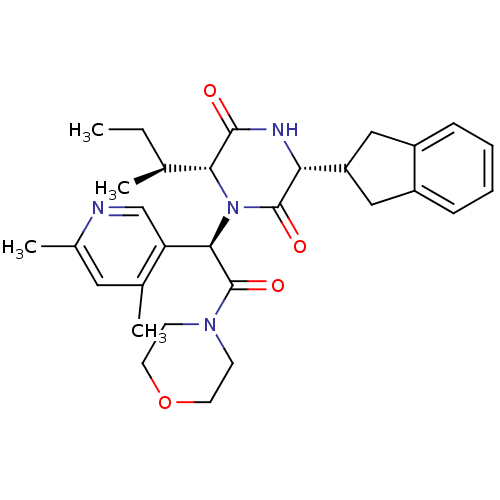
(CHEMBL2037508)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-5-18(2)26-28(35)32-25(23-15-21-8-6-7-9-22(21)16-23)29(36)34(26)27(24-17-31-20(4)14-19(24)3)30(37)33-10-12-38-13-11-33/h6-9,14,17-18,23,25-27H,5,10-13,15-16H2,1-4H3,(H,32,35)/t18-,25+,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384837

(CHEMBL2037515)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-6-18(7-2)24-27(34)31-23(21-14-19-10-8-9-11-20(19)15-21)28(35)32(24)25(26(33)29-5)22-13-12-16(3)30-17(22)4/h8-13,18,21,23-25H,6-7,14-15H2,1-5H3,(H,29,33)(H,31,34)/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384818
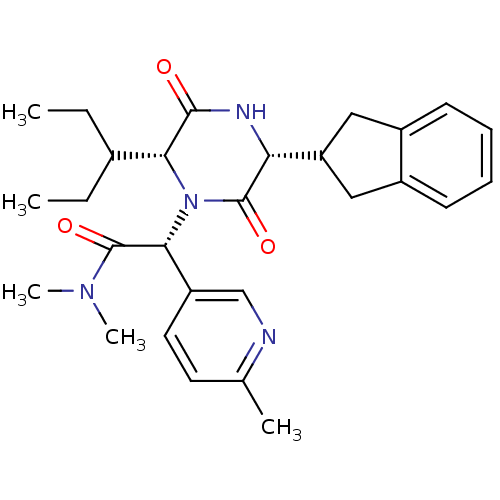
(CHEMBL2037513)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-6-18(7-2)24-26(33)30-23(22-14-19-10-8-9-11-20(19)15-22)27(34)32(24)25(28(35)31(4)5)21-13-12-17(3)29-16-21/h8-13,16,18,22-25H,6-7,14-15H2,1-5H3,(H,30,33)/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384815
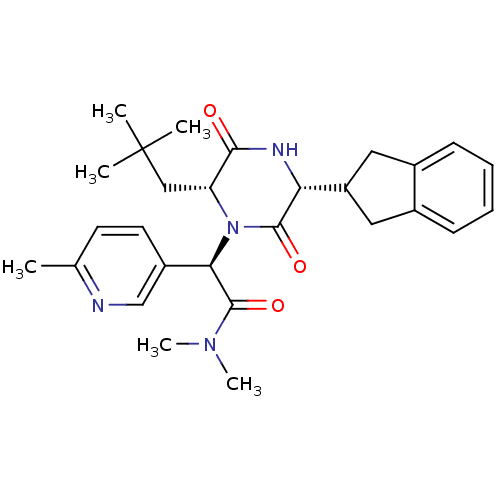
(CHEMBL2037496)Show SMILES CN(C)C(=O)[C@H](N1[C@H](CC(C)(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)c1ccc(C)nc1 |r| Show InChI InChI=1S/C28H36N4O3/c1-17-11-12-20(16-29-17)24(27(35)31(5)6)32-22(15-28(2,3)4)25(33)30-23(26(32)34)21-13-18-9-7-8-10-19(18)14-21/h7-12,16,21-24H,13-15H2,1-6H3,(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384824
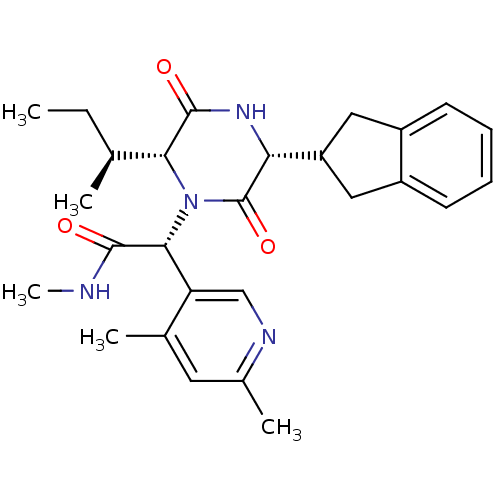
(CHEMBL2037506)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)NC)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-15(2)23-26(33)30-22(20-12-18-9-7-8-10-19(18)13-20)27(34)31(23)24(25(32)28-5)21-14-29-17(4)11-16(21)3/h7-11,14-15,20,22-24H,6,12-13H2,1-5H3,(H,28,32)(H,30,33)/t15-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50190528
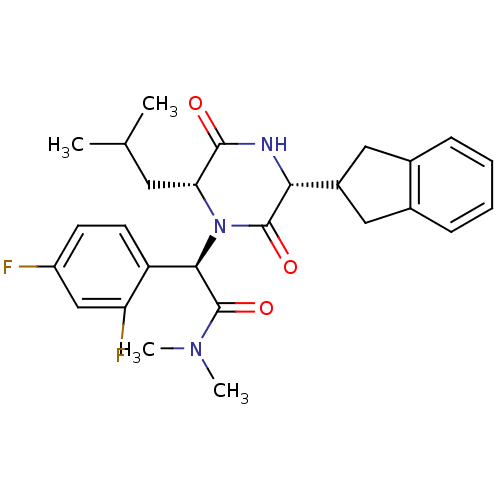
((2R)-2-(2,4-difluorophenyl)-2-[(3R,6R)-3-(2,3-dihy...)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(F)cc2F)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 Show InChI InChI=1S/C27H31F2N3O3/c1-15(2)11-22-25(33)30-23(18-12-16-7-5-6-8-17(16)13-18)26(34)32(22)24(27(35)31(3)4)20-10-9-19(28)14-21(20)29/h5-10,14-15,18,22-24H,11-13H2,1-4H3,(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384838
