

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM50409115 (LONAPRISAN) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-B progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50409115 (LONAPRISAN) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.00360 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-A progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-B progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antgonist potency in transactivation assay in neuroblastoma cells expressing human PR-A progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101232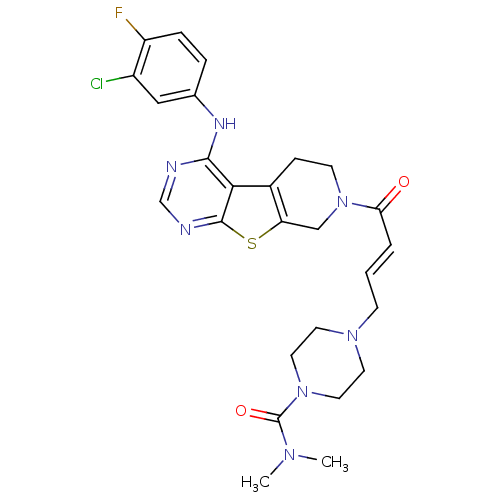 (US8524722, 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101243 (US8524722, 143) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101241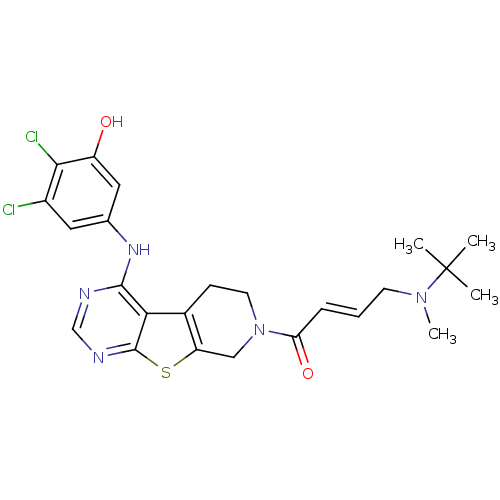 (US8524722, 134) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101240 (US8524722, 123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101237 (US8524722, 90) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101239 (US8524722, 119) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101230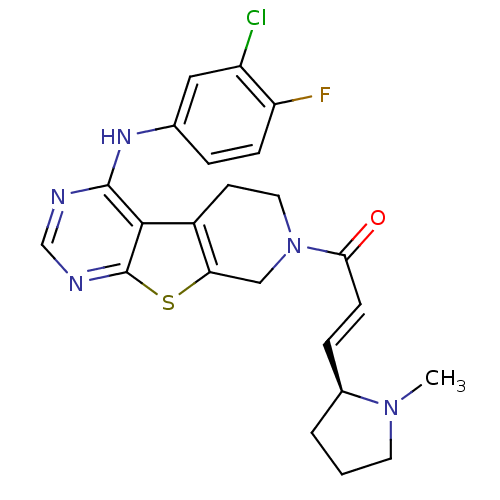 (US8524722, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101228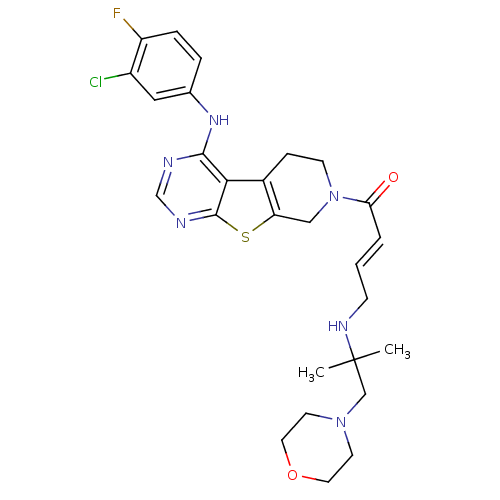 (US8524722, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101226 (US8524722, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101231 (US8524722, 31) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101227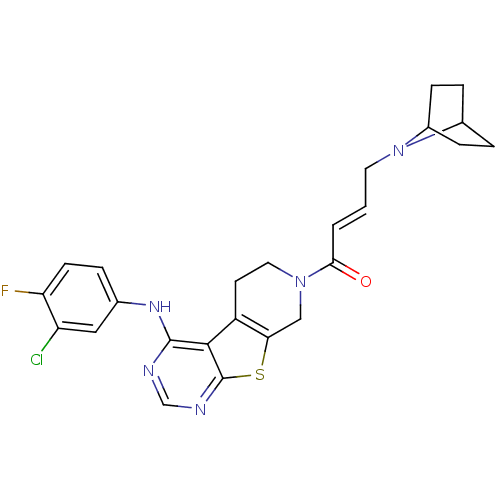 (US8524722, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101234 (US8524722, 84) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101229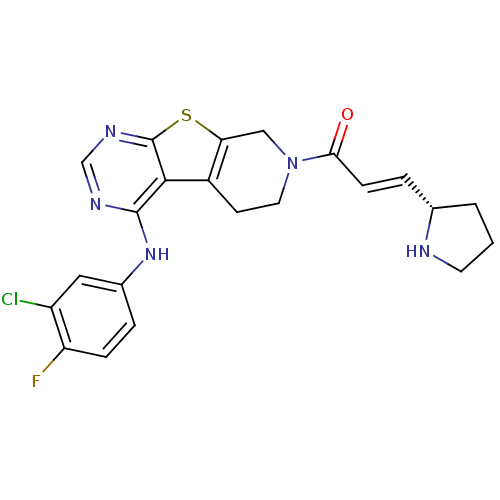 (US8524722, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (MOUSE) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in NIH3T3 cells expressing glucocorticoid receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101244 (US8524722, 148) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50330241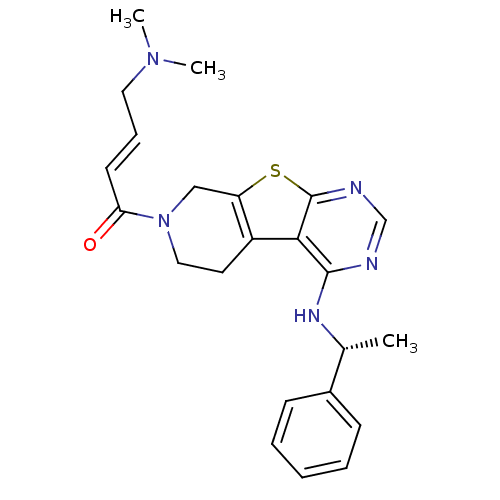 ((E)-4-(Dimethylamino)-1-(4-[(1R)-1-phenylethyl]ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101235 (US8524722, 86) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101245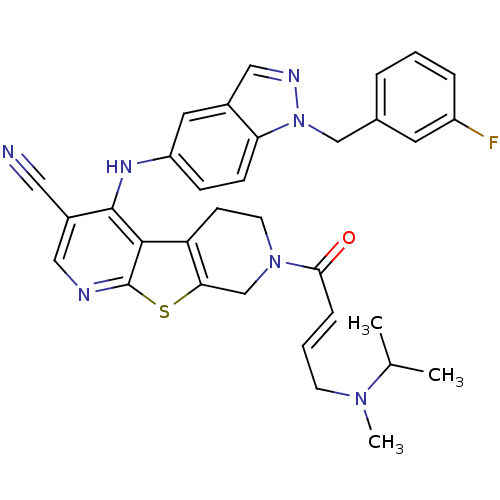 (US8524722, 151) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101242 (US8524722, 139) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101238 (US8524722, 95) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in CV-1 cells expressing androgen receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101233 (US8524722, 81) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (MOUSE) | BDBM50409115 (LONAPRISAN) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in NIH3T3 cells expressing glucocorticoid receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM101236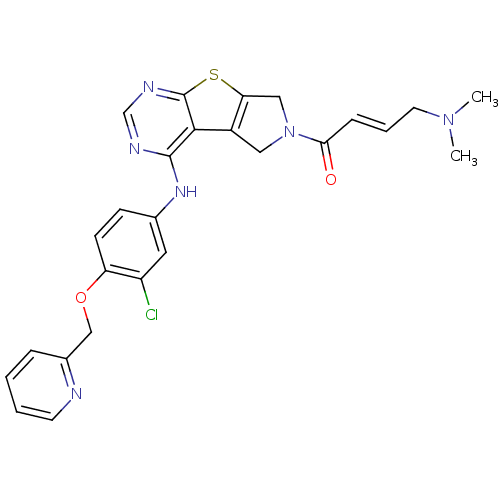 (US8524722, 87) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description EGFR inhibitory activity of compounds of the present invention is quantified employing the EGFR HTRF assay. | US Patent US8524722 (2013) BindingDB Entry DOI: 10.7270/Q2B856RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50409115 (LONAPRISAN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in