 Found 529 hits with Last Name = 'hoang' and Initial = 't'
Found 529 hits with Last Name = 'hoang' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458766

(CHEMBL4212386)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1ccc(C(=O)N[C@@H](CC(O)=O)C(O)=O)c(OCC)c1 |r| Show InChI InChI=1S/C30H40N4O11/c1-4-7-8-9-19(22(5-2)34(43)17-35)27(38)31-16-32-29(40)24-13-12-23(45-24)18-10-11-20(25(14-18)44-6-3)28(39)33-21(30(41)42)15-26(36)37/h10-14,17,19,21-22,43H,4-9,15-16H2,1-3H3,(H,31,38)(H,32,40)(H,33,39)(H,36,37)(H,41,42)/t19-,21+,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tolloid-like protein 1
(Homo sapiens) | BDBM50458766

(CHEMBL4212386)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1ccc(C(=O)N[C@@H](CC(O)=O)C(O)=O)c(OCC)c1 |r| Show InChI InChI=1S/C30H40N4O11/c1-4-7-8-9-19(22(5-2)34(43)17-35)27(38)31-16-32-29(40)24-13-12-23(45-24)18-10-11-20(25(14-18)44-6-3)28(39)33-21(30(41)42)15-26(36)37/h10-14,17,19,21-22,43H,4-9,15-16H2,1-3H3,(H,31,38)(H,32,40)(H,33,39)(H,36,37)(H,41,42)/t19-,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tolloid-like protein 2
(Homo sapiens) | BDBM50458766

(CHEMBL4212386)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1ccc(C(=O)N[C@@H](CC(O)=O)C(O)=O)c(OCC)c1 |r| Show InChI InChI=1S/C30H40N4O11/c1-4-7-8-9-19(22(5-2)34(43)17-35)27(38)31-16-32-29(40)24-13-12-23(45-24)18-10-11-20(25(14-18)44-6-3)28(39)33-21(30(41)42)15-26(36)37/h10-14,17,19,21-22,43H,4-9,15-16H2,1-3H3,(H,31,38)(H,32,40)(H,33,39)(H,36,37)(H,41,42)/t19-,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458771

(CHEMBL4214046)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1cc(OCC)cc(c1)P(O)(O)=O |r| Show InChI InChI=1S/C25H36N3O9P/c1-4-7-8-9-20(21(5-2)28(32)16-29)24(30)26-15-27-25(31)23-11-10-22(37-23)17-12-18(36-6-3)14-19(13-17)38(33,34)35/h10-14,16,20-21,32H,4-9,15H2,1-3H3,(H,26,30)(H,27,31)(H2,33,34,35)/t20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | |
Tolloid-like protein 1
(Homo sapiens) | BDBM50458771

(CHEMBL4214046)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1cc(OCC)cc(c1)P(O)(O)=O |r| Show InChI InChI=1S/C25H36N3O9P/c1-4-7-8-9-20(21(5-2)28(32)16-29)24(30)26-15-27-25(31)23-11-10-22(37-23)17-12-18(36-6-3)14-19(13-17)38(33,34)35/h10-14,16,20-21,32H,4-9,15H2,1-3H3,(H,26,30)(H,27,31)(H2,33,34,35)/t20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | |
Tolloid-like protein 2
(Homo sapiens) | BDBM50458771

(CHEMBL4214046)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1cc(OCC)cc(c1)P(O)(O)=O |r| Show InChI InChI=1S/C25H36N3O9P/c1-4-7-8-9-20(21(5-2)28(32)16-29)24(30)26-15-27-25(31)23-11-10-22(37-23)17-12-18(36-6-3)14-19(13-17)38(33,34)35/h10-14,16,20-21,32H,4-9,15H2,1-3H3,(H,26,30)(H,27,31)(H2,33,34,35)/t20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502628

(CHEMBL4469630)Show SMILES CC(C)(C)CN1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C23H30N4O2/c1-21(2,3)13-26-15-23(29-20(26)28)9-5-8-22(4,12-23)14-27-16-25-18-7-6-17(11-24)10-19(18)27/h6-7,10,16H,5,8-9,12-15H2,1-4H3/t22-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458763

(CHEMBL4202848)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1cc(OCC)cc(c1)C(O)=O |r| Show InChI InChI=1S/C26H35N3O8/c1-4-7-8-9-20(21(5-2)29(35)16-30)24(31)27-15-28-25(32)23-11-10-22(37-23)17-12-18(26(33)34)14-19(13-17)36-6-3/h10-14,16,20-21,35H,4-9,15H2,1-3H3,(H,27,31)(H,28,32)(H,33,34)/t20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458764

(CHEMBL4209125)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1ccc(C(O)=O)c(OCC)c1 |r| Show InChI InChI=1S/C26H35N3O8/c1-4-7-8-9-18(20(5-2)29(35)16-30)24(31)27-15-28-25(32)22-13-12-21(37-22)17-10-11-19(26(33)34)23(14-17)36-6-3/h10-14,16,18,20,35H,4-9,15H2,1-3H3,(H,27,31)(H,28,32)(H,33,34)/t18-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458773

(CHEMBL4204567)Show SMILES CCCCC[C@H](CN(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1cccc(OCC)c1 |r| Show InChI InChI=1S/C23H31N3O6/c1-3-5-6-8-18(14-26(30)16-27)22(28)24-15-25-23(29)21-12-11-20(32-21)17-9-7-10-19(13-17)31-4-2/h7,9-13,16,18,30H,3-6,8,14-15H2,1-2H3,(H,24,28)(H,25,29)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458771