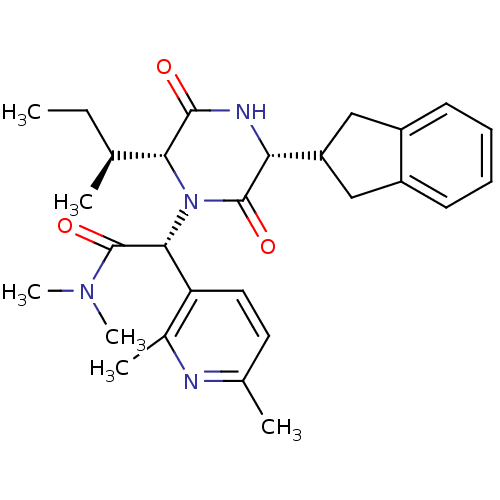
(CHEMBL2037510)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-7-16(2)24-26(33)30-23(21-14-19-10-8-9-11-20(19)15-21)27(34)32(24)25(28(35)31(5)6)22-13-12-17(3)29-18(22)4/h8-13,16,21,23-25H,7,14-15H2,1-6H3,(H,30,33)/t16-,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384834
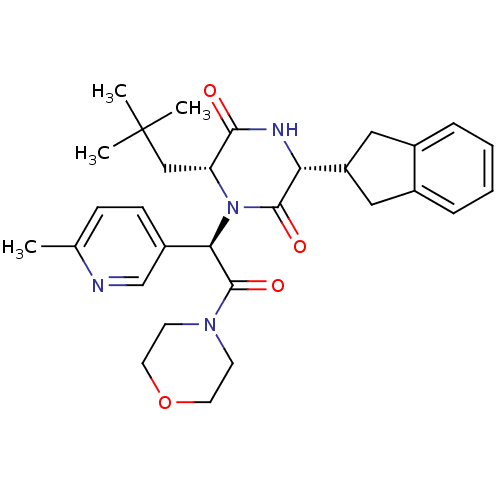
(CHEMBL2037497)Show SMILES Cc1ccc(cn1)[C@@H](N1[C@H](CC(C)(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C30H38N4O4/c1-19-9-10-22(18-31-19)26(29(37)33-11-13-38-14-12-33)34-24(17-30(2,3)4)27(35)32-25(28(34)36)23-15-20-7-5-6-8-21(20)16-23/h5-10,18,23-26H,11-17H2,1-4H3,(H,32,35)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8336
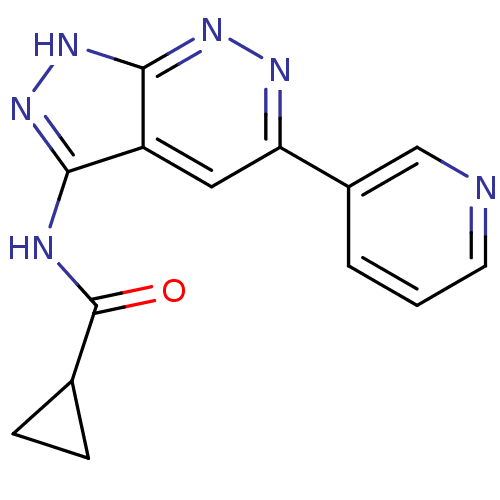
(N-[5-(pyridin-3-yl)-1H-pyrazolo[3,4-c]pyridazin-3-...)Show InChI InChI=1S/C14H12N6O/c21-14(8-3-4-8)16-12-10-6-11(9-2-1-5-15-7-9)17-19-13(10)20-18-12/h1-2,5-8H,3-4H2,(H2,16,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... |
Bioorg Med Chem Lett 13: 1581-4 (2003)
Article DOI: 10.1016/s0960-894x(03)00135-5
BindingDB Entry DOI: 10.7270/Q2BK19JV |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384819
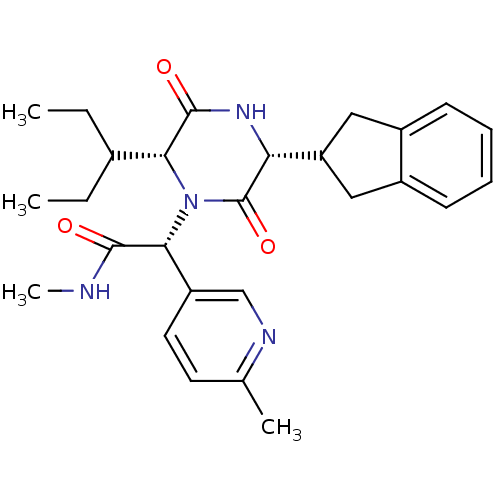
(CHEMBL2037512)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-5-17(6-2)23-26(33)30-22(21-13-18-9-7-8-10-19(18)14-21)27(34)31(23)24(25(32)28-4)20-12-11-16(3)29-15-20/h7-12,15,17,21-24H,5-6,13-14H2,1-4H3,(H,28,32)(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125265
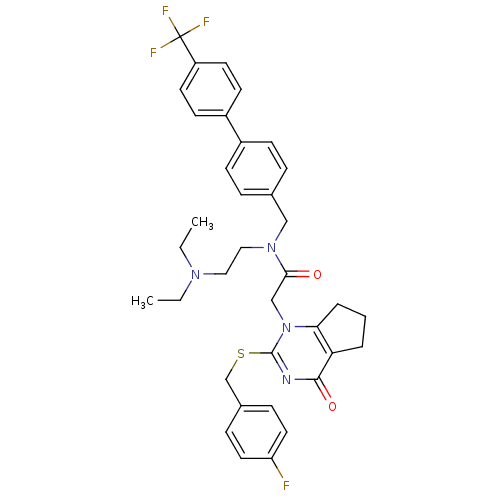
(CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H38F4N4O2S/c1-3-42(4-2)20-21-43(22-25-8-12-27(13-9-25)28-14-16-29(17-15-28)36(38,39)40)33(45)23-44-32-7-5-6-31(32)34(46)41-35(44)47-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8337

(N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...)Show SMILES Fc1cccc(c1F)-c1cc2c(NC(=O)C3CCCC3)n[nH]c2nn1 Show InChI InChI=1S/C17H15F2N5O/c18-12-7-3-6-10(14(12)19)13-8-11-15(22-24-16(11)23-21-13)20-17(25)9-4-1-2-5-9/h3,6-9H,1-2,4-5H2,(H2,20,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... |
Bioorg Med Chem Lett 13: 1581-4 (2003)
Article DOI: 10.1016/s0960-894x(03)00135-5