CV-1 cells expressing androgen receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50241922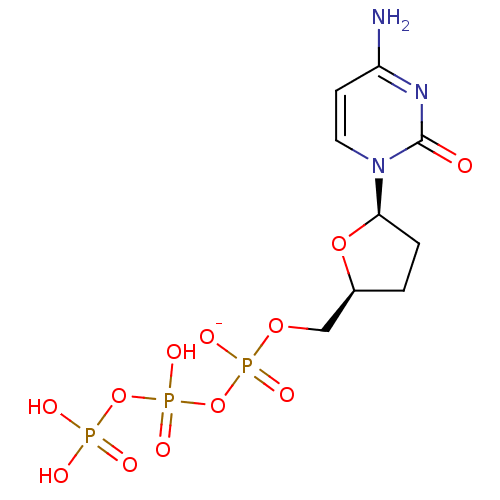 (CHEMBL486991 | ddCTP SODIUM | sodium ((2S,5R)-5-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Scientific Research (CNRS)-Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of human recombinant DNA polymerase beta assessed as fluorescein-12-dCTP incorporation by fluorimetry | J Nat Prod 68: 979-84 (2005) Article DOI: 10.1021/np049676o BindingDB Entry DOI: 10.7270/Q2VT1RVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50241929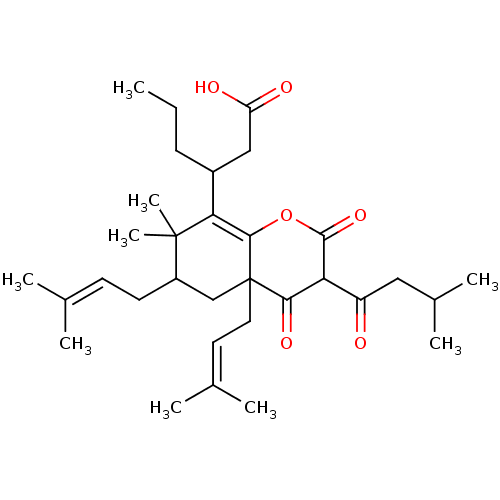 (CHEMBL520861 | Mahureone D) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Scientific Research (CNRS)-Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of human recombinant DNA polymerase beta assessed as fluorescein-12-dCTP incorporation by fluorimetry | J Nat Prod 68: 979-84 (2005) Article DOI: 10.1021/np049676o BindingDB Entry DOI: 10.7270/Q2VT1RVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50241923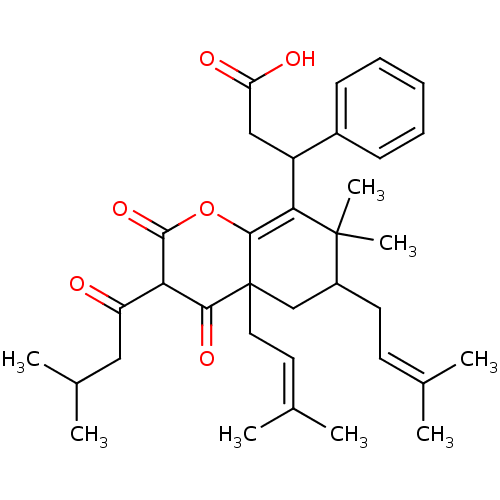 (CHEMBL521208 | mahureone A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Scientific Research (CNRS)-Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of human recombinant DNA polymerase beta assessed as fluorescein-12-dCTP incorporation by fluorimetry | J Nat Prod 68: 979-84 (2005) Article DOI: 10.1021/np049676o BindingDB Entry DOI: 10.7270/Q2VT1RVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50241924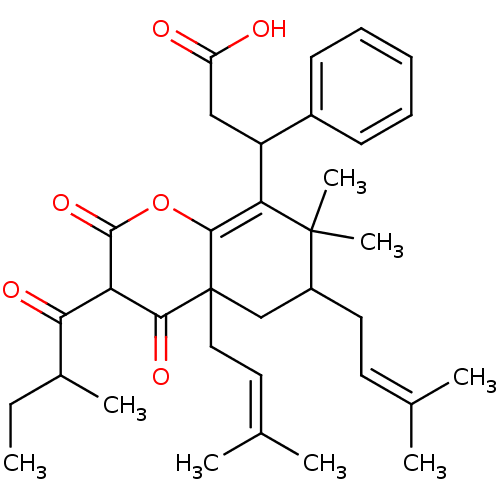 (CHEMBL486797 | Mahureone B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Scientific