

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human recombinant 5-hydroxytryptamine 1B receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50165355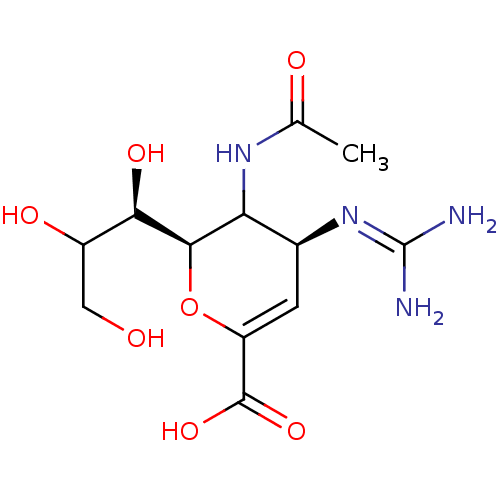 ((2R,4S)-3-(acetylamino)-4-{[(E)-amino(imino)methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against neuraminidase of influenza A/Mem/Bel/71 (H3N1) virus starin; (n=5) | J Med Chem 48: 2964-71 (2005) Article DOI: 10.1021/jm040891b BindingDB Entry DOI: 10.7270/Q2CJ8D10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50165355 ((2R,4S)-3-(acetylamino)-4-{[(E)-amino(imino)methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.48 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against neuraminidase of influenza A/PR/8/34 (H1N1) virus starin; (n=5) | J Med Chem 48: 2964-71 (2005) Article DOI: 10.1021/jm040891b BindingDB Entry DOI: 10.7270/Q2CJ8D10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50033383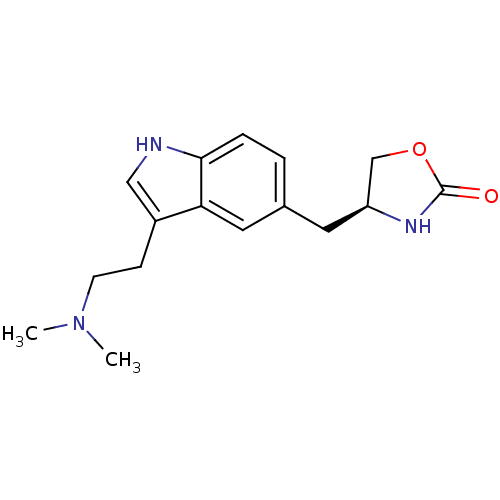 ((S)-4-((3-(2-(dimethylamino)ethyl)-1H-indol-5-yl)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50165355 ((2R,4S)-3-(acetylamino)-4-{[(E)-amino(imino)methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against neuraminidase of influenza A/Chicken/Vietnam/8/2004(H5N1) virus starin; (n=5) | J Med Chem 