BindingDB Entry DOI: 10.7270/Q2BK19JV |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384820
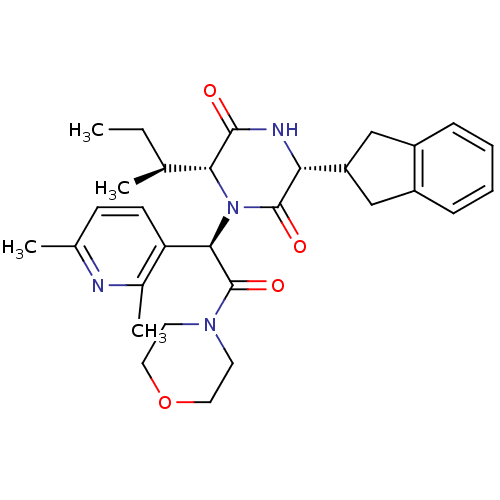
(EPELSIBAN | GSK557296B)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-5-18(2)26-28(35)32-25(23-16-21-8-6-7-9-22(21)17-23)29(36)34(26)27(24-11-10-19(3)31-20(24)4)30(37)33-12-14-38-15-13-33/h6-11,18,23,25-27H,5,12-17H2,1-4H3,(H,32,35)/t18-,25+,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384803
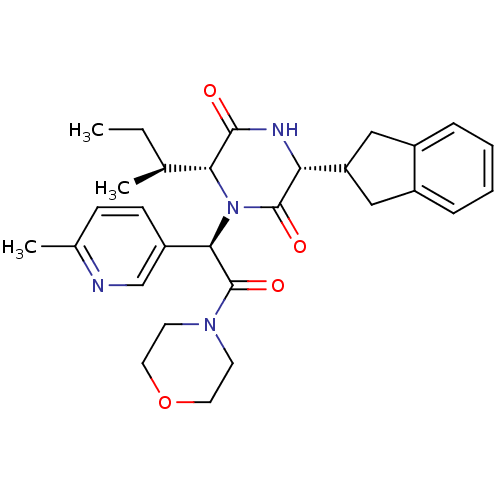
(CHEMBL2037501)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-4-18(2)25-27(34)31-24(23-15-20-7-5-6-8-21(20)16-23)28(35)33(25)26(22-10-9-19(3)30-17-22)29(36)32-11-13-37-14-12-32/h5-10,17-18,23-26H,4,11-16H2,1-3H3,(H,31,34)/t18-,24+,25+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8339

(N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...)Show SMILES CCN1CCC(CC(=O)Nc2n[nH]c3nnc(cc23)-c2cccc(F)c2F)CC1 Show InChI InChI=1S/C20H22F2N6O/c1-2-28-8-6-12(7-9-28)10-17(29)23-19-14-11-16(24-26-20(14)27-25-19)13-4-3-5-15(21)18(13)22/h3-5,11-12H,2,6-10H2,1H3,(H2,23,25,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... |
Bioorg Med Chem Lett 13: 1581-4 (2003)
Article DOI: 10.1016/s0960-894x(03)00135-5
BindingDB Entry DOI: 10.7270/Q2BK19JV |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384805
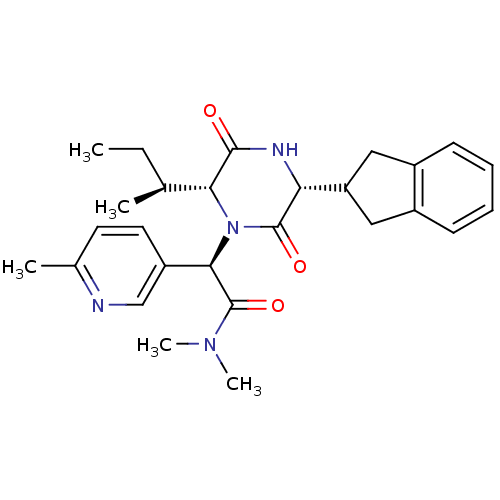
(CHEMBL2037499)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-16(2)23-25(32)29-22(21-13-18-9-7-8-10-19(18)14-21)26(33)31(23)24(27(34)30(4)5)20-12-11-17(3)28-15-20/h7-12,15-16,21-24H,6,13-14H2,1-5H3,(H,29,32)/t16-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384836
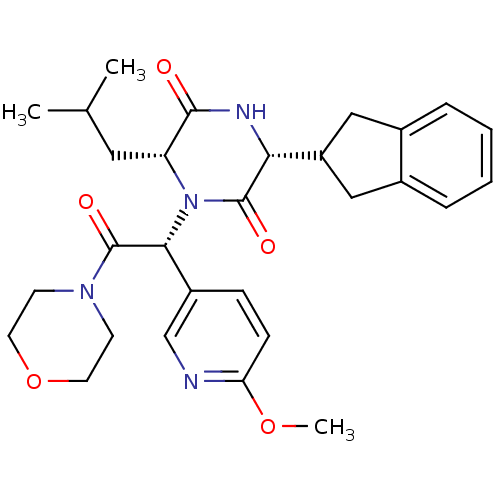
(CHEMBL2037487)Show SMILES COc1ccc(cn1)[C@@H](N1[C@H](CC(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C29H36N4O5/c1-18(2)14-23-27(34)31-25(22-15-19-6-4-5-7-20(19)16-22)28(35)33(23)26(21-8-9-24(37-3)30-17-21)29(36)32-10-12-38-13-11-32/h4-9,17-18,22-23,25-26H,10-16H2,1-3H3,(H,31,34)/t23-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384812
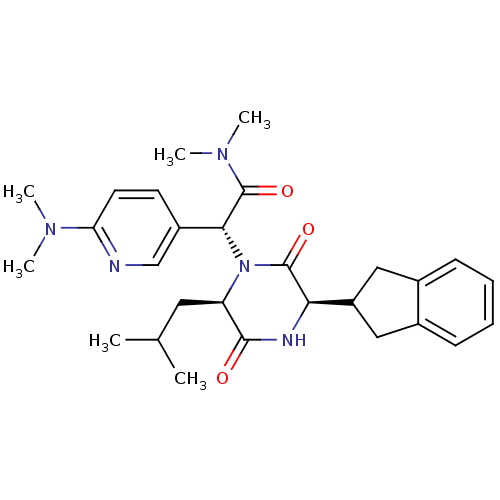