(CHEMBL4214046)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1cc(OCC)cc(c1)P(O)(O)=O |r| Show InChI InChI=1S/C25H36N3O9P/c1-4-7-8-9-20(21(5-2)28(32)16-29)24(30)26-15-27-25(31)23-11-10-22(37-23)17-12-18(36-6-3)14-19(13-17)38(33,34)35/h10-14,16,20-21,32H,4-9,15H2,1-3H3,(H,26,30)(H,27,31)(H2,33,34,35)/t20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458766

(CHEMBL4212386)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1ccc(C(=O)N[C@@H](CC(O)=O)C(O)=O)c(OCC)c1 |r| Show InChI InChI=1S/C30H40N4O11/c1-4-7-8-9-19(22(5-2)34(43)17-35)27(38)31-16-32-29(40)24-13-12-23(45-24)18-10-11-20(25(14-18)44-6-3)28(39)33-21(30(41)42)15-26(36)37/h10-14,17,19,21-22,43H,4-9,15-16H2,1-3H3,(H,31,38)(H,32,40)(H,33,39)(H,36,37)(H,41,42)/t19-,21+,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458765
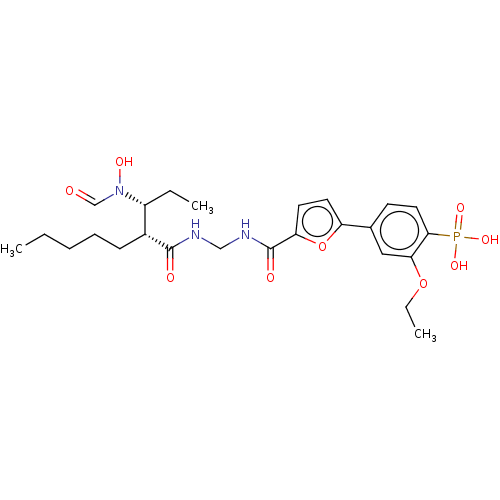
(CHEMBL4205697)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1ccc(c(OCC)c1)P(O)(O)=O |r| Show InChI InChI=1S/C25H36N3O9P/c1-4-7-8-9-18(19(5-2)28(32)16-29)24(30)26-15-27-25(31)21-12-11-20(37-21)17-10-13-23(38(33,34)35)22(14-17)36-6-3/h10-14,16,18-19,32H,4-9,15H2,1-3H3,(H,26,30)(H,27,31)(H2,33,34,35)/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458775
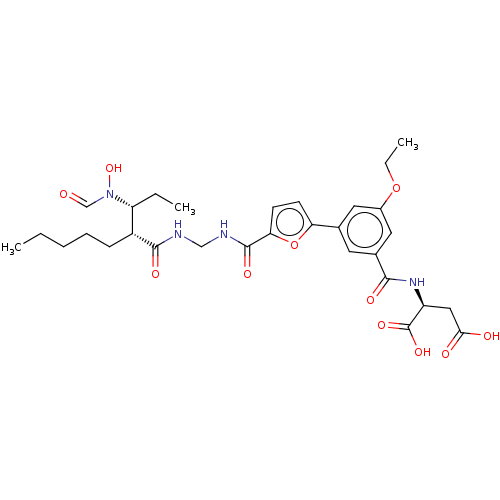
(CHEMBL4218415)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1cc(OCC)cc(c1)C(=O)N[C@@H](CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C30H40N4O11/c1-4-7-8-9-21(23(5-2)34(43)17-35)28(39)31-16-32-29(40)25-11-10-24(45-25)18-12-19(14-20(13-18)44-6-3)27(38)33-22(30(41)42)15-26(36)37/h10-14,17,21-23,43H,4-9,15-16H2,1-3H3,(H,31,39)(H,32,40)(H,33,38)(H,36,37)(H,41,42)/t21-,22+,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged PI4K-alpha (1 to 2044) (unknown origin) using D-myo-phosphatidylinositol as substrate preincubated for 30 mins followed by s... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458774
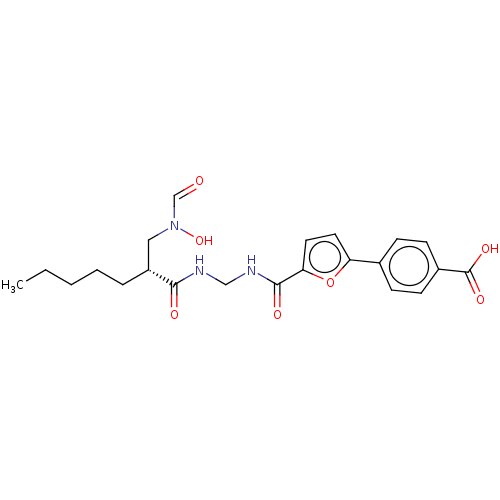
(CHEMBL4202714)Show SMILES CCCCC[C@H](CN(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C22H27N3O7/c1-2-3-4-5-17(12-25(31)14-26)20(27)23-13-24-21(28)19-11-10-18(32-19)15-6-8-16(9-7-15)22(29)30/h6-11,14,17,31H,2-5,12-13H2,1H3,(H,23,27)(H,24,28)(H,29,30)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502635
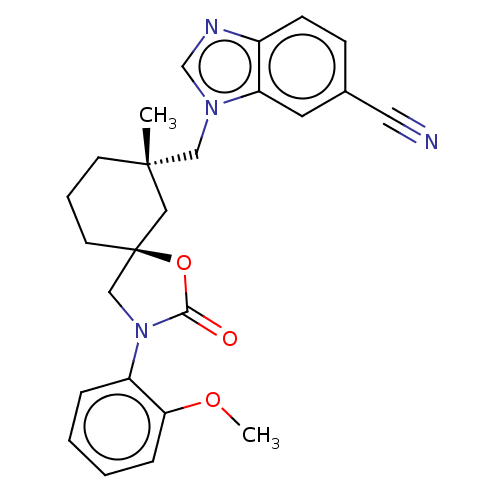
(CHEMBL4521512)Show SMILES COc1ccccc1N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C25H26N4O3/c1-24(15-28-17-27-19-9-8-18(13-26)12-21(19)28)10-5-11-25(14-24)16-29(23(30)32-25)20-6-3-4-7-22(20)31-2/h3-4,6-9,12,17H,5,10-11,14-16H2,1-2H3/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502648
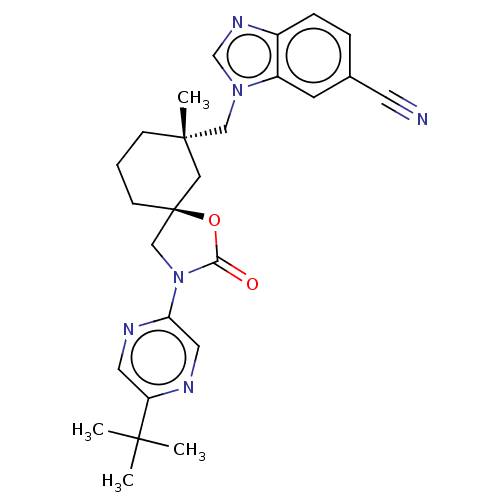
(CHEMBL4588831)Show SMILES CC(C)(C)c1cnc(cn1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C26H30N6O2/c1-24(2,3)21-12-29-22(13-28-21)32-16-26(34-23(32)33)9-5-8-25(4,14-26)15-31-17-30-19-7-6-18(11-27)10-20(19)31/h6-7,10,12-13,17H,5,8-9,14-16H2,1-4H3/t25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502626
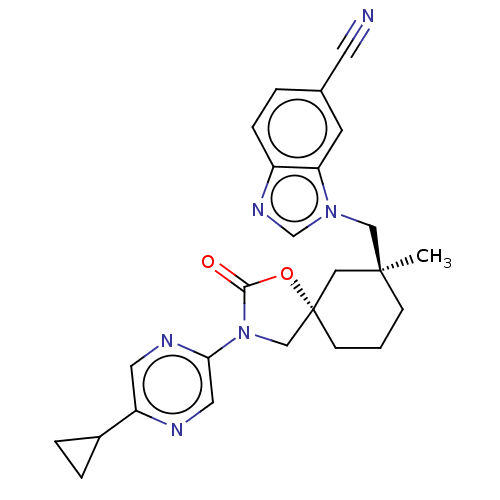
(CHEMBL4536058)Show SMILES C[C@]1(Cn2cnc3ccc(cc23)C#N)CCC[C@@]2(CN(C(=O)O2)c2cnc(cn2)C2CC2)C1 |r| Show InChI InChI=1S/C25H26N6O2/c1-24(14-30-16-29-19-6-3-17(10-26)9-21(19)30)7-2-8-25(13-24)15-31(23(32)33-25)22-12-27-20(11-28-22)18-4-5-18/h3,6,9,11-12,16,18H,2,4-5,7-8,13-15H2,1H3/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502649
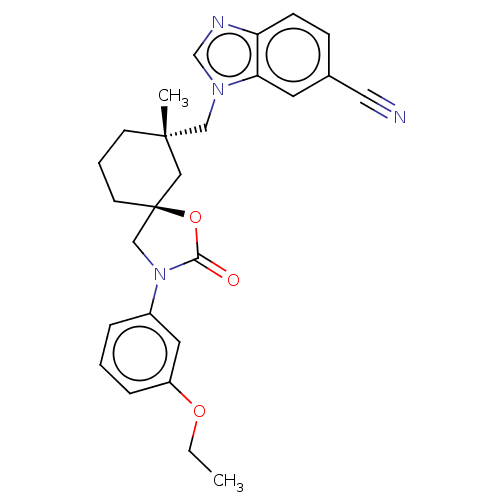
(CHEMBL4532369)Show SMILES CCOc1cccc(c1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C26H28N4O3/c1-3-32-21-7-4-6-20(13-21)30-17-26(33-24(30)31)11-5-10-25(2,15-26)16-29-18-28-22-9-8-19(14-27)12-23(22)29/h4,6-9,12-13,18H,3,5,10-11,15-17H2,1-2H3/t25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50502640

