 Found 166 hits with Last Name = 'horova' and Initial = 'a'
Found 166 hits with Last Name = 'horova' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50336116

(Ambenonium dichloride | CHEMBL1669479)Show SMILES C[N+](C)(CCNC(=O)C(=O)NCC[N+](C)(C)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C24H32Cl2N4O2/c1-29(2,17-19-9-5-7-11-21(19)25)15-13-27-23(31)24(32)28-14-16-30(3,4)18-20-10-6-8-12-22(20)26/h5-12H,13-18H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50262988
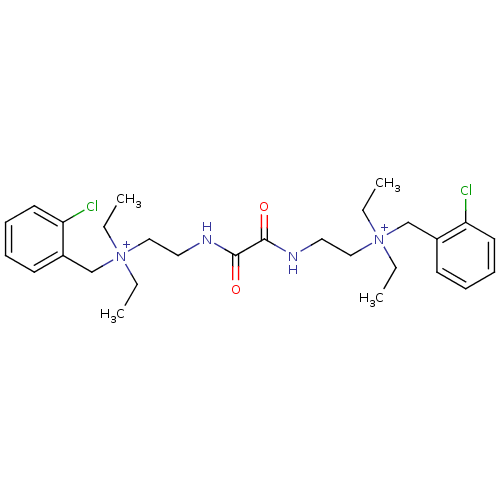
(CHEMBL1200541 | N-(2-chlorobenzyl)-2-(2-(2-((2-chl...)Show SMILES CC[N+](CC)(CCNC(=O)C(=O)NCC[N+](CC)(CC)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C28H40Cl2N4O2/c1-5-33(6-2,21-23-13-9-11-15-25(23)29)19-17-31-27(35)28(36)32-18-20-34(7-3,8-4)22-24-14-10-12-16-26(24)30/h9-16H,5-8,17-22H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50262988

(CHEMBL1200541 | N-(2-chlorobenzyl)-2-(2-(2-((2-chl...)Show SMILES CC[N+](CC)(CCNC(=O)C(=O)NCC[N+](CC)(CC)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C28H40Cl2N4O2/c1-5-33(6-2,21-23-13-9-11-15-25(23)29)19-17-31-27(35)28(36)32-18-20-34(7-3,8-4)22-24-14-10-12-16-26(24)30/h9-16H,5-8,17-22H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50336116

(Ambenonium dichloride | CHEMBL1669479)Show SMILES C[N+](C)(CCNC(=O)C(=O)NCC[N+](C)(C)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C24H32Cl2N4O2/c1-29(2,17-19-9-5-7-11-21(19)25)15-13-27-23(31)24(32)28-14-16-30(3,4)18-20-10-6-8-12-22(20)26/h5-12H,13-18H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Non competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005114

(CHEMBL3093804)Show SMILES [Br-].[Br-].C(CCCCCC[n+]1cccc2ccccc12)CCCCC[n+]1ccccc1 Show InChI InChI=1S/C26H36N2.2BrH/c1(3-5-7-12-20-27-21-13-9-14-22-27)2-4-6-8-15-23-28-24-16-18-25-17-10-11-19-26(25)28;;/h9-11,13-14,16-19,21-22,24H,1-8,12,15,20,23H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Non competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005114

(CHEMBL3093804)Show SMILES [Br-].[Br-].C(CCCCCC[n+]1cccc2ccccc12)CCCCC[n+]1ccccc1 Show InChI InChI=1S/C26H36N2.2BrH/c1(3-5-7-12-20-27-21-13-9-14-22-27)2-4-6-8-15-23-28-24-16-18-25-17-10-11-19-26(25)28;;/h9-11,13-14,16-19,21-22,24H,1-8,12,15,20,23H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119797

