 Found 1047 hits with Last Name = 'holmes' and Initial = 'a'
Found 1047 hits with Last Name = 'holmes' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400528
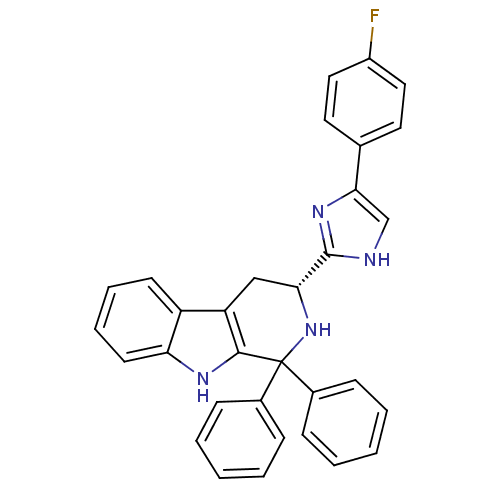
(CHEMBL2204934)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)[C@H]1Cc2c([nH]c3ccccc23)C(N1)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C32H25FN4/c33-24-17-15-21(16-18-24)29-20-34-31(36-29)28-19-26-25-13-7-8-14-27(25)35-30(26)32(37-28,22-9-3-1-4-10-22)23-11-5-2-6-12-23/h1-18,20,28,35,37H,19H2,(H,34,36)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400518

(CHEMBL2204942)Show SMILES Cn1cc(cn1)[C@H]1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H21FN6/c1-31-13-15(11-27-31)22-23-18(17-4-2-3-5-19(17)28-23)10-20(29-22)24-26-12-21(30-24)14-6-8-16(25)9-7-14/h2-9,11-13,20,22,28-29H,10H2,1H3,(H,26,30)/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50389590
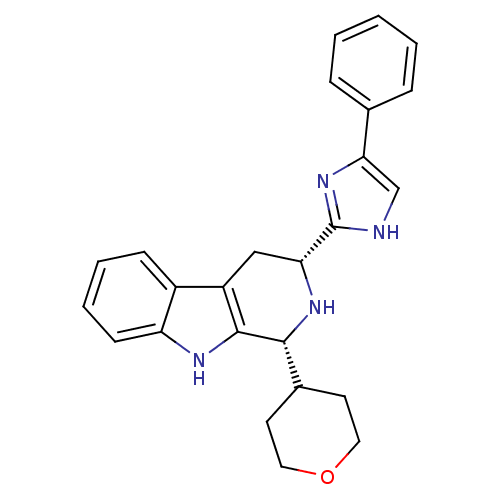
(CHEMBL2069502)Show SMILES C1CC(CCO1)[C@H]1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C25H26N4O/c1-2-6-16(7-3-1)22-15-26-25(29-22)21-14-19-18-8-4-5-9-20(18)27-24(19)23(28-21)17-10-12-30-13-11-17/h1-9,15,17,21,23,27-28H,10-14H2,(H,26,29)/t21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 369 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400519

(CHEMBL2204941)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)[C@H]1Cc2c([nH]c3ccccc23)[C@H](N1)C1CCOCC1 |r| Show InChI InChI=1S/C25H25FN4O/c26-17-7-5-15(6-8-17)22-14-27-25(30-22)21-13-19-18-3-1-2-4-20(18)28-24(19)23(29-21)16-9-11-31-12-10-16/h1-8,14,16,21,23,28-29H,9-13H2,(H,27,30)/t21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400520

(CHEMBL2204932)Show SMILES Cc1nc(no1)[C@H]1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H19FN6O/c1-12-26-23(30-31-12)21-20-16(15-4-2-3-5-17(15)27-20)10-18(28-21)22-25-11-19(29-22)13-6-8-14(24)9-7-13/h2-9,11,18,21,27-28H,10H2,1H3,(H,25,29)/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 385 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400526