(CHEMBL4470585)Show SMILES CC(C)(O)c1cnc(cn1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C25H28N6O3/c1-23(2,33)20-11-28-21(12-27-20)31-15-25(34-22(31)32)8-4-7-24(3,13-25)14-30-16-29-18-6-5-17(10-26)9-19(18)30/h5-6,9,11-12,16,33H,4,7-8,13-15H2,1-3H3/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of rat TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye bas... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502643
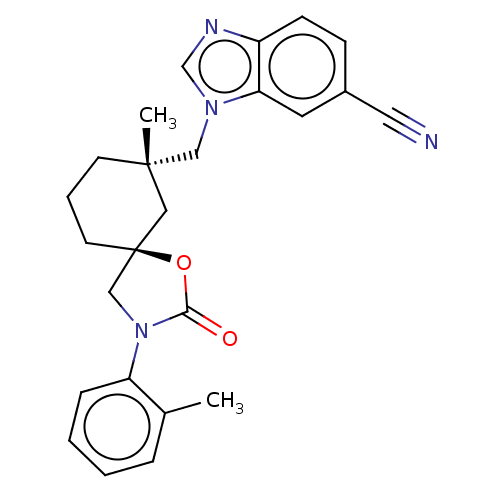
(CHEMBL4541827)Show SMILES Cc1ccccc1N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C25H26N4O2/c1-18-6-3-4-7-21(18)29-16-25(31-23(29)30)11-5-10-24(2,14-25)15-28-17-27-20-9-8-19(13-26)12-22(20)28/h3-4,6-9,12,17H,5,10-11,14-16H2,1-2H3/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502640