(1,1'-(decane-1,10-diyl)diquinolinium iodide | 1,10...)Show SMILES C(CCCCC[n+]1cccc2ccccc12)CCCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C28H34N2/c1(3-5-11-21-29-23-13-17-25-15-7-9-19-27(25)29)2-4-6-12-22-30-24-14-18-26-16-8-10-20-28(26)30/h7-10,13-20,23-24H,1-6,11-12,21-22H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133473
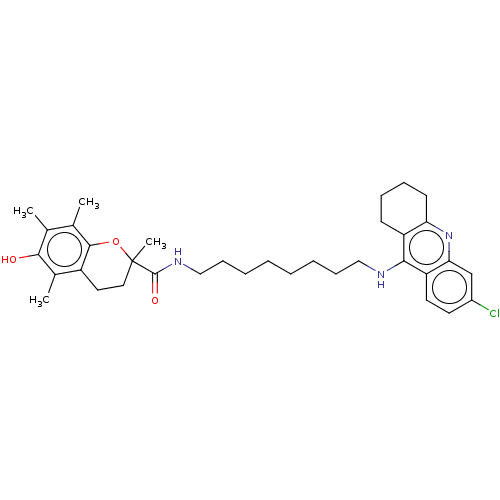
(CHEMBL3632994)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H46ClN3O3/c1-22-23(2)33-26(24(3)32(22)40)17-18-35(4,42-33)34(41)38-20-12-8-6-5-7-11-19-37-31-27-13-9-10-14-29(27)39-30-21-25(36)15-16-28(30)31/h15-16,21,40H,5-14,17-20H2,1-4H3,(H,37,39)(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Non competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119797

(1,1'-(decane-1,10-diyl)diquinolinium iodide | 1,10...)Show SMILES C(CCCCC[n+]1cccc2ccccc12)CCCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C28H34N2/c1(3-5-11-21-29-23-13-17-25-15-7-9-19-27(25)29)2-4-6-12-22-30-24-14-18-26-16-8-10-20-28(26)30/h7-10,13-20,23-24H,1-6,11-12,21-22H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005115
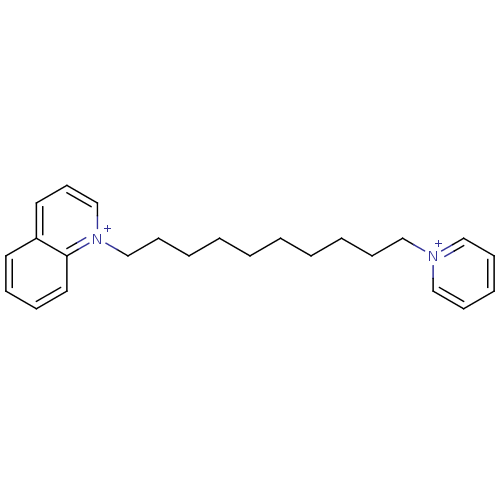
(CHEMBL3093802)Show SMILES [Br-].[Br-].C(CCCCC[n+]1cccc2ccccc12)CCCC[n+]1ccccc1 Show InChI InChI=1S/C24H32N2.2BrH/c1(3-5-10-18-25-19-11-7-12-20-25)2-4-6-13-21-26-22-14-16-23-15-8-9-17-24(23)26;;/h7-9,11-12,14-17,19-20,22H,1-6,10,13,18,21H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Non competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005115

(CHEMBL3093802)Show SMILES [Br-].[Br-].C(CCCCC[n+]1cccc2ccccc12)CCCC[n+]1ccccc1 Show InChI InChI=1S/C24H32N2.2BrH/c1(3-5-10-18-25-19-11-7-12-20-25)2-4-6-13-21-26-22-14-16-23-15-8-9-17-24(23)26;;/h7-9,11-12,14-17,19-20,22H,1-6,10,13,18,21H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM120262
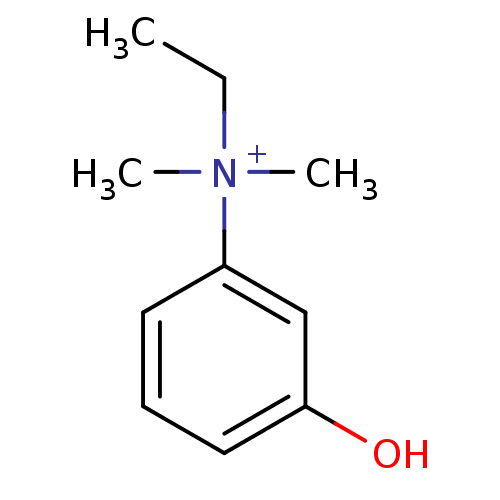
(EDROPHONIUM BROMIDE | EDROPHONIUM CHLORIDE | Edrop...)Show InChI InChI=1S/C10H15NO/c1-4-11(2,3)9-6-5-7-10(12)8-9/h5-8H,4H2,1-3H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50339942