(CHEMBL2204937)Show SMILES Cc1nc(no1)[C@]1(N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 462 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400525
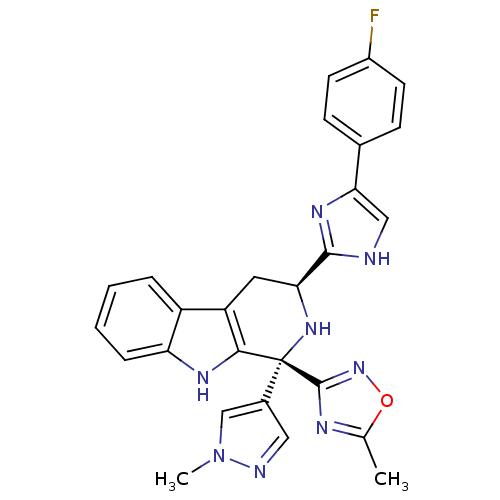
(CHEMBL2204938)Show SMILES Cc1nc(no1)[C@@]1(N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 543 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400523
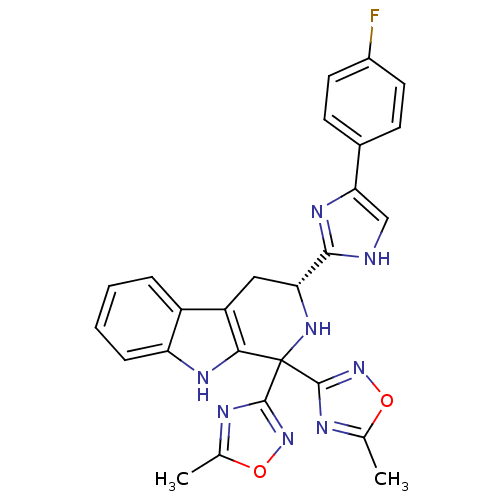
(CHEMBL2204940)Show SMILES Cc1nc(no1)C1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1noc(C)n1 |r| Show InChI InChI=1S/C26H21FN8O2/c1-13-29-24(34-36-13)26(25-30-14(2)37-35-25)22-18(17-5-3-4-6-19(17)31-22)11-20(33-26)23-28-12-21(32-23)15-7-9-16(27)10-8-15/h3-10,12,20,31,33H,11H2,1-2H3,(H,28,32)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400527
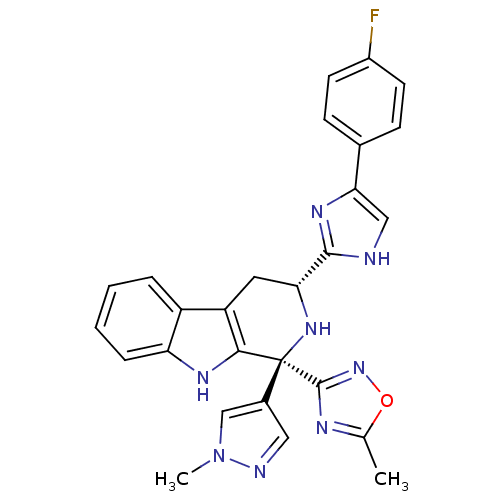
(CHEMBL2204936)Show SMILES Cc1nc(no1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400517
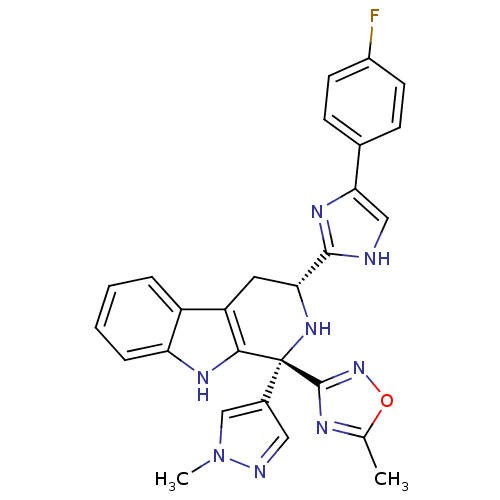
(CHEMBL2204935)Show SMILES Cc1nc(no1)[C@@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400522
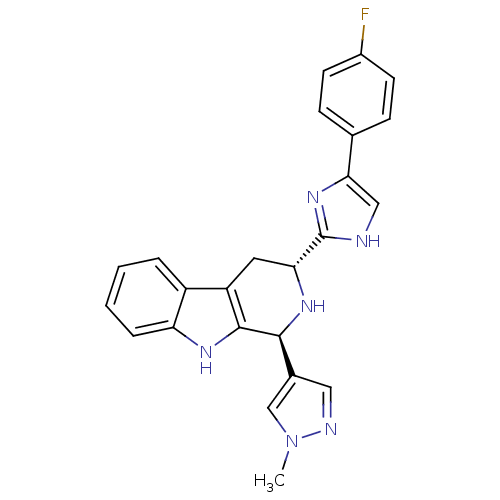
(CHEMBL2204931)Show SMILES Cn1cc(cn1)[C@@H]1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H21FN6/c1-31-13-15(11-27-31)22-23-18(17-4-2-3-5-19(17)28-23)10-20(29-22)24-26-12-21(30-24)14-6-8-16(25)9-7-14/h2-9,11-13,20,22,28-29H,10H2,1H3,(H,26,30)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400521
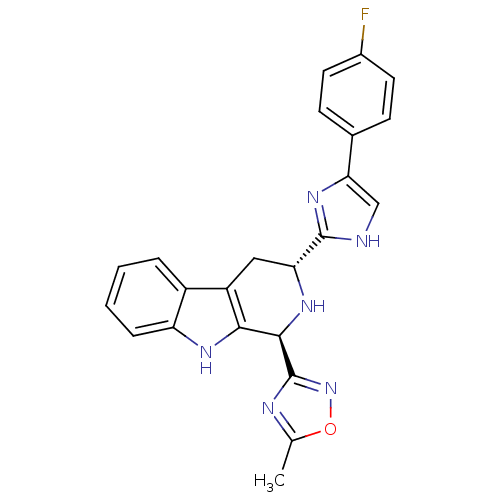
(CHEMBL2204933)Show SMILES Cc1nc(no1)[C@@H]1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H19FN6O/c1-12-26-23(30-31-12)21-20-16(15-4-2-3-5-17(15)27-20)10-18(28-21)22-25-11-19(29-22)13-6-8-14(24)9-7-13/h2-9,11,18,21,27-28H,10H2,1H3,(H,25,29)/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400524
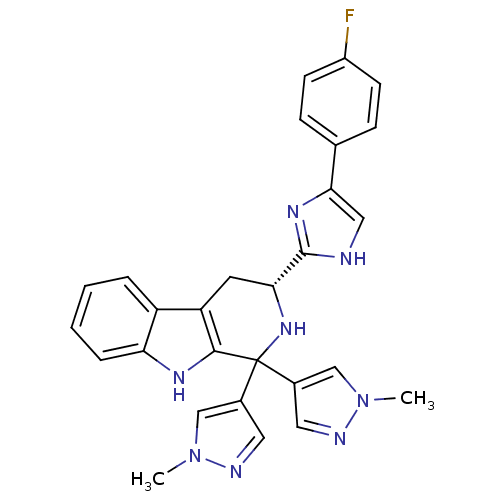
(CHEMBL2204939)Show SMILES Cn1cc(cn1)C1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C28H25FN8/c1-36-15-18(12-31-36)28(19-13-32-37(2)16-19)26-22(21-5-3-4-6-23(21)33-26)11-24(35-28)27-30-14-25(34-27)17-7-9-20(29)10-8-17/h3-10,12-16,24,33,35H,11H2,1-2H3,(H,30,34)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301603