(CHEMBL4470585)Show SMILES CC(C)(O)c1cnc(cn1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C25H28N6O3/c1-23(2,33)20-11-28-21(12-27-20)31-15-25(34-22(31)32)8-4-7-24(3,13-25)14-30-16-29-18-6-5-17(10-26)9-19(18)30/h5-6,9,11-12,16,33H,4,7-8,13-15H2,1-3H3/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502653

(CHEMBL4514491)Show SMILES C[C@]1(Cn2cnc3ccc(cc23)C#N)CCC[C@@]2(CN(C(=O)O2)c2ccccc2Cl)C1 |r| Show InChI InChI=1S/C24H23ClN4O2/c1-23(14-28-16-27-19-8-7-17(12-26)11-21(19)28)9-4-10-24(13-23)15-29(22(30)31-24)20-6-3-2-5-18(20)25/h2-3,5-8,11,16H,4,9-10,13-15H2,1H3/t23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502636
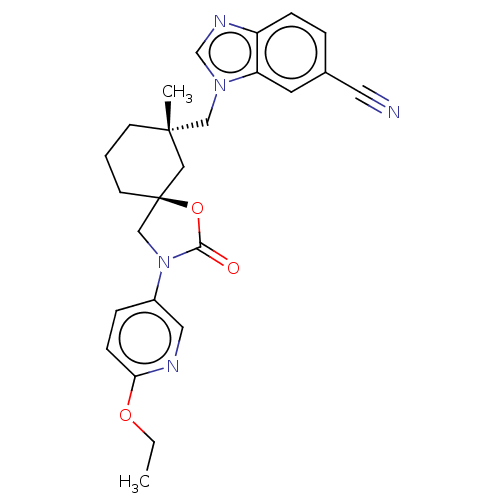
(CHEMBL4588158)Show SMILES CCOc1ccc(cn1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C25H27N5O3/c1-3-32-22-8-6-19(13-27-22)30-16-25(33-23(30)31)10-4-9-24(2,14-25)15-29-17-28-20-7-5-18(12-26)11-21(20)29/h5-8,11,13,17H,3-4,9-10,14-16H2,1-2H3/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50426532
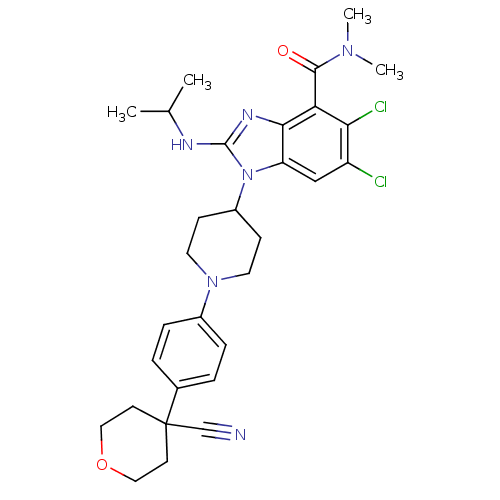
(CHEMBL2323936)Show SMILES CC(C)Nc1nc2c(C(=O)N(C)C)c(Cl)c(Cl)cc2n1C1CCN(CC1)c1ccc(cc1)C1(CCOCC1)C#N Show InChI InChI=1S/C30H36Cl2N6O2/c1-19(2)34-29-35-27-24(17-23(31)26(32)25(27)28(39)36(3)4)38(29)22-9-13-37(14-10-22)21-7-5-20(6-8-21)30(18-33)11-15-40-16-12-30/h5-8,17,19,22H,9-16H2,1-4H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV4 expressed in BacMam virus infected HEK MSRII cells assessed as inhibition of GSK1016790A-induced calci... |
ACS Med Chem Lett 4: 293-6 (2013)
Article DOI: 10.1021/ml300449k
BindingDB Entry DOI: 10.7270/Q2VT1TDB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502637

(CHEMBL4469982)Show SMILES CCOc1cnc(cn1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-3-32-21-12-26-20(11-27-21)30-15-24(33-22(30)31)8-4-7-23(2,13-24)14-29-16-28-18-6-5-17(10-25)9-19(18)29/h5-6,9,11-12,16H,3-4,7-8,13-15H2,1-2H3/t23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50294706

(4-((2-chlorobenzyl)(3-methyl-1-(1-methyl-1H-tetraz...)Show SMILES CC(C)CC(N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1)c1nnnn1C Show InChI InChI=1S/C21H22Cl2N6/c1-14(2)10-20(21-25-26-27-28(21)3)29(13-16-6-4-5-7-18(16)22)17-9-8-15(12-24)19(23)11-17/h4-9,11,14,20H,10,13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay |
Bioorg Med Chem Lett 19: 2637-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.146
BindingDB Entry DOI: 10.7270/Q2FX79GM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM50296010