(CHEMBL2037489)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(nc2)N(C)C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H37N5O3/c1-17(2)13-22-26(34)30-24(21-14-18-9-7-8-10-19(18)15-21)27(35)33(22)25(28(36)32(5)6)20-11-12-23(29-16-20)31(3)4/h7-12,16-17,21-22,24-25H,13-15H2,1-6H3,(H,30,34)/t22-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384811
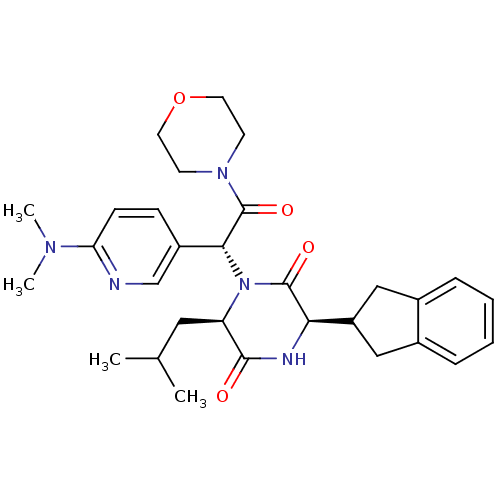
(CHEMBL2037490)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(nc2)N(C)C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H39N5O4/c1-19(2)15-24-28(36)32-26(23-16-20-7-5-6-8-21(20)17-23)29(37)35(24)27(30(38)34-11-13-39-14-12-34)22-9-10-25(31-18-22)33(3)4/h5-10,18-19,23-24,26-27H,11-17H2,1-4H3,(H,32,36)/t24-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384835
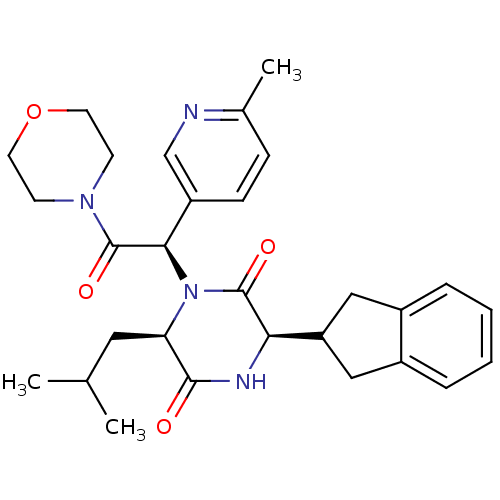
(CHEMBL2037492)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-18(2)14-24-27(34)31-25(23-15-20-6-4-5-7-21(20)16-23)28(35)33(24)26(22-9-8-19(3)30-17-22)29(36)32-10-12-37-13-11-32/h4-9,17-18,23-26H,10-16H2,1-3H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384821
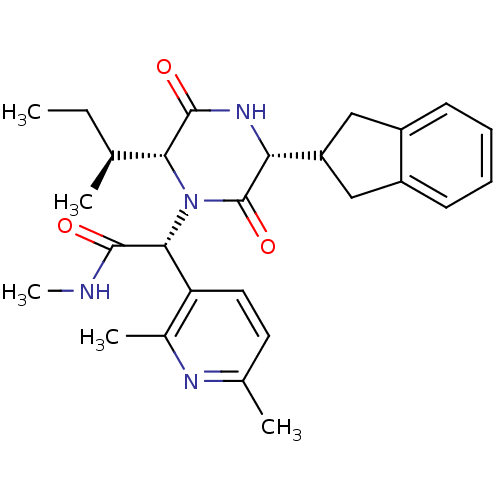
(CHEMBL2037509)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-15(2)23-26(33)30-22(20-13-18-9-7-8-10-19(18)14-20)27(34)31(23)24(25(32)28-5)21-12-11-16(3)29-17(21)4/h7-12,15,20,22-24H,6,13-14H2,1-5H3,(H,28,32)(H,30,33)/t15-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384814
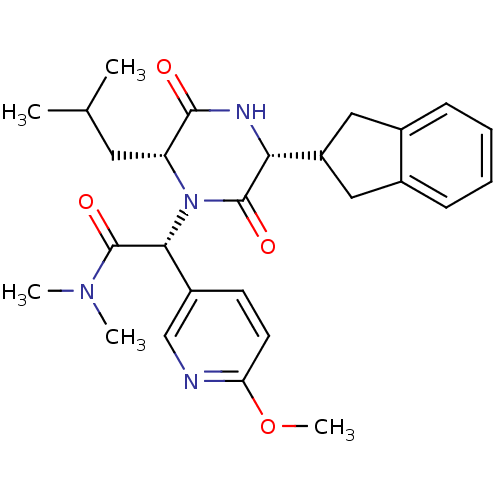
(CHEMBL2037486)Show SMILES COc1ccc(cn1)[C@@H](N1[C@H](CC(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N(C)C |r| Show InChI InChI=1S/C27H34N4O4/c1-16(2)12-21-25(32)29-23(20-13-17-8-6-7-9-18(17)14-20)26(33)31(21)24(27(34)30(3)4)19-10-11-22(35-5)28-15-19/h6-11,15-16,20-21,23-24H,12-14H2,1-5H3,(H,29,32)/t21-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384804
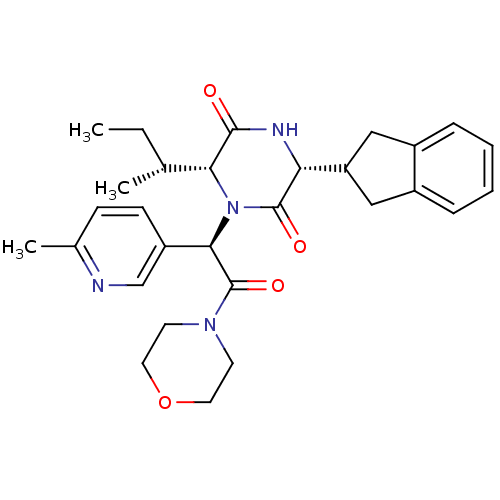
(CHEMBL2037500)Show SMILES CC[C@@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-4-18(2)25-27(34)31-24(23-15-20-7-5-6-8-21(20)16-23)28(35)33(25)26(22-10-9-19(3)30-17-22)29(36)32-11-13-37-14-12-32/h5-10,17-18,23-26H,4,11-16H2,1-3H3,(H,31,34)/t18-,24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384809

(CHEMBL2037493)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N2CCCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O3/c1-18(2)14-24-27(34)31-25(23-15-20-8-4-5-9-21(20)16-23)28(35)33(24)26(22-11-10-19(3)30-17-22)29(36)32-12-6-7-13-32/h4-5,8-11,17-18,23-26H,6-7,12-16H2,1-3H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361648