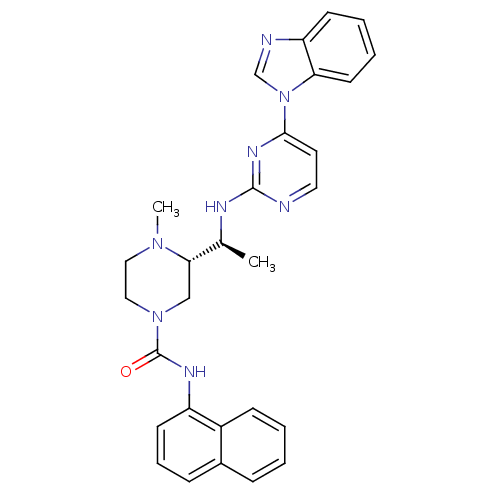
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2ccccc12)[C@@H]1CN(CCN1C)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C29H30N8O/c1-20(32-28-30-15-14-27(34-28)37-19-31-24-11-5-6-13-25(24)37)26-18-36(17-16-35(26)2)29(38)33-23-12-7-9-21-8-3-4-10-22(21)23/h3-15,19-20,26H,16-18H2,1-2H3,(H,33,38)(H,30,32,34)/t20-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301619
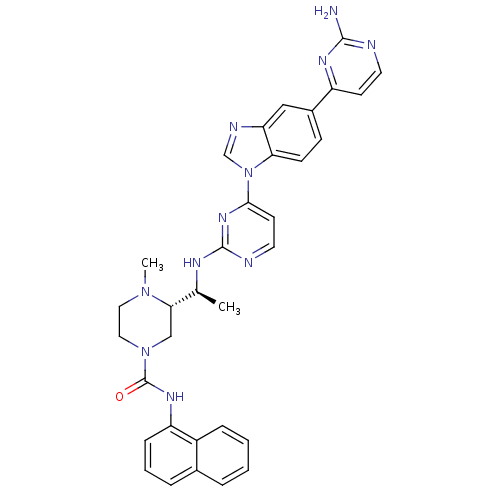
((S)-3-((S)-1-(4-(5-(2-aminopyrimidin-4-yl)-1H-benz...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2cc(ccc12)-c1ccnc(N)n1)[C@@H]1CN(CCN1C)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C33H33N11O/c1-21(29-19-43(17-16-42(29)2)33(45)40-26-9-5-7-22-6-3-4-8-24(22)26)38-32-36-15-13-30(41-32)44-20-37-27-18-23(10-11-28(27)44)25-12-14-35-31(34)39-25/h3-15,18,20-21,29H,16-17,19H2,1-2H3,(H,40,45)(H2,34,35,39)(H,36,38,41)/t21-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301604
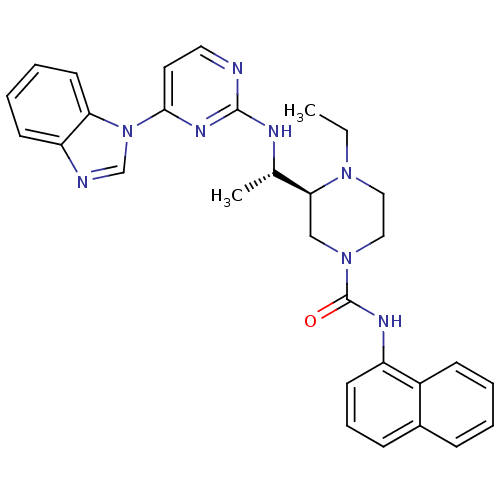
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES CCN1CCN(C[C@H]1[C@H](C)Nc1nccc(n1)-n1cnc2ccccc12)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C30H32N8O/c1-3-36-17-18-37(30(39)34-24-13-8-10-22-9-4-5-11-23(22)24)19-27(36)21(2)33-29-31-16-15-28(35-29)38-20-32-25-12-6-7-14-26(25)38/h4-16,20-21,27H,3,17-19H2,1-2H3,(H,34,39)(H,31,33,35)/t21-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301624
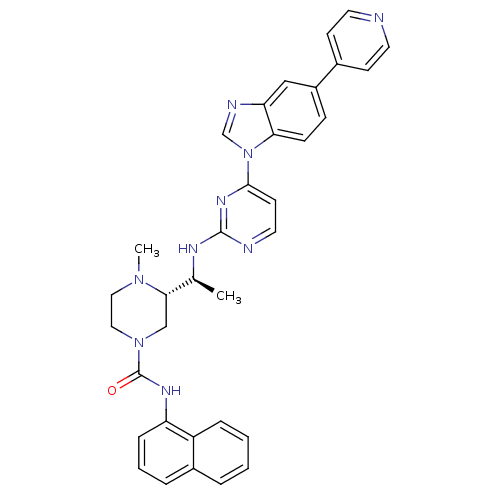
((S)-4-methyl-N(S)-4-methyl-N-(naphthalen-1-yl)-3-(...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2cc(ccc12)-c1ccncc1)[C@@H]1CN(CCN1C)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C34H33N9O/c1-23(31-21-42(19-18-41(31)2)34(44)39-28-9-5-7-25-6-3-4-8-27(25)28)38-33-36-17-14-32(40-33)43-22-37-29-20-26(10-11-30(29)43)24-12-15-35-16-13-24/h3-17,20,22-23,31H,18-19,21H2,1-2H3,(H,39,44)(H,36,38,40)/t23-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM50400525