(2-isobutyl-4-(5-phenyl-1H-pyrrolo[2,3-b]pyridin-3-...)Show SMILES CC(C)Cc1cc(ccc1C(O)=O)-c1c[nH]c2ncc(cc12)-c1ccccc1 Show InChI InChI=1S/C24H22N2O2/c1-15(2)10-18-11-17(8-9-20(18)24(27)28)22-14-26-23-21(22)12-19(13-25-23)16-6-4-3-5-7-16/h3-9,11-15H,10H2,1-2H3,(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SGK1 by fluorescence polarization assay |
Bioorg Med Chem Lett 19: 4441-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.051
BindingDB Entry DOI: 10.7270/Q2WQ03VC |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50304973

((S)-4-((2-bromobenzyl)(1-(methylsulfonyl)pyrrolidi...)Show SMILES CS(=O)(=O)N1CC[C@@H](C1)N(Cc1ccccc1Br)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C19H19BrClN3O2S/c1-27(25,26)23-9-8-17(13-23)24(12-15-4-2-3-5-18(15)20)16-7-6-14(11-22)19(21)10-16/h2-7,10,17H,8-9,12-13H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PR |
Bioorg Med Chem Lett 20: 371-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.092
BindingDB Entry DOI: 10.7270/Q2057G17 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502642
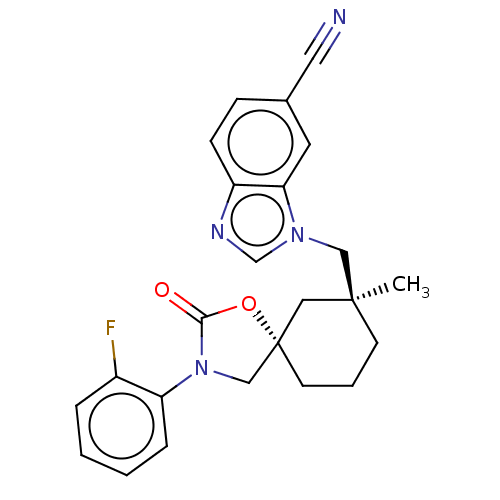
(CHEMBL4560266)Show SMILES C[C@]1(Cn2cnc3ccc(cc23)C#N)CCC[C@@]2(CN(C(=O)O2)c2ccccc2F)C1 |r| Show InChI InChI=1S/C24H23FN4O2/c1-23(14-28-16-27-19-8-7-17(12-26)11-21(19)28)9-4-10-24(13-23)15-29(22(30)31-24)20-6-3-2-5-18(20)25/h2-3,5-8,11,16H,4,9-10,13-15H2,1H3/t23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50502646
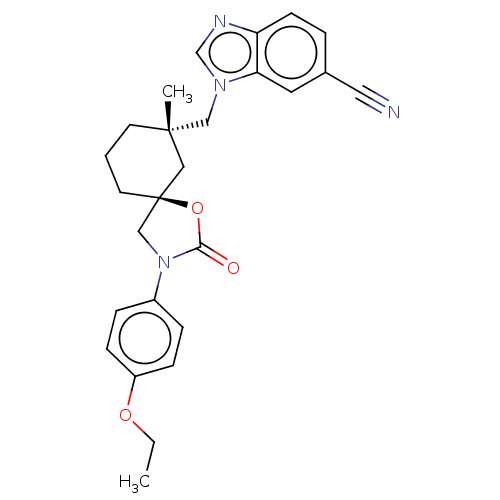
(CHEMBL4579838)Show SMILES CCOc1ccc(cc1)N1C[C@@]2(CCC[C@](C)(Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C26H28N4O3/c1-3-32-21-8-6-20(7-9-21)30-17-26(33-24(30)31)12-4-11-25(2,15-26)16-29-18-28-22-10-5-19(14-27)13-23(22)29/h5-10,13,18H,3-4,11-12,15-17H2,1-2H3/t25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye b... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Rattus norvegicus) | BDBM50502650
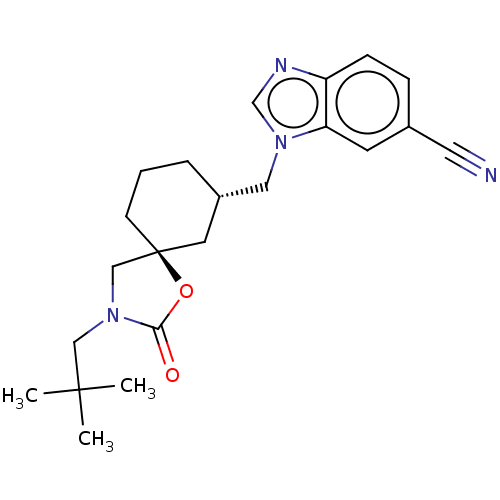
(CHEMBL4549613)Show SMILES CC(C)(C)CN1C[C@@]2(CCC[C@H](Cn3cnc4ccc(cc34)C#N)C2)OC1=O |r| Show InChI InChI=1S/C22H28N4O2/c1-21(2,3)13-26-14-22(28-20(26)27)8-4-5-17(10-22)12-25-15-24-18-7-6-16(11-23)9-19(18)25/h6-7,9,15,17H,4-5,8,10,12-14H2,1-3H3/t17-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of rat TRPV4 expressed in baculovirus infected HEK/MSR 2 cells pre-incubated for 10 mins followed by GSK634775A addition by Fura-2 dye bas... |
ACS Med Chem Lett 10: 1228-1233 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00274
BindingDB Entry DOI: 10.7270/Q2RX9GBF |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50304972