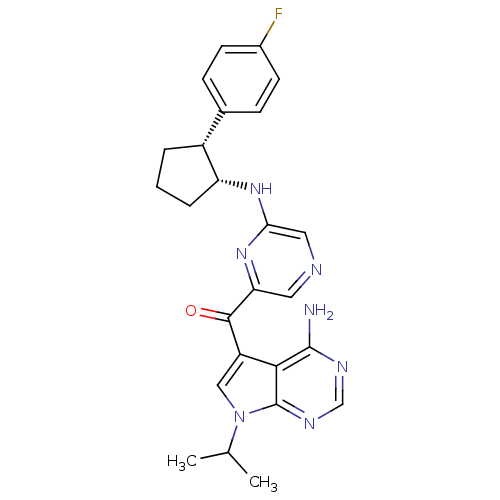
(CHEMBL1940246)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H26FN7O/c1-14(2)33-12-18(22-24(27)29-13-30-25(22)33)23(34)20-10-28-11-21(32-20)31-19-5-3-4-17(19)15-6-8-16(26)9-7-15/h6-14,17,19H,3-5H2,1-2H3,(H,31,32)(H2,27,29,30)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361649
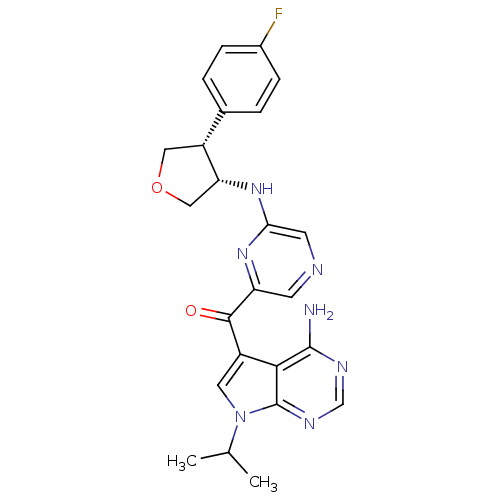
(CHEMBL1938415)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3COC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24FN7O2/c1-13(2)32-9-16(21-23(26)28-12-29-24(21)32)22(33)18-7-27-8-20(30-18)31-19-11-34-10-17(19)14-3-5-15(25)6-4-14/h3-9,12-13,17,19H,10-11H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361642
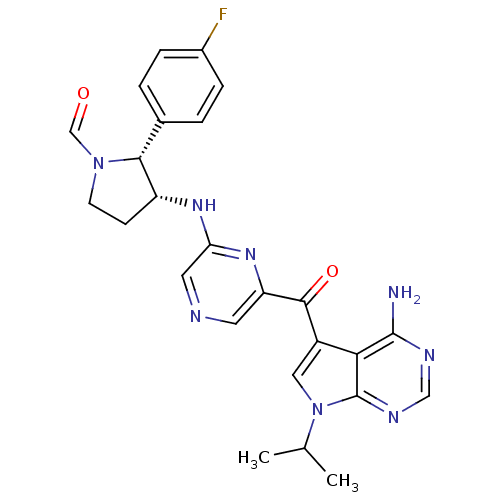
(CHEMBL1940251)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN(C=O)[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H25FN8O2/c1-14(2)34-11-17(21-24(27)29-12-30-25(21)34)23(36)19-9-28-10-20(32-19)31-18-7-8-33(13-35)22(18)15-3-5-16(26)6-4-15/h3-6,9-14,18,22H,7-8H2,1-2H3,(H,31,32)(H2,27,29,30)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384801

(CHEMBL2037504)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2cc(C)cnc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-7-17(3)24-26(33)30-23(21-13-19-10-8-9-11-20(19)14-21)27(34)32(24)25(28(35)31(5)6)22-12-16(2)15-29-18(22)4/h8-12,15,17,21,23-25H,7,13-14H2,1-6H3,(H,30,33)/t17-,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384839
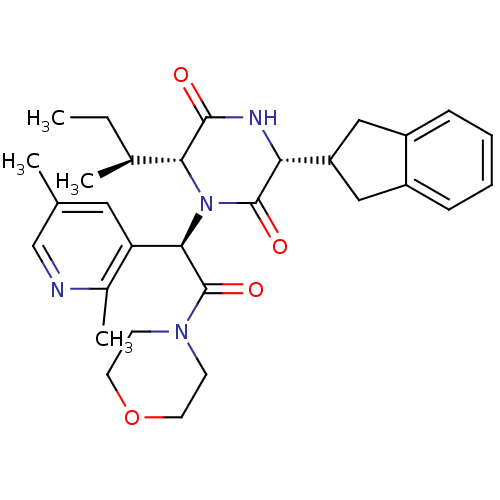
(CHEMBL2037505)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2cc(C)cnc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-5-19(3)26-28(35)32-25(23-15-21-8-6-7-9-22(21)16-23)29(36)34(26)27(24-14-18(2)17-31-20(24)4)30(37)33-10-12-38-13-11-33/h6-9,14,17,19,23,25-27H,5,10-13,15-16H2,1-4H3,(H,32,35)/t19-,25+,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361641
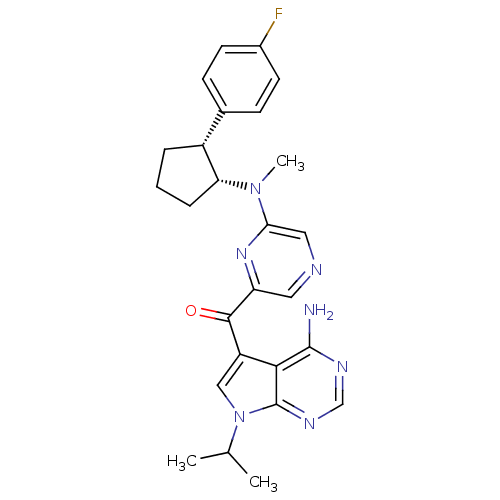