(CHEMBL2204938)Show SMILES Cc1nc(no1)[C@@]1(N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of mouse SSTR3 transfected in CHO cells assessed as inhibition of SRIF-induced reduction of cAMP accumulation after 45 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50062005
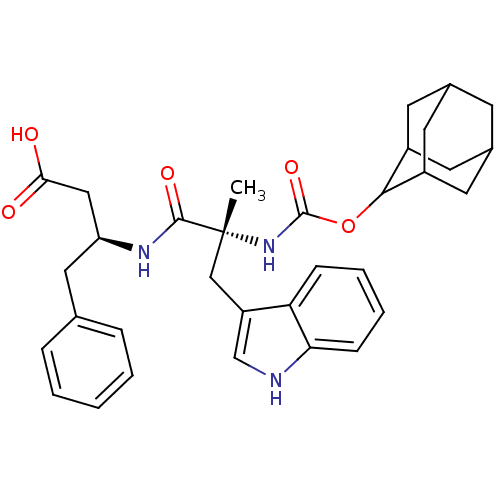
((S)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@H](CC(O)=O)Cc1ccccc1 |wU:1.13,wD:29.33,1.0,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:21:16:19.18.25,20:19:16:21.23.22,(10.8,-2.25,;10.81,-3.63,;10.9,-5.18,;12.26,-5.88,;11.02,-6.79,;11.49,-8.25,;13.03,-8.24,;14.06,-9.39,;15.56,-9.07,;16.04,-7.61,;15.01,-6.46,;13.52,-6.79,;9.43,-3.12,;8.15,-3.96,;8.17,-5.43,;6.76,-3.28,;5.47,-4.12,;5.46,-5.67,;4.44,-6.95,;3.04,-6.37,;1.53,-6.79,;2.74,-5.52,;4.05,-6.01,;2.72,-4.03,;4.08,-3.55,;3.02,-4.78,;12.1,-2.95,;12.07,-1.57,;13.48,-3.63,;14.76,-2.78,;14.67,-1.25,;15.43,.09,;14.65,1.43,;16.98,.11,;16.14,-3.47,;17.43,-2.62,;17.34,-1.25,;18.6,-.23,;20,-.92,;20.1,-2.46,;18.81,-3.31,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-25-19-34-28-10-6-5-9-27(25)28,31(39)35-26(17-29(37)38)16-20-7-3-2-4-8-20)36-32(40)41-30-23-12-21-11-22(14-23)15-24(30)13-21/h2-10,19,21-24,26,30,34H,11-18H2,1H3,(H,35,39)(H,36,40)(H,37,38)/t21?,22?,23?,24?,26-,30?,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against radioligand [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 41: 38-45 (1998)
Article DOI: 10.1021/jm970065l
BindingDB Entry DOI: 10.7270/Q2GT5NV6 |
More data for this
Ligand-Target Pair | |
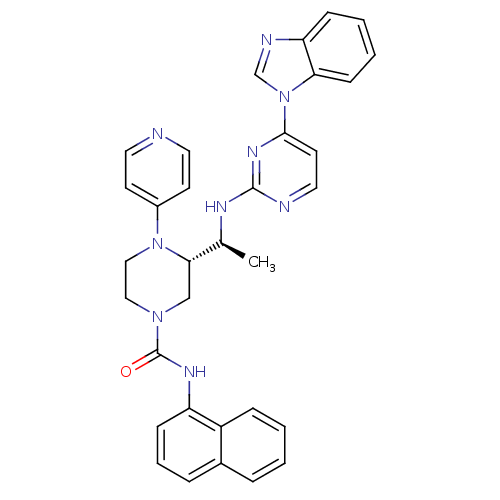
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301605
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES CC(C)CN1CCN(C[C@H]1[C@H](C)Nc1nccc(n1)-n1cnc2ccccc12)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C32H36N8O/c1-22(2)19-38-17-18-39(32(41)36-26-13-8-10-24-9-4-5-11-25(24)26)20-29(38)23(3)35-31-33-16-15-30(37-31)40-21-34-27-12-6-7-14-28(27)40/h4-16,21-23,29H,17-20H2,1-3H3,(H,36,41)(H,33,35,37)/t23-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
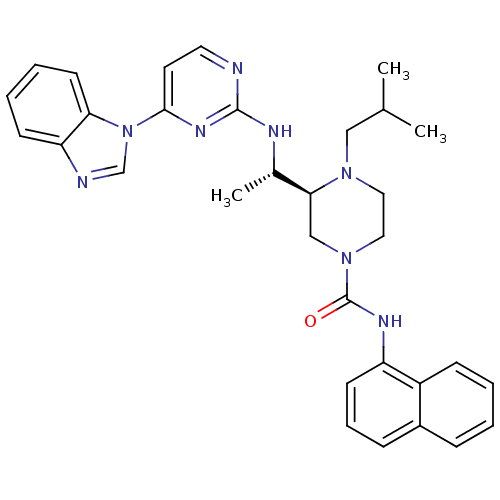
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301607
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2ccccc12)[C@@H]1CN(CCN1c1ccncc1)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C33H31N9O/c1-23(37-32-35-18-15-31(39-32)42-22-36-28-10-4-5-12-29(28)42)30-21-40(19-20-41(30)25-13-16-34-17-14-25)33(43)38-27-11-6-8-24-7-2-3-9-26(24)27/h2-18,22-23,30H,19-21H2,1H3,(H,38,43)(H,35,37,39)/t23-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301594
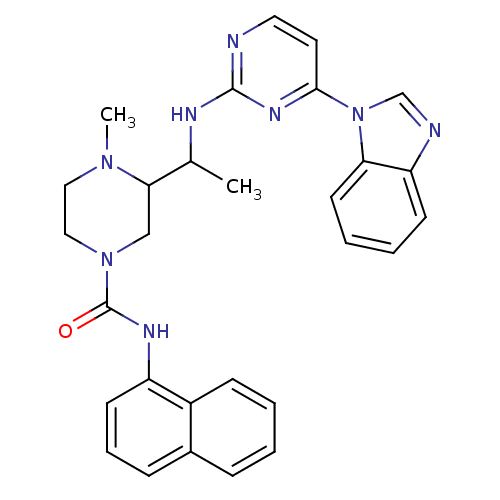
(3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CN(CCN1C)C(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C29H30N8O/c1-20(32-28-30-15-14-27(34-28)37-19-31-24-11-5-6-13-25(24)37)26-18-36(17-16-35(26)2)29(38)33-23-12-7-9-21-8-3-4-10-22(21)23/h3-15,19-20,26H,16-18H2,1-2H3,(H,33,38)(H,30,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301594