((S)-2-chloro-4-((2-chlorobenzyl)(1-(methylsulfonyl...)Show SMILES CS(=O)(=O)N1CC[C@@H](C1)N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C19H19Cl2N3O2S/c1-27(25,26)23-9-8-17(13-23)24(12-15-4-2-3-5-18(15)20)16-7-6-14(11-22)19(21)10-16/h2-7,10,17H,8-9,12-13H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PR |
Bioorg Med Chem Lett 20: 371-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.092
BindingDB Entry DOI: 10.7270/Q2057G17 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50298032

((S)-2-chloro-4-((2-chlorobenzyl)(1-(cyanomethyl)py...)Show SMILES Clc1ccccc1CN([C@H]1CCN(CC#N)C1)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C20H18Cl2N4/c21-19-4-2-1-3-16(19)13-26(18-7-9-25(14-18)10-8-23)17-6-5-15(12-24)20(22)11-17/h1-6,11,18H,7,9-10,13-14H2/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to PR by fluorescence polarization based competition binding assay |
Bioorg Med Chem Lett 19: 4777-80 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.055
BindingDB Entry DOI: 10.7270/Q2TB16XG |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458758

(CHEMBL4207907)Show SMILES CCCCC[C@H](CN(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1cccc(c1)C(O)=O |r| Show InChI InChI=1S/C22H27N3O7/c1-2-3-4-6-17(12-25(31)14-26)20(27)23-13-24-21(28)19-10-9-18(32-19)15-7-5-8-16(11-15)22(29)30/h5,7-11,14,17,31H,2-4,6,12-13H2,1H3,(H,23,27)(H,24,28)(H,29,30)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal 6His/flag-tagged BMP1 (121 to 721 residues) expressed in CHO cells using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50304978

((S)-methyl 3-((3-chloro-4-cyanophenyl)(2-chloroben...)Show SMILES COC(=O)N1CC[C@@H](C1)N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C20H19Cl2N3O2/c1-27-20(26)24-9-8-17(13-24)25(12-15-4-2-3-5-18(15)21)16-7-6-14(11-23)19(22)10-16/h2-7,10,17H,8-9,12-13H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PR |
Bioorg Med Chem Lett 20: 371-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.092
BindingDB Entry DOI: 10.7270/Q2057G17 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50304987
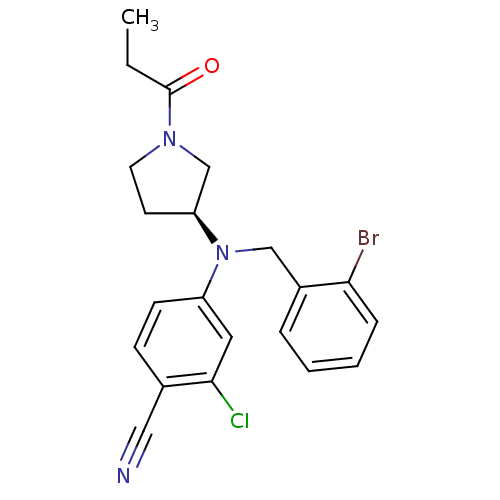
((S)-4-((2-bromobenzyl)(1-propionylpyrrolidin-3-yl)...)Show SMILES CCC(=O)N1CC[C@@H](C1)N(Cc1ccccc1Br)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C21H21BrClN3O/c1-2-21(27)25-10-9-18(14-25)26(13-16-5-3-4-6-19(16)22)17-8-7-15(12-24)20(23)11-17/h3-8,11,18H,2,9-10,13-14H2,1H3/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PR |
Bioorg Med Chem Lett 20: 371-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.092
BindingDB Entry DOI: 10.7270/Q2057G17 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50298033

((S)-ethyl 2-(3-((3-chloro-4-cyanophenyl)(2-chlorob...)Show SMILES CCOC(=O)CN1CC[C@@H](C1)N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C22H23Cl2N3O2/c1-2-29-22(28)15-26-10-9-19(14-26)27(13-17-5-3-4-6-20(17)23)18-8-7-16(12-25)21(24)11-18/h3-8,11,19H,2,9-10,13-15H2,1H3/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to PR by fluorescence polarization based competition binding assay |
Bioorg Med Chem Lett 19: 4777-80 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.055
BindingDB Entry DOI: 10.7270/Q2TB16XG |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50298208