48: 2964-71 (2005) Article DOI: 10.1021/jm040891b BindingDB Entry DOI: 10.7270/Q2CJ8D10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50473516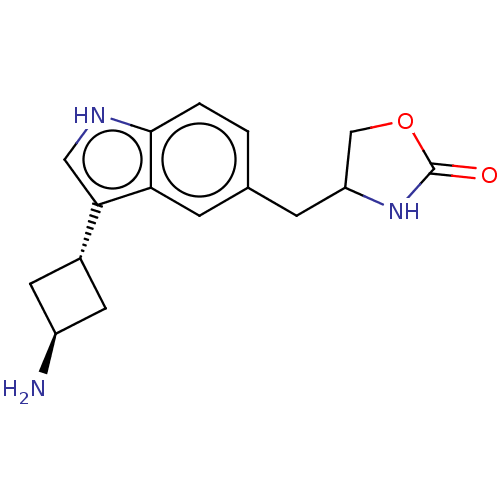 (CHEMBL2110299) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50380884 (CHEMBL2018954) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50380881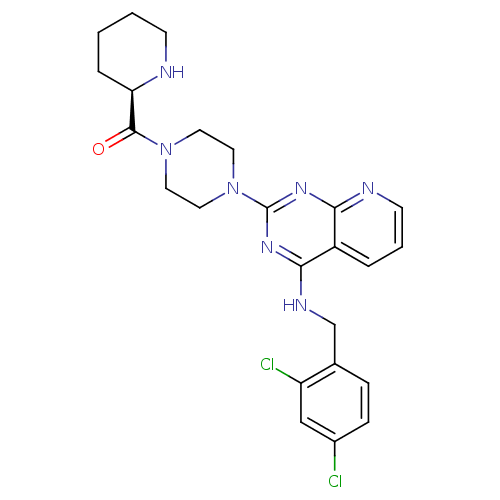 (CHEMBL2018953) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50033383 ((S)-4-((3-(2-(dimethylamino)ethyl)-1H-indol-5-yl)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human recombinant 5-hydroxytryptamine 1B receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490519 (CHEMBL2326625) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490519 (CHEMBL2326625) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490515 (CHEMBL2326611) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50473505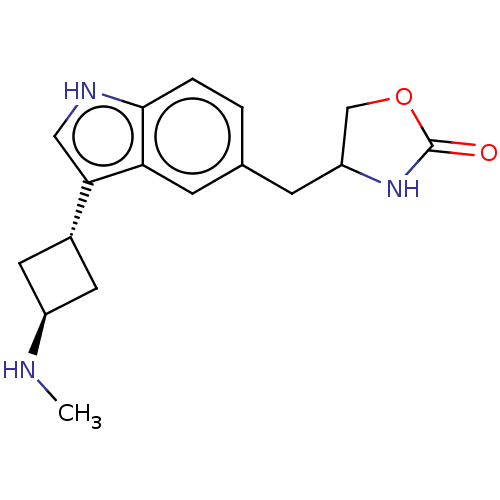 (CHEMBL2368256) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50473521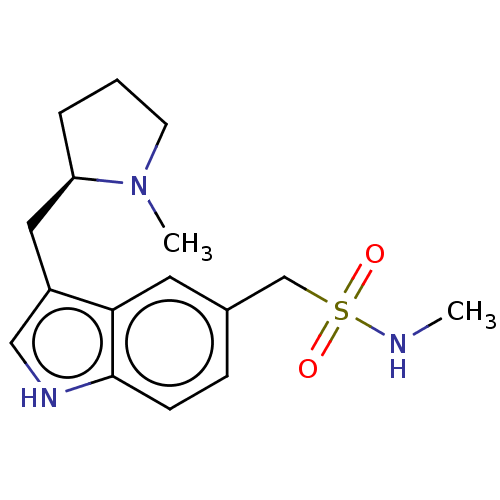 (CHEMBL159332 | CP-122288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50165356 ((2R,4S)-4-carbamimidamido-2-[(1R)-1-({[12-({[(1R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.86 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against