(1,1'-(naphthalene-2,7-diylbis(methylene))diquinoli...)Show SMILES C(c1ccc2ccc(C[n+]3cccc4ccccc34)cc2c1)[n+]1cccc2ccccc12 Show InChI InChI=1S/C30H24N2/c1-3-11-29-26(7-1)9-5-17-31(29)21-23-13-15-25-16-14-24(20-28(25)19-23)22-32-18-6-10-27-8-2-4-12-30(27)32/h1-20H,21-22H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM120262

(EDROPHONIUM BROMIDE | EDROPHONIUM CHLORIDE | Edrop...)Show InChI InChI=1S/C10H15NO/c1-4-11(2,3)9-6-5-7-10(12)8-9/h5-8H,4H2,1-3H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM120262

(EDROPHONIUM BROMIDE | EDROPHONIUM CHLORIDE | Edrop...)Show InChI InChI=1S/C10H15NO/c1-4-11(2,3)9-6-5-7-10(12)8-9/h5-8H,4H2,1-3H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50339942

(1,1'-(naphthalene-2,7-diylbis(methylene))diquinoli...)Show SMILES C(c1ccc2ccc(C[n+]3cccc4ccccc34)cc2c1)[n+]1cccc2ccccc12 Show InChI InChI=1S/C30H24N2/c1-3-11-29-26(7-1)9-5-17-31(29)21-23-13-15-25-16-14-24(20-28(25)19-23)22-32-18-6-10-27-8-2-4-12-30(27)32/h1-20H,21-22H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8958
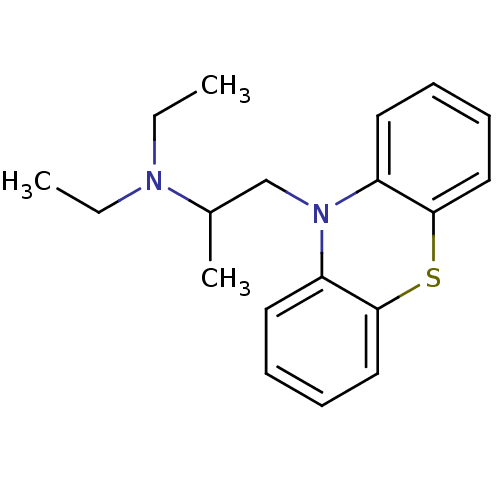
(10-(2-diethylaminopropyl)phenothiazine | CHEMBL120...)Show InChI InChI=1S/C19H24N2S/c1-4-20(5-2)15(3)14-21-16-10-6-8-12-18(16)22-19-13-9-7-11-17(19)21/h6-13,15H,4-5,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8958

(10-(2-diethylaminopropyl)phenothiazine | CHEMBL120...)Show InChI InChI=1S/C19H24N2S/c1-4-20(5-2)15(3)14-21-16-10-6-8-12-18(16)22-19-13-9-7-11-17(19)21/h6-13,15H,4-5,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.21E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50262988

(CHEMBL1200541 | N-(2-chlorobenzyl)-2-(2-(2-((2-chl...)Show SMILES CC[N+](CC)(CCNC(=O)C(=O)NCC[N+](CC)(CC)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C28H40Cl2N4O2/c1-5-33(6-2,21-23-13-9-11-15-25(23)29)19-17-31-27(35)28(36)32-18-20-34(7-3,8-4)22-24-14-10-12-16-26(24)30/h9-16H,5-8,17-22H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte recombinant AChE by modified Ellman's method |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50336116

(Ambenonium dichloride | CHEMBL1669479)Show SMILES C[N+](C)(CCNC(=O)C(=O)NCC[N+](C)(C)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C24H32Cl2N4O2/c1-29(2,17-19-9-5-7-11-21(19)25)15-13-27-23(31)24(32)28-14-16-30(3,4)18-20-10-6-8-12-22(20)26/h5-12H,13-18H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119797