((S)-2-chloro-4-((2-chloro-5-fluorobenzyl)(1-ethyl-...)Show SMILES CCN1C[C@H](CC1=O)N(Cc1cc(F)ccc1Cl)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C20H18Cl2FN3O/c1-2-25-12-17(9-20(25)27)26(11-14-7-15(23)4-6-18(14)21)16-5-3-13(10-24)19(22)8-16/h3-8,17H,2,9,11-12H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to progesterone receptor |
Bioorg Med Chem Lett 19: 4664-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.081
BindingDB Entry DOI: 10.7270/Q25H7G9T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50298207
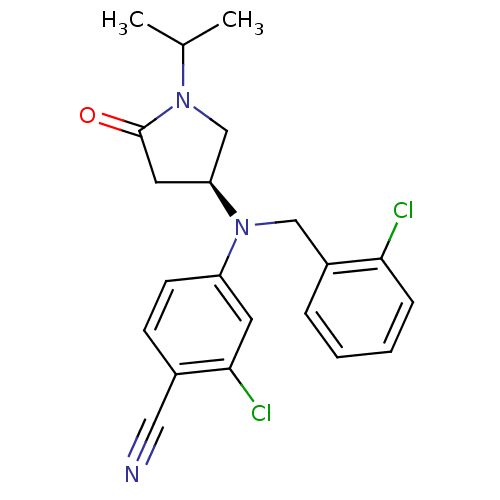
((S)-2-chloro-4-((2-chlorobenzyl)(1-isopropyl-5-oxo...)Show SMILES CC(C)N1C[C@H](CC1=O)N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C21H21Cl2N3O/c1-14(2)25-13-18(10-21(25)27)26(12-16-5-3-4-6-19(16)22)17-8-7-15(11-24)20(23)9-17/h3-9,14,18H,10,12-13H2,1-2H3/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to progesterone receptor |
Bioorg Med Chem Lett 19: 4664-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.081
BindingDB Entry DOI: 10.7270/Q25H7G9T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50298210
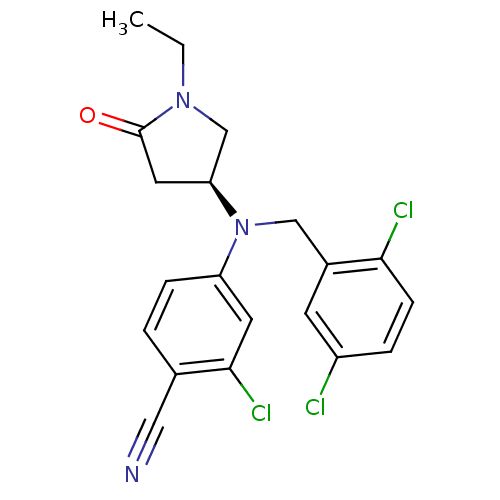
((S)-2-chloro-4-((2,5-dichlorobenzyl)(1-ethyl-5-oxo...)Show SMILES CCN1C[C@H](CC1=O)N(Cc1cc(Cl)ccc1Cl)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C20H18Cl3N3O/c1-2-25-12-17(9-20(25)27)26(11-14-7-15(21)4-6-18(14)22)16-5-3-13(10-24)19(23)8-16/h3-8,17H,2,9,11-12H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to progesterone receptor |
Bioorg Med Chem Lett 19: 4664-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.081
BindingDB Entry DOI: 10.7270/Q25H7G9T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50304977

((S)-methyl 3-((3-chloro-4-cyanophenyl)(2-methylben...)Show SMILES COC(=O)N1CC[C@@H](C1)N(Cc1ccccc1C)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C21H22ClN3O2/c1-15-5-3-4-6-17(15)13-25(18-8-7-16(12-23)20(22)11-18)19-9-10-24(14-19)21(26)27-2/h3-8,11,19H,9-10,13-14H2,1-2H3/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PR |
Bioorg Med Chem Lett 20: 371-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.092
BindingDB Entry DOI: 10.7270/Q2057G17 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50304976

((S)-methyl 3-((3-chloro-4-cyanophenyl)(2-fluoroben...)Show SMILES COC(=O)N1CC[C@@H](C1)N(Cc1ccccc1F)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C20H19ClFN3O2/c1-27-20(26)24-9-8-17(13-24)25(12-15-4-2-3-5-19(15)22)16-7-6-14(11-23)18(21)10-16/h2-7,10,17H,8-9,12-13H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PR |
Bioorg Med Chem Lett 20: 371-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.092
BindingDB Entry DOI: 10.7270/Q2057G17 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50304975
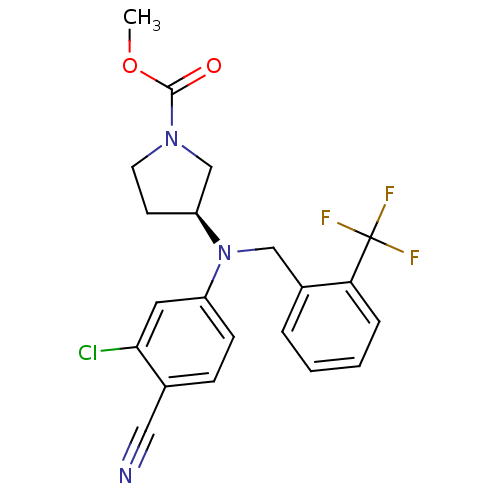
((S)-methyl 3-((3-chloro-4-cyanophenyl)(2-(trifluor...)Show SMILES COC(=O)N1CC[C@@H](C1)N(Cc1ccccc1C(F)(F)F)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C21H19ClF3N3O2/c1-30-20(29)27-9-8-17(13-27)28(16-7-6-14(11-26)19(22)10-16)12-15-4-2-3-5-18(15)21(23,24)25/h2-7,10,17H,8-9,12-13H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PR |
Bioorg Med Chem Lett 20: 371-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.092
BindingDB Entry DOI: 10.7270/Q2057G17 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50304964
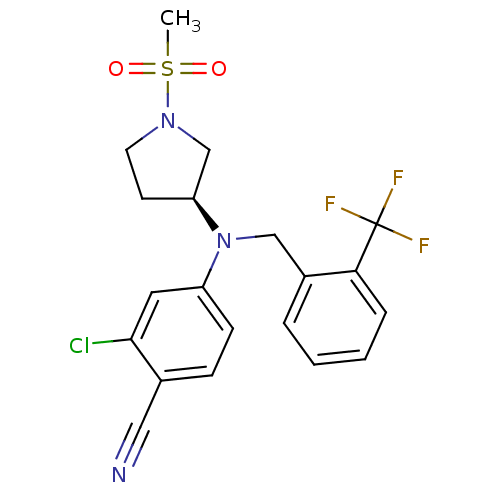
((S)-2-chloro-4-((1-(methylsulfonyl)pyrrolidin-3-yl...)Show SMILES CS(=O)(=O)N1CC[C@@H](C1)N(Cc1ccccc1C(F)(F)F)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C20H19ClF3N3O2S/c1-30(28,29)26-9-8-17(13-26)27(16-7-6-14(11-25)19(21)10-16)12-15-4-2-3-5-18(15)20(22,23)24/h2-7,10,17H,8-9,12-13H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PR |
Bioorg Med Chem Lett 20: 371-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.092
BindingDB Entry DOI: 10.7270/Q2057G17 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50294715