neuraminidase of influenza A/Mem/Bel/71 (H3N1) virus starin; (n=5) | J Med Chem 48: 2964-71 (2005) Article DOI: 10.1021/jm040891b BindingDB Entry DOI: 10.7270/Q2CJ8D10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50473512 (CHEMBL2368254) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human recombinant 5-hydroxytryptamine 1B receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490500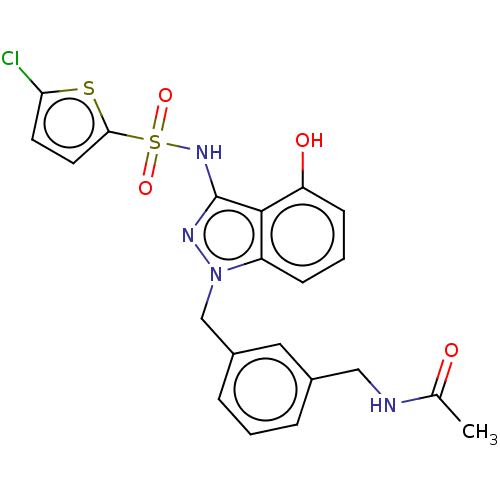 (CHEMBL2321924) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490524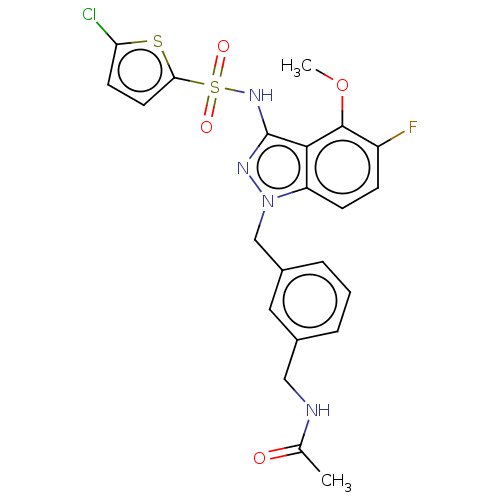 (CHEMBL2326630) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50473512 (CHEMBL2368254) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50473521 (CHEMBL159332 | CP-122288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human recombinant 5-hydroxytryptamine 1B receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50473508 (CHEMBL158638) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50473503 (CHEMBL2110300) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490511 (CHEMBL2326624) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490530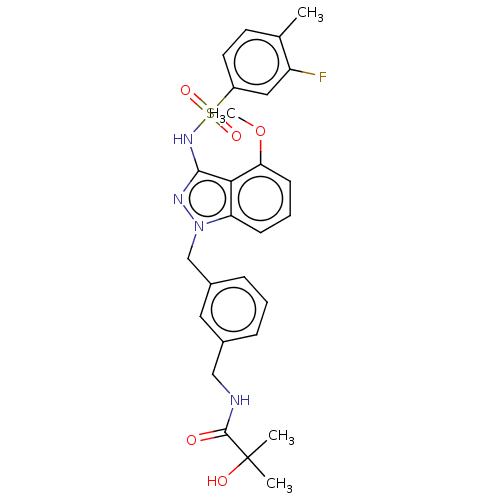 (CHEMBL2326926) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490506 (CHEMBL2326633) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490512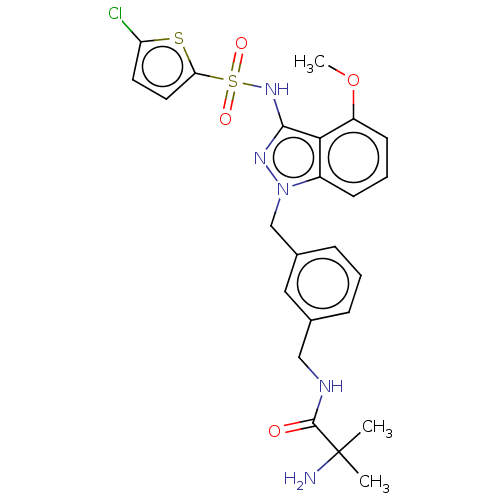 (CHEMBL2326621) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490520 (CHEMBL2326620) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490531 (CHEMBL2326622) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50473516 (CHEMBL2110299) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human recombinant 5-hydroxytryptamine 1B receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490518 (CHEMBL2326613) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490522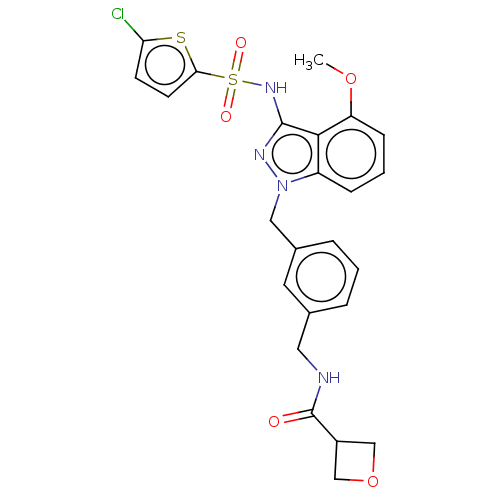 (CHEMBL2326616) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50165356 ((2R,4S)-4-carbamimidamido-2-[(1R)-1-({[12-({[(1R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21.7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against neuraminidase of influenza A/PR/8/34 (H1N1) virus starin; (n=5) | J Med Chem 48: 2964-71 (2005) Article DOI: 10.1021/jm040891b BindingDB Entry DOI: 10.7270/Q2CJ8D10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490517 (CHEMBL2326623) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50473504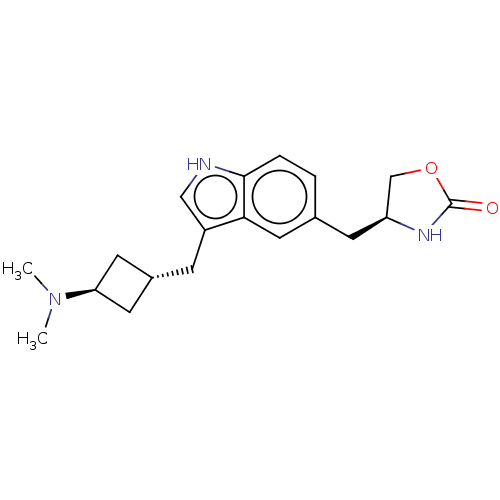 (CHEMBL3084967) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50380867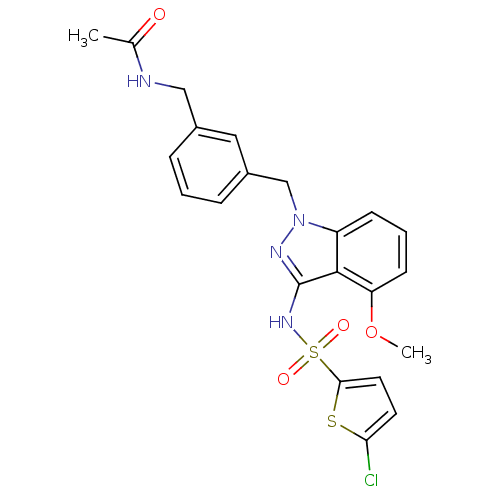 (CHEMBL2018964) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490525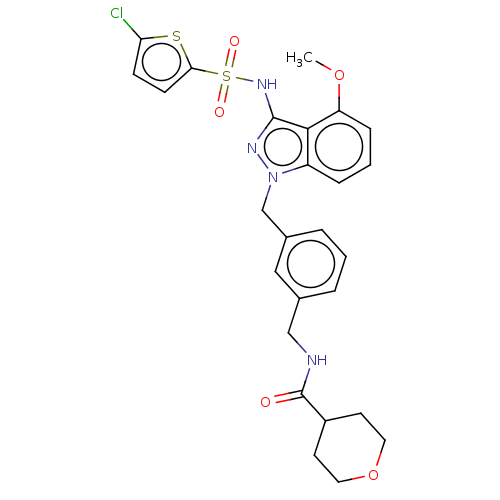 (CHEMBL2326619) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50473505 (CHEMBL2368256) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human recombinant 5-hydroxytryptamine 1B receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490529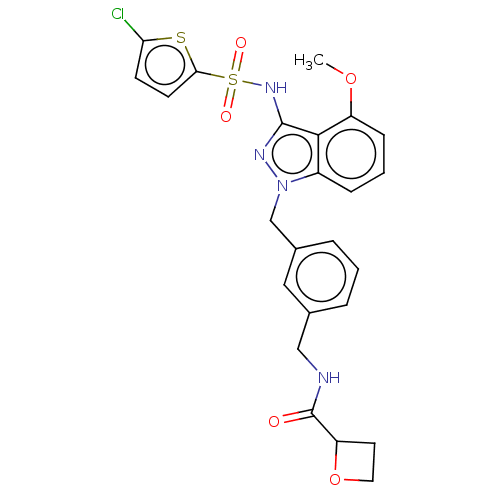 (CHEMBL2326617) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490502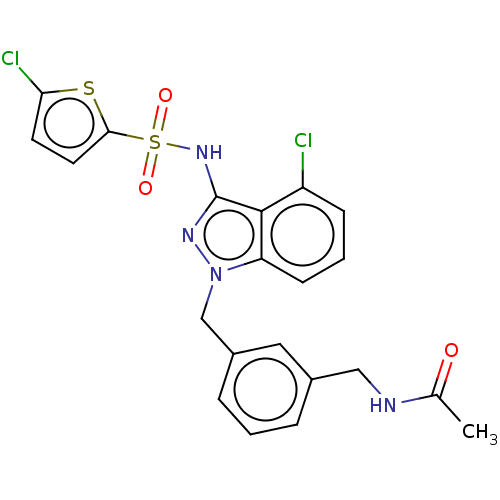 (CHEMBL2326608) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490516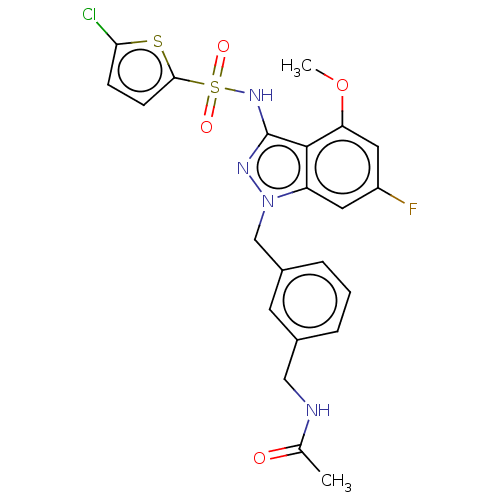 (CHEMBL2326632) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490507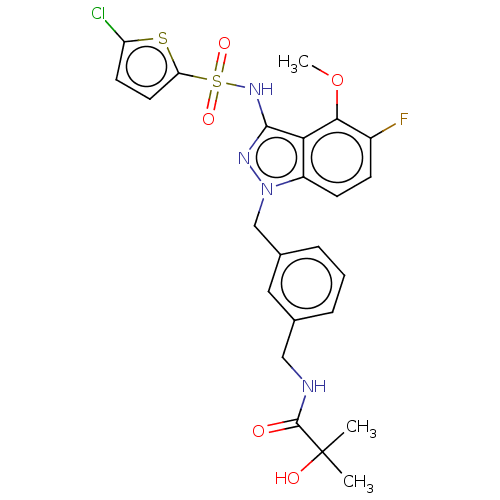 (CHEMBL2326631) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50380880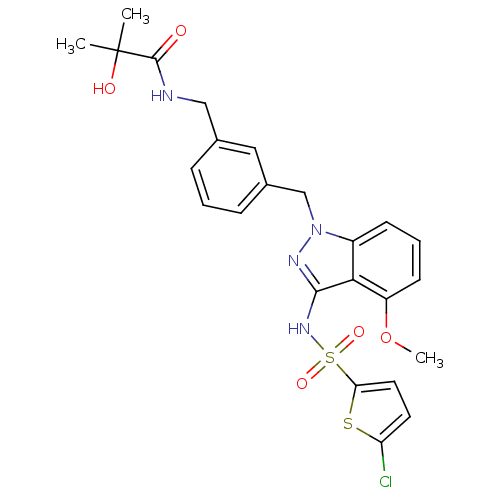 (CHEMBL2018969) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490513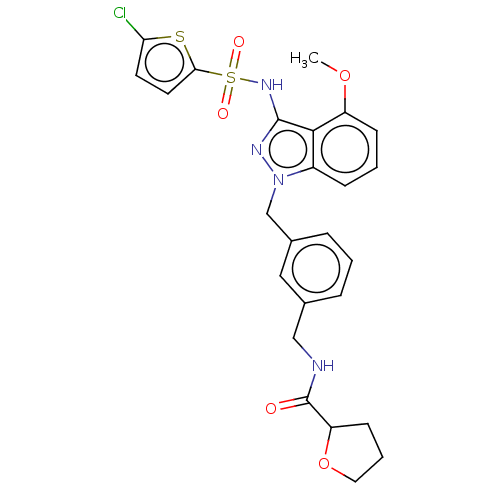 (CHEMBL2326618) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50473508 (CHEMBL158638) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human recombinant 5-hydroxytryptamine 1B receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50490504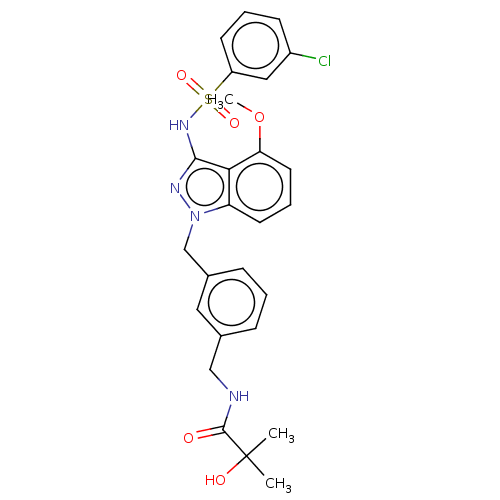 (CHEMBL2326924) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR4 receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | J Med Chem 56: 1946-60 (2013) Article DOI: 10.1021/jm301572h BindingDB Entry DOI: 10.7270/Q26W9F07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50473509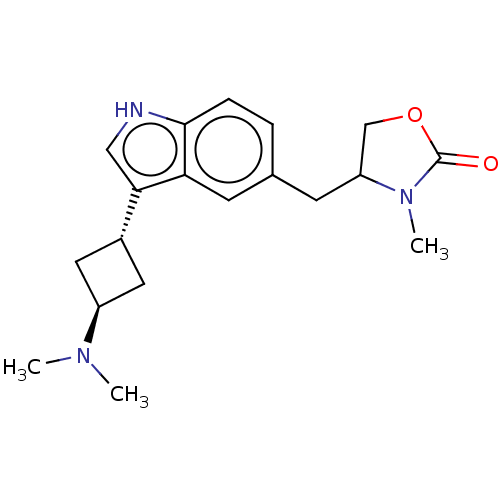 (CHEMBL2368257) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 57.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50473509 (CHEMBL2368257) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50165356 ((2R,4S)-4-carbamimidamido-2-[(1R)-1-({[12-({[(1R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against neuraminidase of influenza A/Chicken/Vietnam/8/2004(H5N1) virus starin; (n=5) | J Med Chem 48: 2964-71 (2005) Article DOI: 10.1021/jm040891b BindingDB Entry DOI: 10.7270/Q2CJ8D10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50473501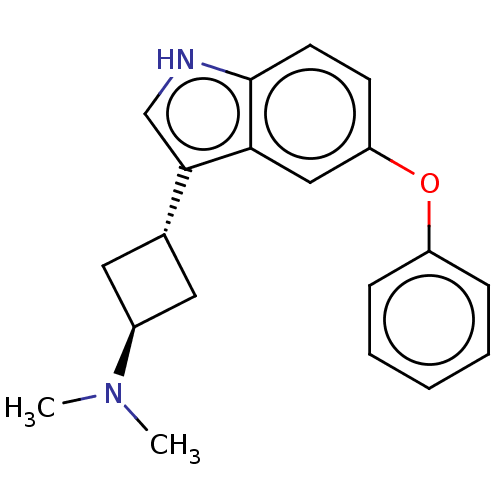 (CHEMBL2368253) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Curated by ChEMBL | Assay Description Binding affinity in CHO-K1 cells transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 44: 681-93 (2001) Article DOI: 10.1021/jm000956k BindingDB Entry DOI: 10.7270/Q2PC354M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 141 total ) | Next | Last >> |