(3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CN(CCN1C)C(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C29H30N8O/c1-20(32-28-30-15-14-27(34-28)37-19-31-24-11-5-6-13-25(24)37)26-18-36(17-16-35(26)2)29(38)33-23-12-7-9-21-8-3-4-10-22(21)23/h3-15,19-20,26H,16-18H2,1-2H3,(H,33,38)(H,30,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM50400517

(CHEMBL2204935)Show SMILES Cc1nc(no1)[C@@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-28 from mouse SSTR3 transfected in CHO cells after 60 to 90 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301588
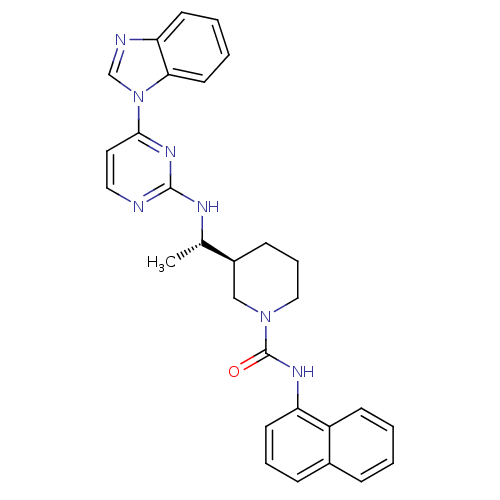
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2ccccc12)[C@H]1CCCN(C1)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C29H29N7O/c1-20(32-28-30-16-15-27(34-28)36-19-31-25-12-4-5-14-26(25)36)22-10-7-17-35(18-22)29(37)33-24-13-6-9-21-8-2-3-11-23(21)24/h2-6,8-9,11-16,19-20,22H,7,10,17-18H2,1H3,(H,33,37)(H,30,32,34)/t20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301618
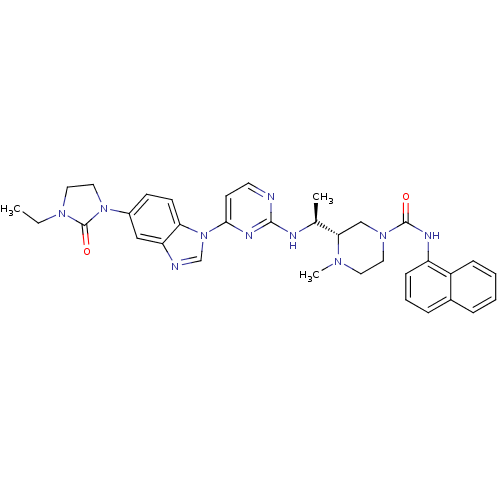
((S)-3-((S)-1-(4-(5-(3-ethyl-2-oxoimidazolidin-1-yl...)Show SMILES CCN1CCN(C1=O)c1ccc2n(cnc2c1)-c1ccnc(N[C@@H](C)[C@@H]2CN(CCN2C)C(=O)Nc2cccc3ccccc23)n1 |r| Show InChI InChI=1S/C34H38N10O2/c1-4-41-18-19-43(34(41)46)25-12-13-29-28(20-25)36-22-44(29)31-14-15-35-32(39-31)37-23(2)30-21-42(17-16-40(30)3)33(45)38-27-11-7-9-24-8-5-6-10-26(24)27/h5-15,20,22-23,30H,4,16-19,21H2,1-3H3,(H,38,45)(H,35,37,39)/t23-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50329956
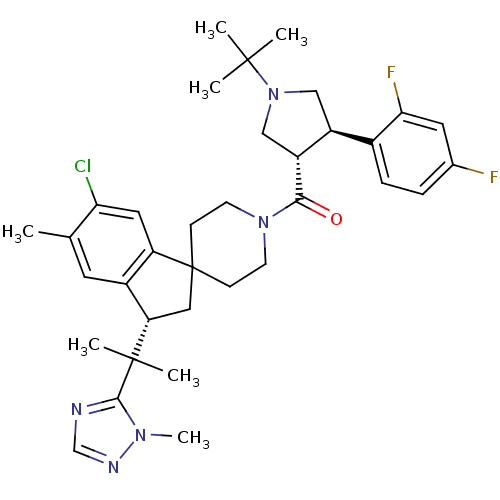
(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES Cc1cc2[C@H](CC3(CCN(CC3)C(=O)[C@@H]3CN(C[C@H]3c3ccc(F)cc3F)C(C)(C)C)c2cc1Cl)C(C)(C)c1ncnn1C |r| Show InChI InChI=1S/C35H44ClF2N5O/c1-21-14-24-27(16-29(21)36)35(17-28(24)34(5,6)32-39-20-40-41(32)7)10-12-42(13-11-35)31(44)26-19-43(33(2,3)4)18-25(26)23-9-8-22(37)15-30(23)38/h8-9,14-16,20,25-26,28H,10-13,17-19H2,1-7H3/t25-,26+,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MC4 receptor |
Bioorg Med Chem Lett 20: 6524-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.049
BindingDB Entry DOI: 10.7270/Q24M94SF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50329959
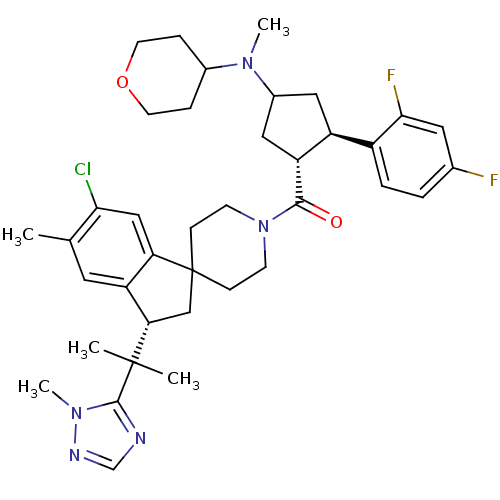
((6-chloro-5-methyl-3-(2-(1-methyl-1H-1,2,4-triazol...)Show SMILES CN(C1C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC2(C[C@@H](c3cc(C)c(Cl)cc23)C(C)(C)c2ncnn2C)CC1)C1CCOCC1 |r| Show InChI InChI=1S/C38H48ClF2N5O2/c1-23-16-30-31(20-33(23)39)38(21-32(30)37(2,3)36-42-22-43-45(36)5)10-12-46(13-11-38)35(47)29-19-26(44(4)25-8-14-48-15-9-25)18-28(29)27-7-6-24(40)17-34(27)41/h6-7,16-17,20,22,25-26,28-29,32H,8-15,18-19,21H2,1-5H3/t26?,28-,29+,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MC4 receptor |
Bioorg Med Chem Lett 20: 6524-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.049
BindingDB Entry DOI: 10.7270/Q24M94SF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301608
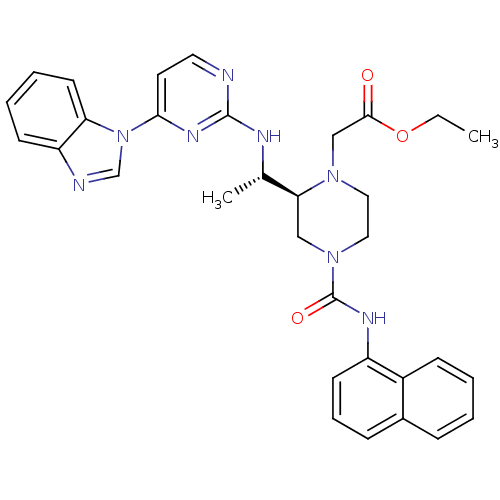
(CHEMBL566507 | ethyl 2-((S)-2-((S)-1-(4-(1H-benzo[...)Show SMILES CCOC(=O)CN1CCN(C[C@H]1[C@H](C)Nc1nccc(n1)-n1cnc2ccccc12)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C32H34N8O3/c1-3-43-30(41)20-38-17-18-39(32(42)36-25-13-8-10-23-9-4-5-11-24(23)25)19-28(38)22(2)35-31-33-16-15-29(37-31)40-21-34-26-12-6-7-14-27(26)40/h4-16,21-22,28H,3,17-20H2,1-2H3,(H,36,42)(H,33,35,37)/t22-,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM50400517