(4-((2-chlorobenzyl)(1-(4-methyl-4H-1,2,4-triazol-3...)Show SMILES CCCC(N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1)c1nncn1C Show InChI InChI=1S/C21H21Cl2N5/c1-3-6-20(21-26-25-14-27(21)2)28(13-16-7-4-5-8-18(16)22)17-10-9-15(12-24)19(23)11-17/h4-5,7-11,14,20H,3,6,13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay |
Bioorg Med Chem Lett 19: 2637-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.146
BindingDB Entry DOI: 10.7270/Q2FX79GM |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50294696
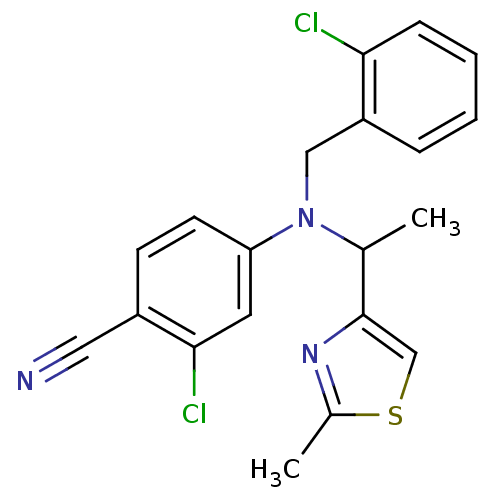
(4-((2-chlorobenzyl)(1-(2-methylthiazol-4-yl)ethyl)...)Show SMILES CC(N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1)c1csc(C)n1 Show InChI InChI=1S/C20H17Cl2N3S/c1-13(20-12-26-14(2)24-20)25(11-16-5-3-4-6-18(16)21)17-8-7-15(10-23)19(22)9-17/h3-9,12-13H,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay |
Bioorg Med Chem Lett 19: 2637-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.146
BindingDB Entry DOI: 10.7270/Q2FX79GM |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50294702

(4-((2-chlorobenzyl)(1-(1-methyl-1H-tetrazol-5-yl)e...)Show SMILES CC(N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1)c1nnnn1C Show InChI InChI=1S/C18H16Cl2N6/c1-12(18-22-23-24-25(18)2)26(11-14-5-3-4-6-16(14)19)15-8-7-13(10-21)17(20)9-15/h3-9,12H,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay |
Bioorg Med Chem Lett 19: 2637-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.146
BindingDB Entry DOI: 10.7270/Q2FX79GM |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50294703

(4-((2-chlorobenzyl)(1-(4-methyl-4H-1,2,4-triazol-3...)Show SMILES CC(N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1)c1nncn1C Show InChI InChI=1S/C19H17Cl2N5/c1-13(19-24-23-12-25(19)2)26(11-15-5-3-4-6-17(15)20)16-8-7-14(10-22)18(21)9-16/h3-9,12-13H,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay |
Bioorg Med Chem Lett 19: 2637-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.146
BindingDB Entry DOI: 10.7270/Q2FX79GM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM50296011
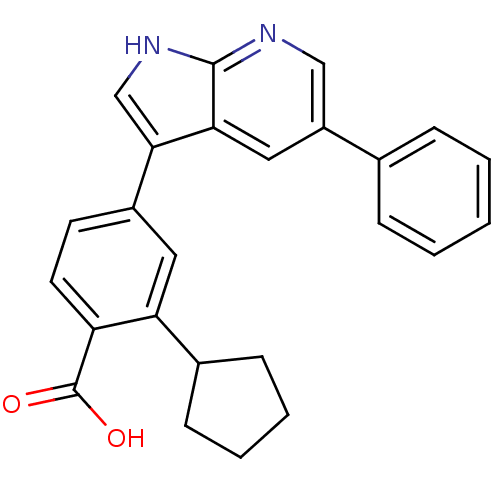
(2-cyclopentyl-4-(5-phenyl-1H-pyrrolo[2,3-b]pyridin...)Show SMILES OC(=O)c1ccc(cc1C1CCCC1)-c1c[nH]c2ncc(cc12)-c1ccccc1 Show InChI InChI=1S/C25H22N2O2/c28-25(29)20-11-10-18(12-21(20)17-8-4-5-9-17)23-15-27-24-22(23)13-19(14-26-24)16-6-2-1-3-7-16/h1-3,6-7,10-15,17H,4-5,8-9H2,(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SGK1 by fluorescence polarization assay |
Bioorg Med Chem Lett 19: 4441-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.051
BindingDB Entry DOI: 10.7270/Q2WQ03VC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data