(CHEMBL1940247)Show SMILES CC(C)n1cc(C(=O)c2cncc(n2)N(C)[C@@H]2CCC[C@@H]2c2ccc(F)cc2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H28FN7O/c1-15(2)34-13-19(23-25(28)30-14-31-26(23)34)24(35)20-11-29-12-22(32-20)33(3)21-6-4-5-18(21)16-7-9-17(27)10-8-16/h7-15,18,21H,4-6H2,1-3H3,(H2,28,30,31)/t18-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8338

(N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...)Show SMILES CCN1CCC(CC1)C(=O)Nc1n[nH]c2nnc(cc12)-c1cccc(F)c1F Show InChI InChI=1S/C19H20F2N6O/c1-2-27-8-6-11(7-9-27)19(28)22-17-13-10-15(23-25-18(13)26-24-17)12-4-3-5-14(20)16(12)21/h3-5,10-11H,2,6-9H2,1H3,(H2,22,24,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... |
Bioorg Med Chem Lett 13: 1581-4 (2003)
Article DOI: 10.1016/s0960-894x(03)00135-5
BindingDB Entry DOI: 10.7270/Q2BK19JV |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50372608
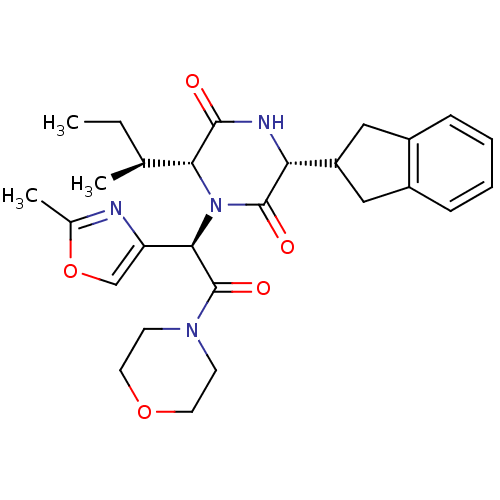
(CHEMBL429736 | GSK-221149A)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2coc(C)n2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 Show InChI InChI=1S/C27H34N4O5/c1-4-16(2)23-25(32)29-22(20-13-18-7-5-6-8-19(18)14-20)26(33)31(23)24(21-15-36-17(3)28-21)27(34)30-9-11-35-12-10-30/h5-8,15-16,20,22-24H,4,9-14H2,1-3H3,(H,29,32)/t16-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human oxytocin receptor |
Bioorg Med Chem Lett 18: 90-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.008
BindingDB Entry DOI: 10.7270/Q2XD12H2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361652
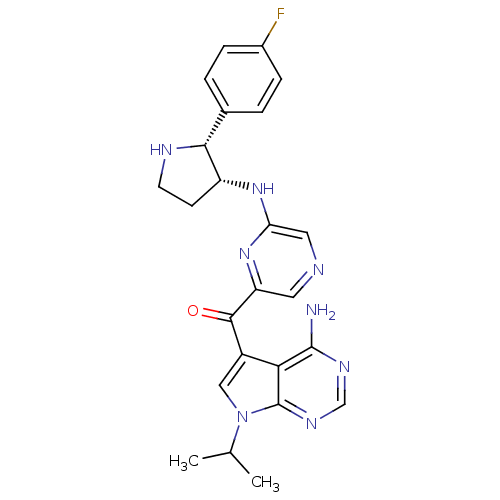
(CHEMBL1940250)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H25FN8O/c1-13(2)33-11-16(20-23(26)29-12-30-24(20)33)22(34)18-9-27-10-19(32-18)31-17-7-8-28-21(17)14-3-5-15(25)6-4-14/h3-6,9-13,17,21,28H,7-8H2,1-2H3,(H,31,32)(H2,26,29,30)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384806

(CHEMBL2037498)Show SMILES CC[C@@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-16(2)23-25(32)29-22(21-13-18-9-7-8-10-19(18)14-21)26(33)31(23)24(27(34)30(4)5)20-12-11-17(3)28-15-20/h7-12,15-16,21-24H,6,13-14H2,1-5H3,(H,29,32)/t16-,22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384833
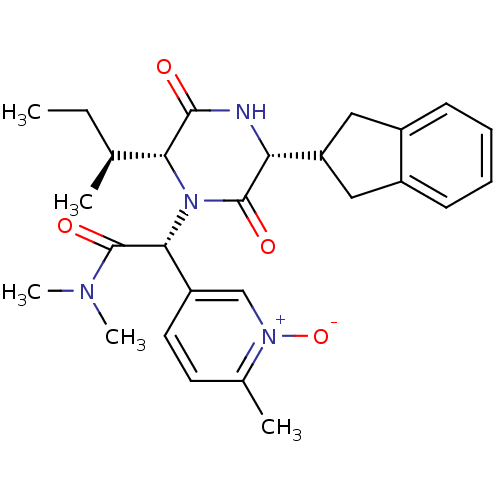
