

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50240305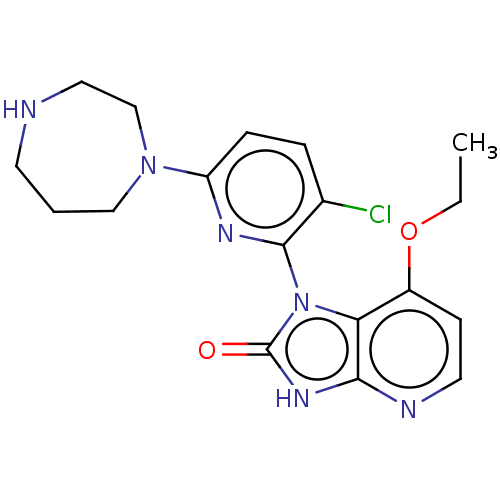 (CHEMBL4082370) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub... | Bioorg Med Chem Lett 27: 2497-2501 (2017) Article DOI: 10.1016/j.bmcl.2017.03.099 BindingDB Entry DOI: 10.7270/Q21G0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50240307 (CHEMBL4065996) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub... | Bioorg Med Chem Lett 27: 2497-2501 (2017) Article DOI: 10.1016/j.bmcl.2017.03.099 BindingDB Entry DOI: 10.7270/Q21G0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98678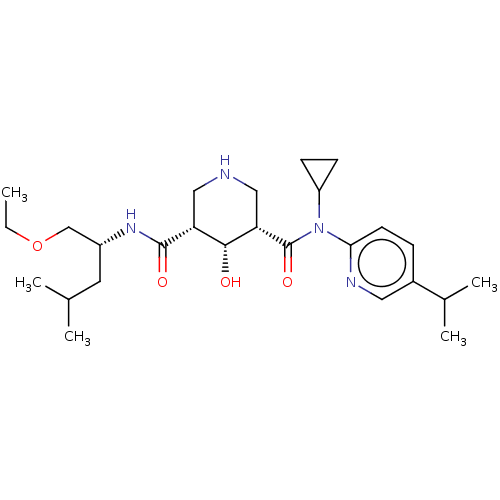 (US8497286, 154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98684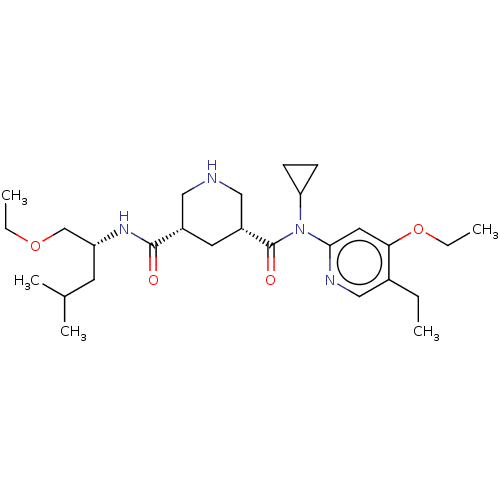 (US8497286, 160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98679 (US8497286, 155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98680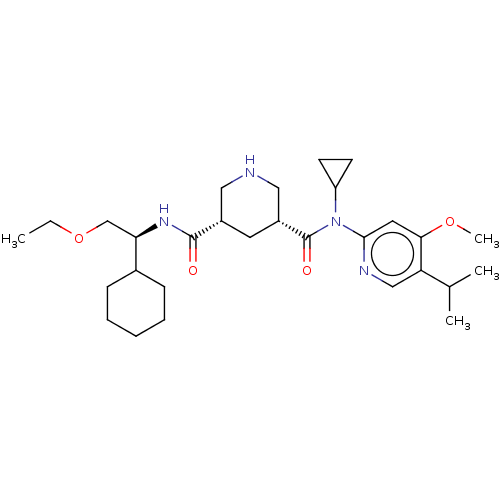 (US8497286, 156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054632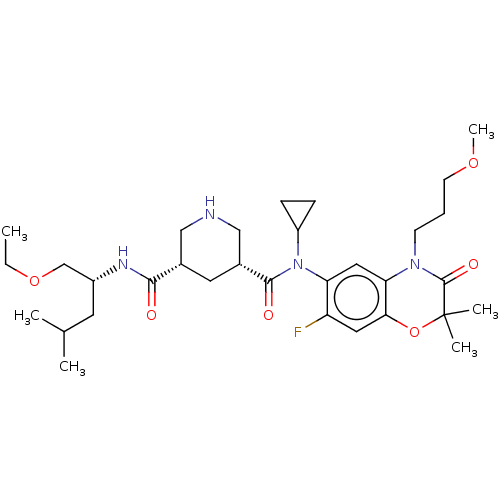 (CHEMBL3318940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054540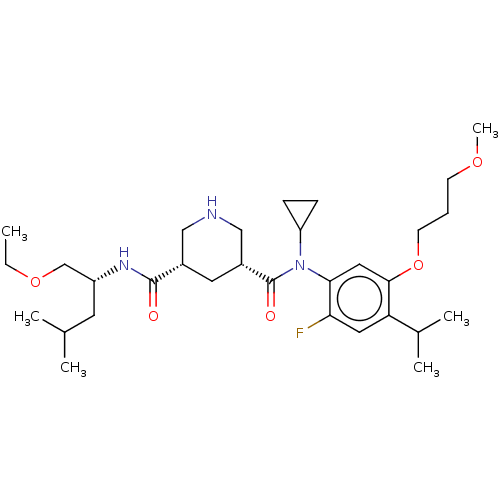 (CHEMBL3318939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98686 (US8497286, 162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98679 (US8497286, 155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98676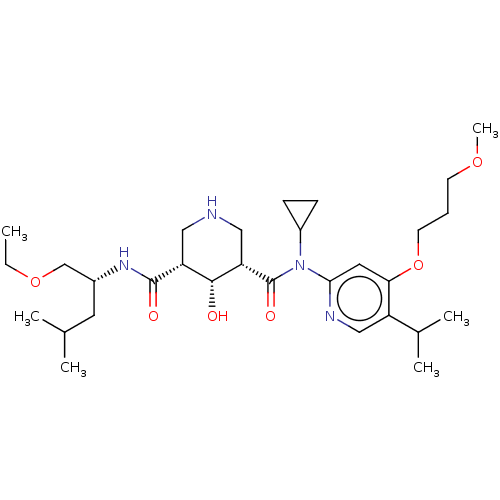 (US8497286, 152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054636 (CHEMBL3318937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054637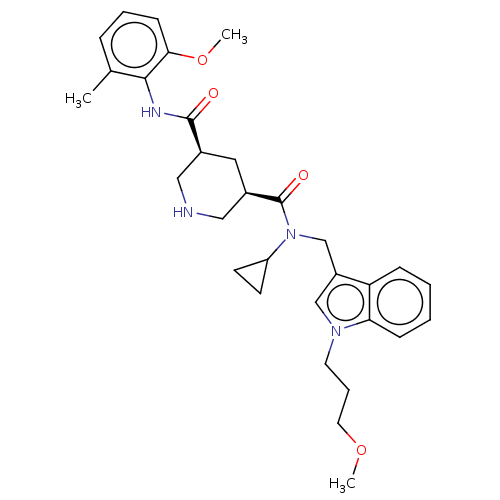 (CHEMBL3318938) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054633 (CHEMBL3318941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98690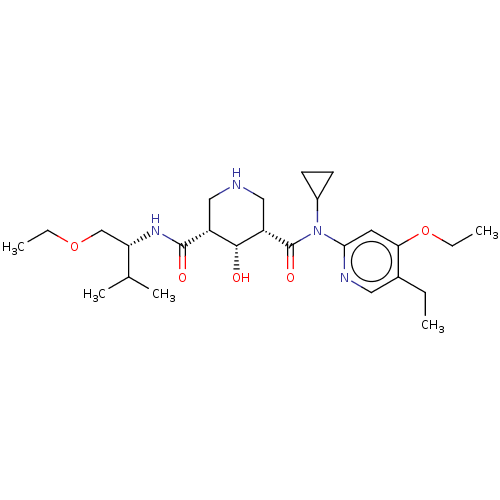 (US8497286, 166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98684 (US8497286, 160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98684 (US8497286, 160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98675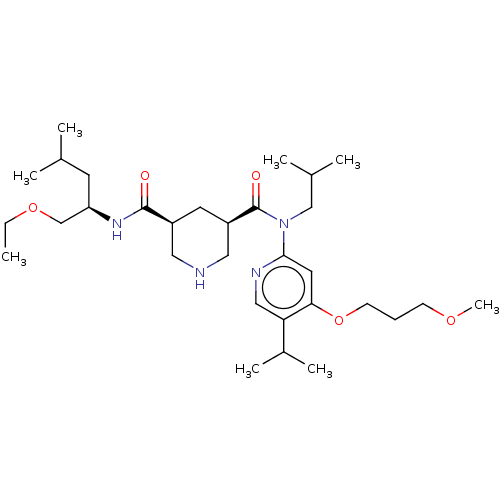 (US8497286, 151) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98686 (US8497286, 162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98685 (US8497286, 161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98682 (US8497286, 158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054634 (CHEMBL3318942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98685 (US8497286, 161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50240298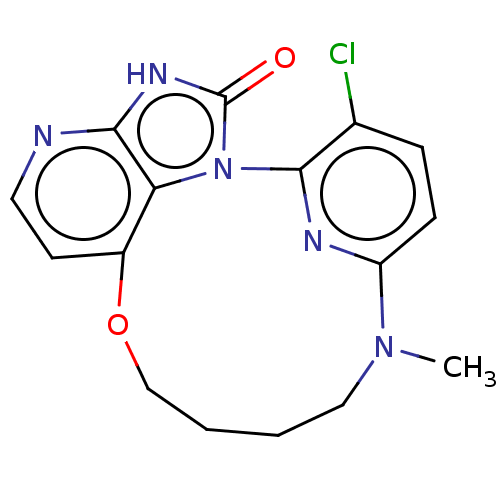 (CHEMBL4092652) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub... | Bioorg Med Chem Lett 27: 2497-2501 (2017) Article DOI: 10.1016/j.bmcl.2017.03.099 BindingDB Entry DOI: 10.7270/Q21G0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98679 (US8497286, 155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98677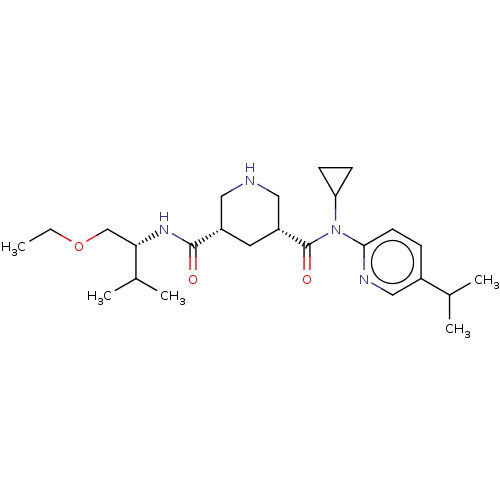 (US8497286, 153) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98673 (US8497286, 149) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98673 (US8497286, 149) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50273289 (2-Cyano-4-(1-methyl-piperidin-4-yloxy)-6-[(spiro[2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054633 (CHEMBL3318941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223939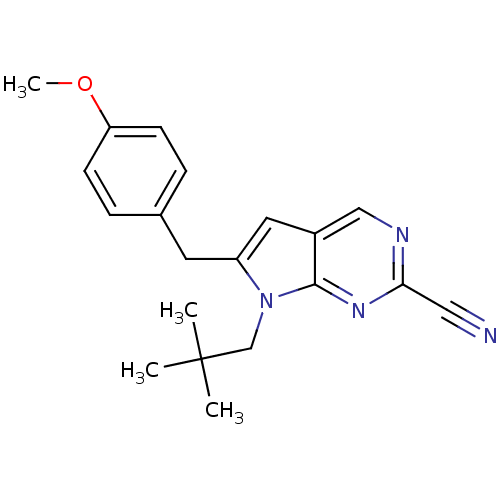 (6-(4-methoxybenzyl)-7-neopentyl-7H-pyrrolo[2,3-d]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223914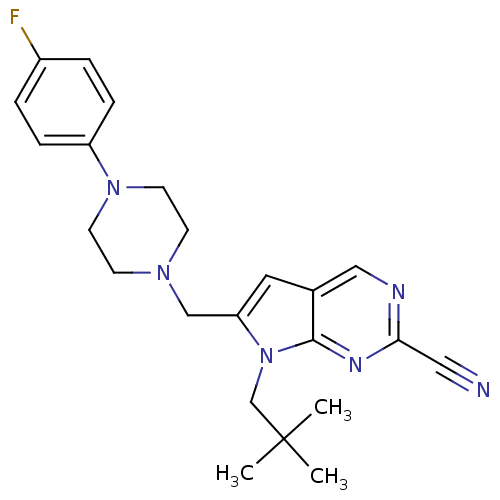 (6-((4-(4-fluorophenyl)piperazin-1-yl)methyl)-7-neo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054636 (CHEMBL3318937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50260529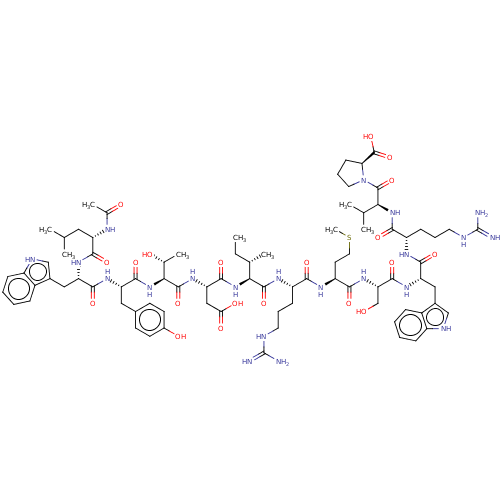 (CHEMBL4077626) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of C-terminal biotin-labelled BCoR (Arg498 to 514Pro residues) binding to recombinant FLAG-tagged BCL6 BTB (5 to 129 residues) (unknown or... | Bioorg Med Chem 25: 4876-4886 (2017) Article DOI: 10.1016/j.bmc.2017.07.037 BindingDB Entry DOI: 10.7270/Q2G44SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223921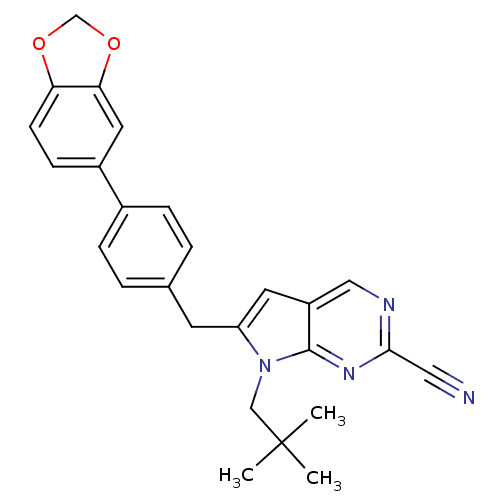 (6-(4-(benzo[d][1,3]dioxol-5-yl)benzyl)-7-neopentyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223935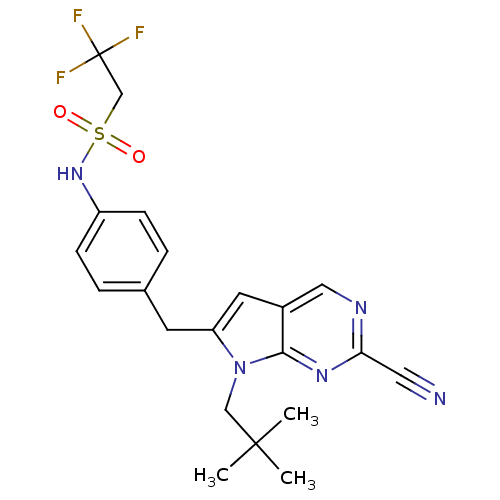 (CHEMBL399842 | N-(4-((2-cyano-7-neopentyl-7H-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98673 (US8497286, 149) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223915 (6-benzyl-7-neopentyl-7H-pyrrolo[2,3-d]pyrimidine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223925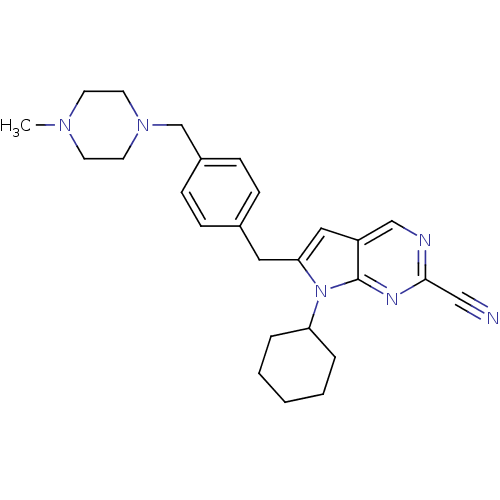 (6-(4-((4-methylpiperazin-1-yl)methyl)benzyl)-7-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223919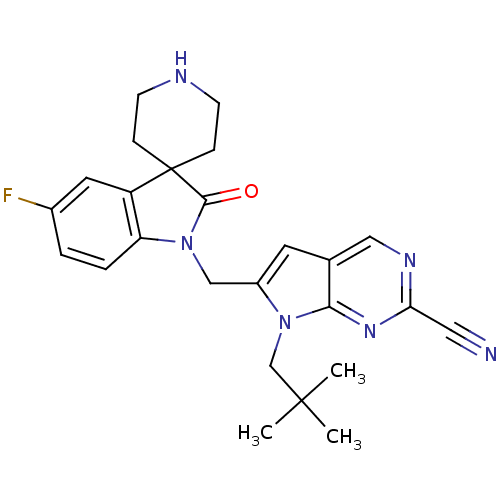 (7-(2,2-dimethylpropyl)-6-[(5-fluoro-2-oxospiro[ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50252500 (6-(4-chlorobenzyl)-7-(3,3-dimethylbutyl)-7H-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054540 (CHEMBL3318939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98683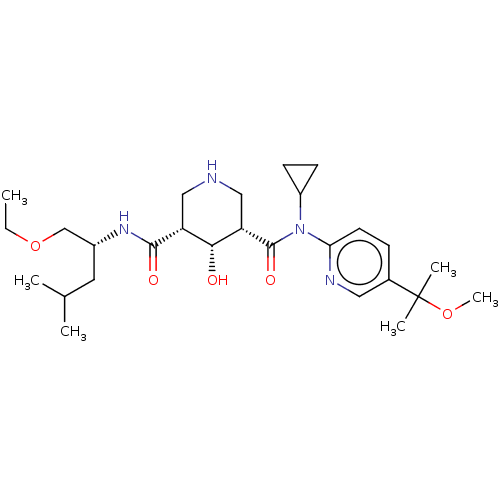 (US8497286, 159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50251889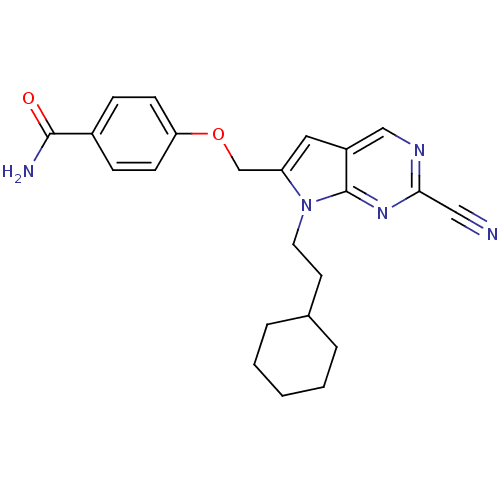 (4-((2-cyano-7-(2-cyclohexylethyl)-7H-pyrrolo[2,3-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem Lett 18: 3959-62 (2008) Article DOI: 10.1016/j.bmcl.2008.06.009 BindingDB Entry DOI: 10.7270/Q23N235B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50272786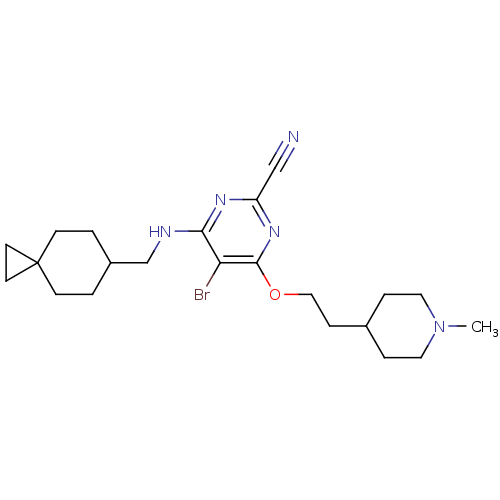 (5-Bromo-4-[2-(1-methyl-piperidin-4-yl)-ethoxy]-6-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98685 (US8497286, 161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263668 (2-Cyano-4-[2-(1-methyl-piperidin-4-yl)-ethoxy]-6-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50273288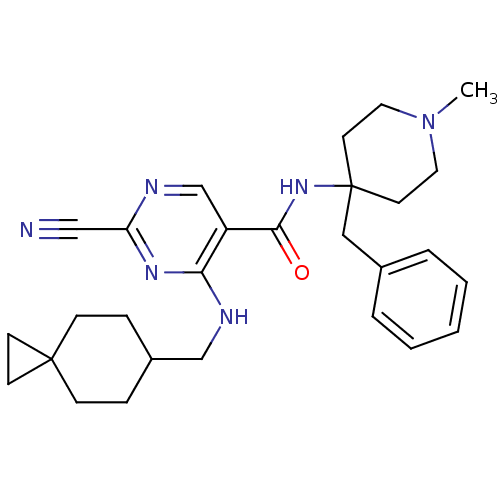 (2-Cyano-4-[(spiro[2.5]oct-6-ylmethyl)-amino]-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 433 total ) | Next | Last >> |