Research (CNRS)-Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of human recombinant DNA polymerase beta assessed as fluorescein-12-dCTP incorporation by fluorimetry | J Nat Prod 68: 979-84 (2005) Article DOI: 10.1021/np049676o BindingDB Entry DOI: 10.7270/Q2VT1RVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50241930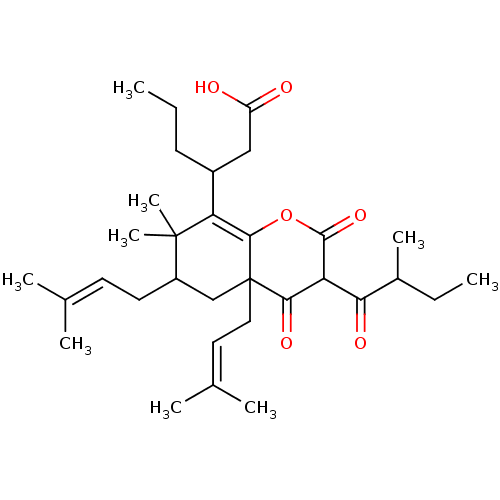 (CHEMBL486194 | Mahureone E) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Scientific Research (CNRS)-Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of human recombinant DNA polymerase beta assessed as fluorescein-12-dCTP incorporation by fluorimetry | J Nat Prod 68: 979-84 (2005) Article DOI: 10.1021/np049676o BindingDB Entry DOI: 10.7270/Q2VT1RVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50602335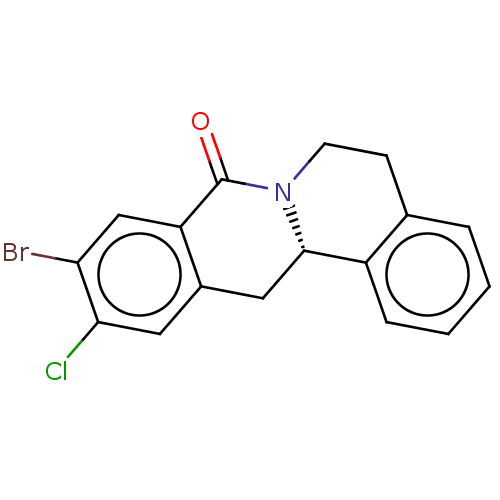 (CHEMBL5186309) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00956 BindingDB Entry DOI: 10.7270/Q2HM5DH2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM50602335 (CHEMBL5186309) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0580 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00956 BindingDB Entry DOI: 10.7270/Q2HM5DH2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50602335 (CHEMBL5186309) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00956 BindingDB Entry DOI: 10.7270/Q2HM5DH2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM50602335 (CHEMBL5186309) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 71 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00956 BindingDB Entry DOI: 10.7270/Q2HM5DH2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||