(CHEMBL2037502)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)[n+]([O-])c2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O4/c1-6-16(2)23-25(32)28-22(21-13-18-9-7-8-10-19(18)14-21)26(33)31(23)24(27(34)29(4)5)20-12-11-17(3)30(35)15-20/h7-12,15-16,21-24H,6,13-14H2,1-5H3,(H,28,32)/t16-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384802

(CHEMBL2037503)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)NC)c2cc(C)cnc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-16(3)23-26(33)30-22(20-12-18-9-7-8-10-19(18)13-20)27(34)31(23)24(25(32)28-5)21-11-15(2)14-29-17(21)4/h7-11,14,16,20,22-24H,6,12-13H2,1-5H3,(H,28,32)(H,30,33)/t16-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361650
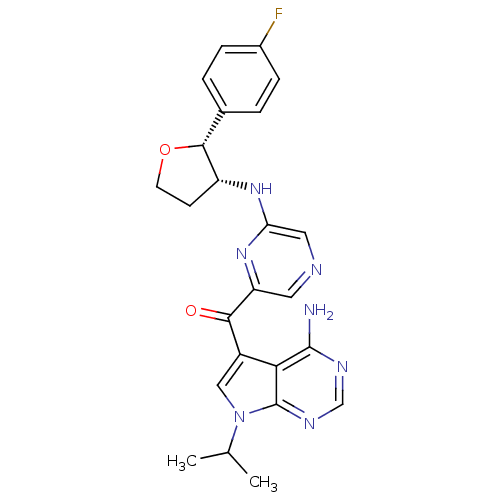
(CHEMBL1940248)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCO[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24FN7O2/c1-13(2)32-11-16(20-23(26)28-12-29-24(20)32)21(33)18-9-27-10-19(31-18)30-17-7-8-34-22(17)14-3-5-15(25)6-4-14/h3-6,9-13,17,22H,7-8H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361644
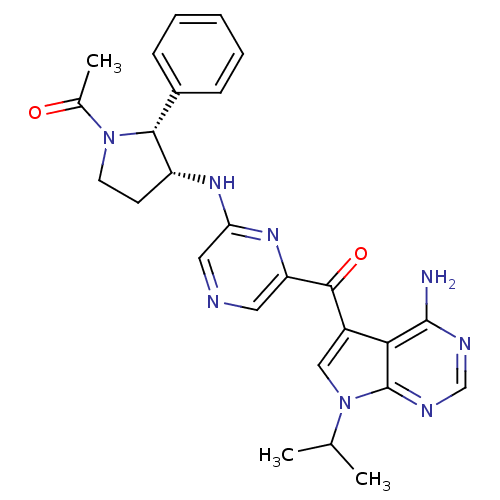
(CHEMBL1940253)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN([C@@H]3c3ccccc3)C(C)=O)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H28N8O2/c1-15(2)34-13-18(22-25(27)29-14-30-26(22)34)24(36)20-11-28-12-21(32-20)31-19-9-10-33(16(3)35)23(19)17-7-5-4-6-8-17/h4-8,11-15,19,23H,9-10H2,1-3H3,(H,31,32)(H2,27,29,30)/t19-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361643
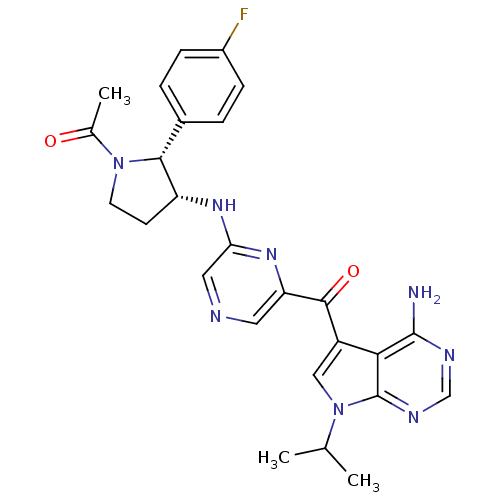
(CHEMBL1940252)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN([C@@H]3c3ccc(F)cc3)C(C)=O)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H27FN8O2/c1-14(2)35-12-18(22-25(28)30-13-31-26(22)35)24(37)20-10-29-11-21(33-20)32-19-8-9-34(15(3)36)23(19)16-4-6-17(27)7-5-16/h4-7,10-14,19,23H,8-9H2,1-3H3,(H,32,33)(H2,28,30,31)/t19-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384810

(CHEMBL2037491)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-16(2)12-22-25(32)29-23(21-13-18-8-6-7-9-19(18)14-21)26(33)31(22)24(27(34)30(4)5)20-11-10-17(3)28-15-20/h6-11,15-16,21-24H,12-14H2,1-5H3,(H,29,32)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361653
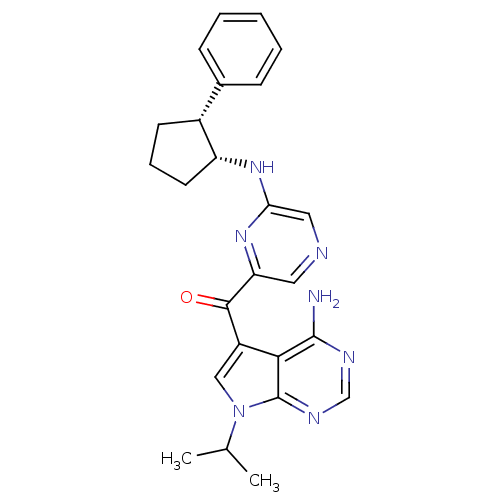