(CHEMBL2204935)Show SMILES Cc1nc(no1)[C@@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of mouse SSTR3 transfected in CHO cells assessed as inhibition of SRIF-induced reduction of cAMP accumulation after 45 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM50400524

(CHEMBL2204939)Show SMILES Cn1cc(cn1)C1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C28H25FN8/c1-36-15-18(12-31-36)28(19-13-32-37(2)16-19)26-22(21-5-3-4-6-23(21)33-26)11-24(35-28)27-30-14-25(34-27)17-7-9-20(29)10-8-17/h3-10,12-16,24,33,35H,11H2,1-2H3,(H,30,34)/t24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-28 from mouse SSTR3 transfected in CHO cells after 60 to 90 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM50400524

(CHEMBL2204939)Show SMILES Cn1cc(cn1)C1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C28H25FN8/c1-36-15-18(12-31-36)28(19-13-32-37(2)16-19)26-22(21-5-3-4-6-23(21)33-26)11-24(35-28)27-30-14-25(34-27)17-7-9-20(29)10-8-17/h3-10,12-16,24,33,35H,11H2,1-2H3,(H,30,34)/t24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of mouse SSTR3 transfected in CHO cells assessed as inhibition of SRIF-induced reduction of cAMP accumulation after 45 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM50400525

(CHEMBL2204938)Show SMILES Cc1nc(no1)[C@@]1(N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-28 from mouse SSTR3 transfected in CHO cells after 60 to 90 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM50400523

(CHEMBL2204940)Show SMILES Cc1nc(no1)C1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1noc(C)n1 |r| Show InChI InChI=1S/C26H21FN8O2/c1-13-29-24(34-36-13)26(25-30-14(2)37-35-25)22-18(17-5-3-4-6-19(17)31-22)11-20(33-26)23-28-12-21(32-23)15-7-9-16(27)10-8-15/h3-10,12,20,31,33H,11H2,1-2H3,(H,28,32)/t20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-28 from mouse SSTR3 transfected in CHO cells after 60 to 90 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM50400527