(CHEMBL1940245)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCC[C@@H]3c3ccccc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H27N7O/c1-15(2)32-13-18(22-24(26)28-14-29-25(22)32)23(33)20-11-27-12-21(31-20)30-19-10-6-9-17(19)16-7-4-3-5-8-16/h3-5,7-8,11-15,17,19H,6,9-10H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361651
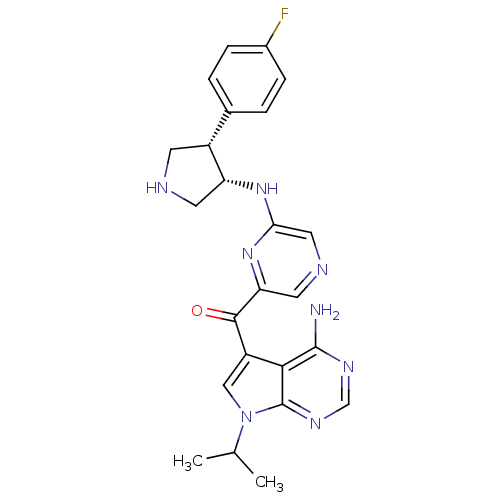
(CHEMBL1940249)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CNC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H25FN8O/c1-13(2)33-11-17(21-23(26)29-12-30-24(21)33)22(34)19-9-28-10-20(32-19)31-18-8-27-7-16(18)14-3-5-15(25)6-4-14/h3-6,9-13,16,18,27H,7-8H2,1-2H3,(H,31,32)(H2,26,29,30)/t16-,18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384813

(CHEMBL2037488)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(=O)[nH]c2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C26H32N4O4/c1-15(2)11-20-24(32)28-22(19-12-16-7-5-6-8-17(16)13-19)25(33)30(20)23(26(34)29(3)4)18-9-10-21(31)27-14-18/h5-10,14-15,19-20,22-23H,11-13H2,1-4H3,(H,27,31)(H,28,32)/t20-,22-,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384831
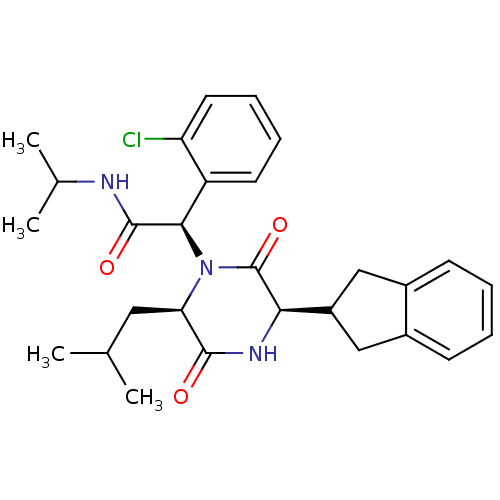
(CHEMBL2035009)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)NC(C)C)c2ccccc2Cl)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H34ClN3O3/c1-16(2)13-23-26(33)31-24(20-14-18-9-5-6-10-19(18)15-20)28(35)32(23)25(27(34)30-17(3)4)21-11-7-8-12-22(21)29/h5-12,16-17,20,23-25H,13-15H2,1-4H3,(H,30,34)(H,31,33)/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human oxytocin receptor assessed as inhibition of oxytocin binding by FLIPR analysis |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50372608

(CHEMBL429736 | GSK-221149A)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2coc(C)n2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 Show InChI InChI=1S/C27H34N4O5/c1-4-16(2)23-25(32)29-22(20-13-18-7-5-6-8-19(18)14-20)26(33)31(23)24(21-15-36-17(3)28-21)27(34)30-9-11-35-12-10-30/h5-8,15-16,20,22-24H,4,9-14H2,1-3H3,(H,29,32)/t16-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant oxytocin receptor |
Bioorg Med Chem Lett 18: 90-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.008
BindingDB Entry DOI: 10.7270/Q2XD12H2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data