(CHEMBL2204936)Show SMILES Cc1nc(no1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-28 from mouse SSTR3 transfected in CHO cells after 60 to 90 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50400517

(CHEMBL2204935)Show SMILES Cc1nc(no1)[C@@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-28 from human SSTR3 transfected in CHO cells after 60 to 90 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50400524

(CHEMBL2204939)Show SMILES Cn1cc(cn1)C1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C28H25FN8/c1-36-15-18(12-31-36)28(19-13-32-37(2)16-19)26-22(21-5-3-4-6-23(21)33-26)11-24(35-28)27-30-14-25(34-27)17-7-9-20(29)10-8-17/h3-10,12-16,24,33,35H,11H2,1-2H3,(H,30,34)/t24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human SSTR3 transfected in CHO cells assessed as inhibition of SRIF-induced reduction of cAMP accumulation after 45 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50400524

(CHEMBL2204939)Show SMILES Cn1cc(cn1)C1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C28H25FN8/c1-36-15-18(12-31-36)28(19-13-32-37(2)16-19)26-22(21-5-3-4-6-23(21)33-26)11-24(35-28)27-30-14-25(34-27)17-7-9-20(29)10-8-17/h3-10,12-16,24,33,35H,11H2,1-2H3,(H,30,34)/t24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-28 from human SSTR3 transfected in CHO cells after 60 to 90 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50400528

(CHEMBL2204934)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)[C@H]1Cc2c([nH]c3ccccc23)C(N1)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C32H25FN4/c33-24-17-15-21(16-18-24)29-20-34-31(36-29)28-19-26-25-13-7-8-14-27(25)35-30(26)32(37-28,22-9-3-1-4-10-22)23-11-5-2-6-12-23/h1-18,20,28,35,37H,19H2,(H,34,36)/t28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-28 from human SSTR3 transfected in CHO cells after 60 to 90 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50400525

(CHEMBL2204938)Show SMILES Cc1nc(no1)[C@@]1(N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human SSTR3 transfected in CHO cells assessed as inhibition of SRIF-induced reduction of cAMP accumulation after 45 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50329958
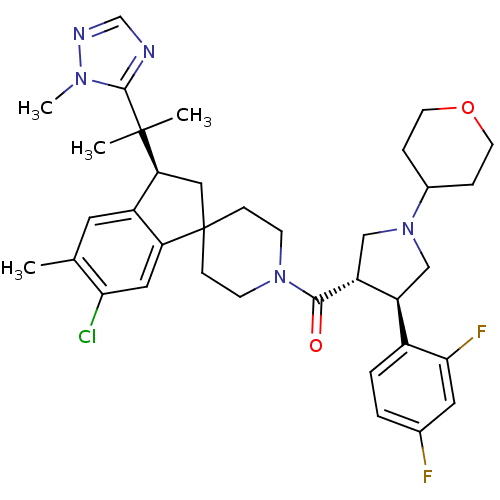
((6-chloro-5-methyl-3-(2-(1-methyl-1H-1,2,4-triazol...)Show SMILES Cc1cc2[C@H](CC3(CCN(CC3)C(=O)[C@@H]3CN(C[C@H]3c3ccc(F)cc3F)C3CCOCC3)c2cc1Cl)C(C)(C)c1ncnn1C |r| Show InChI InChI=1S/C36H44ClF2N5O2/c1-22-15-26-29(17-31(22)37)36(18-30(26)35(2,3)34-40-21-41-42(34)4)9-11-43(12-10-36)33(45)28-20-44(24-7-13-46-14-8-24)19-27(28)25-6-5-23(38)16-32(25)39/h5-6,15-17,21,24,27-28,30H,7-14,18-20H2,1-4H3/t27-,28+,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MC4 receptor |
Bioorg Med Chem Lett 20: 6524-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.049
BindingDB Entry DOI: 10.7270/Q24M94SF |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50400525

(CHEMBL2204938)Show SMILES Cc1nc(no1)[C@@]1(N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-28 from human SSTR3 transfected in CHO cells after 60 to 90 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50400517

(CHEMBL2204935)Show SMILES Cc1nc(no1)[C@@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human SSTR3 transfected in CHO cells assessed as inhibition of SRIF-induced reduction of cAMP accumulation after 45 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50400523

(CHEMBL2204940)Show SMILES Cc1nc(no1)C1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1noc(C)n1 |r| Show InChI InChI=1S/C26H21FN8O2/c1-13-29-24(34-36-13)26(25-30-14(2)37-35-25)22-18(17-5-3-4-6-19(17)31-22)11-20(33-26)23-28-12-21(32-23)15-7-9-16(27)10-8-15/h3-10,12,20,31,33H,11H2,1-2H3,(H,28,32)/t20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-28 from human SSTR3 transfected in CHO cells after 60 to 90 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50062000
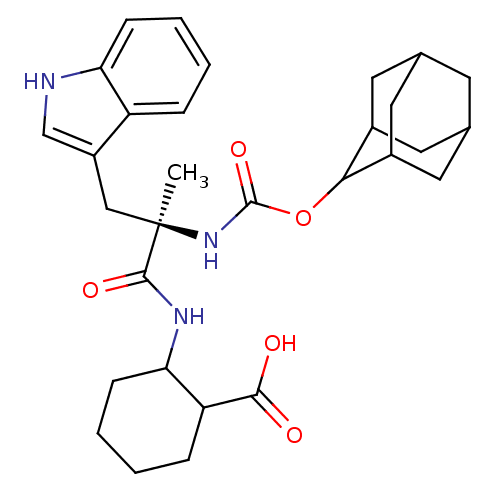
(2-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1H-in...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC1CCCCC1C(O)=O |wU:1.13,wD:1.0,TLB:15:16:23:19.25.20,18:19:23:16.17.22,THB:18:17:23:19.25.20,20:21:16:19.18.25,20:19:16:21.23.22,(1.86,-7.02,;1.88,-8.39,;1.95,-9.94,;3.33,-10.64,;2.08,-11.55,;2.56,-13.03,;4.11,-13.02,;5.13,-14.17,;6.63,-13.85,;7.1,-12.37,;6.07,-11.24,;4.57,-11.55,;.49,-7.88,;-.8,-8.73,;-.77,-10.19,;-2.19,-8.04,;-3.48,-8.9,;-3.48,-10.43,;-4.51,-11.72,;-5.92,-11.15,;-7.41,-11.57,;-6.22,-10.29,;-4.89,-10.78,;-6.22,-8.8,;-4.87,-8.32,;-5.92,-9.55,;3.16,-7.71,;3.15,-6.33,;4.41,-8.44,;5.74,-7.68,;7.08,-8.44,;8.41,-7.69,;8.43,-6.14,;7.08,-5.37,;5.74,-6.13,;4.64,-5.03,;5.04,-3.54,;3.15,-5.42,)| Show InChI InChI=1S/C30H39N3O5/c1-30(15-21-16-31-24-8-4-2-6-22(21)24,28(36)32-25-9-5-3-7-23(25)27(34)35)33-29(37)38-26-19-11-17-10-18(13-19)14-20(26)12-17/h2,4,6,8,16-20,23,25-26,31H,3,5,7,9-15H2,1H3,(H,32,36)(H,33,37)(H,34,35)/t17?,18?,19?,20?,23?,25?,26?,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against radioligand [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 41: 38-45 (1998)
Article DOI: 10.1021/jm970065l
BindingDB Entry DOI: 10.7270/Q2GT5NV6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301587
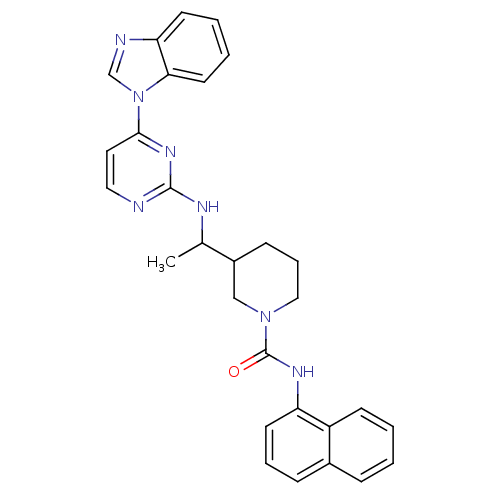
(CHEMBL567885 | rac 3-(1-(4-(1H-benzo[d]imidazol-1-...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CCCN(C1)C(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C29H29N7O/c1-20(32-28-30-16-15-27(34-28)36-19-31-25-12-4-5-14-26(25)36)22-10-7-17-35(18-22)29(37)33-24-13-6-9-21-8-2-3-11-23(21)24/h2-6,8-9,11-16,19-20,22H,7,10,17-18H2,1H3,(H,33,37)(H,30,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301621

((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2ccccc12)[C@]1(C)CN(CCN1C)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C30H32N8O/c1-21(33-28-31-16-15-27(35-28)38-20-32-25-12-6-7-14-26(25)38)30(2)19-37(18-17-36(30)3)29(39)34-24-13-8-10-22-9-4-5-11-23(22)24/h4-16,20-21H,17-19H2,1-3H3,(H,34,39)(H,31,33,35)/t21-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301617
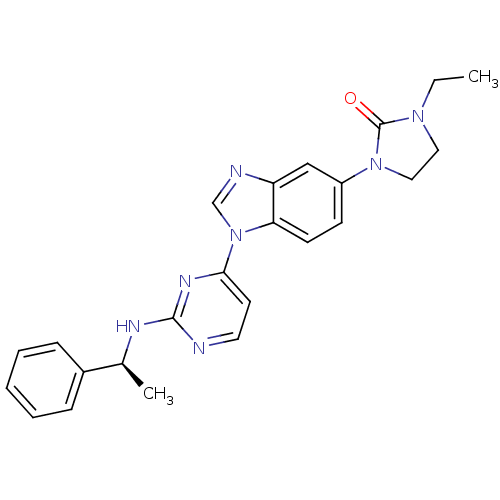
((S)-1-ethyl-3-(1-(2-(1-phenylethylamino)pyrimidin-...)Show SMILES CCN1CCN(C1=O)c1ccc2n(cnc2c1)-c1ccnc(N[C@@H](C)c2ccccc2)n1 |r| Show InChI InChI=1S/C24H25N7O/c1-3-29-13-14-30(24(29)32)19-9-10-21-20(15-19)26-16-31(21)22-11-12-25-23(28-22)27-17(2)18-7-5-4-6-8-18/h4-12,15-17H,3,13-14H2,1-2H3,(H,25,27,28)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50400527

(CHEMBL2204936)Show SMILES Cc1nc(no1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-28 from human SSTR3 transfected in CHO cells after 60 to 90 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50400527

(CHEMBL2204936)Show SMILES Cc1nc(no1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human SSTR3 transfected in CHO cells assessed as inhibition of SRIF-induced reduction of cAMP accumulation after 45 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data