(1,1'-(decane-1,10-diyl)diquinolinium iodide | 1,10...)Show SMILES C(CCCCC[n+]1cccc2ccccc12)CCCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C28H34N2/c1(3-5-11-21-29-23-13-17-25-15-7-9-19-27(25)29)2-4-6-12-22-30-24-14-18-26-16-8-10-20-28(26)30/h7-10,13-20,23-24H,1-6,11-12,21-22H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte recombinant AChE by modified Ellman's method |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005114

(CHEMBL3093804)Show SMILES [Br-].[Br-].C(CCCCCC[n+]1cccc2ccccc12)CCCCC[n+]1ccccc1 Show InChI InChI=1S/C26H36N2.2BrH/c1(3-5-7-12-20-27-21-13-9-14-22-27)2-4-6-8-15-23-28-24-16-18-25-17-10-11-19-26(25)28;;/h9-11,13-14,16-19,21-22,24H,1-8,12,15,20,23H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005115

(CHEMBL3093802)Show SMILES [Br-].[Br-].C(CCCCC[n+]1cccc2ccccc12)CCCC[n+]1ccccc1 Show InChI InChI=1S/C24H32N2.2BrH/c1(3-5-10-18-25-19-11-7-12-20-25)2-4-6-13-21-26-22-14-16-23-15-8-9-17-24(23)26;;/h7-9,11-12,14-17,19-20,22H,1-6,10,13,18,21H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119799
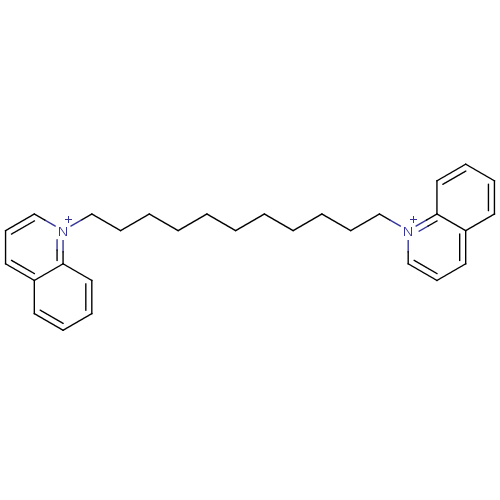
(1,11-di(1-quinoliniumyl)undecane; with dibromide i...)Show SMILES C(CCCCC[n+]1cccc2ccccc12)CCCCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C29H36N2/c1(2-4-6-12-22-30-24-14-18-26-16-8-10-20-28(26)30)3-5-7-13-23-31-25-15-19-27-17-9-11-21-29(27)31/h8-11,14-21,24-25H,1-7,12-13,22-23H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte recombinant AChE by modified Ellman's method |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005116

(CHEMBL3093803)Show SMILES [Br-].[Br-].C(CCCCC[n+]1ccccc1)CCCCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C25H34N2.2BrH/c1(2-4-6-11-19-26-20-12-8-13-21-26)3-5-7-14-22-27-23-15-17-24-16-9-10-18-25(24)27;;/h8-10,12-13,15-18,20-21,23H,1-7,11,14,19,22H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8987

(6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-10-12(7-8)16-11-4-2-1-3-9(11)13(10)15/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119789

(1,1'-(dodecane-1,12-diyl)diquinolinium bromide | 1...)Show SMILES C(CCCCCC[n+]1cccc2ccccc12)CCCCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C30H38N2/c1(3-5-7-13-23-31-25-15-19-27-17-9-11-21-29(27)31)2-4-6-8-14-24-32-26-16-20-28-18-10-12-22-30(28)32/h9-12,15-22,25-26H,1-8,13-14,23-24H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte recombinant AChE by modified Ellman's method |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005181

(CHEMBL3093797)Show SMILES [Br-].[Br-].C(CCCCC[n+]1ccc2ccccc2c1)CCCC[n+]1ccccc1 Show InChI InChI=1S/C24H32N2.2BrH/c1(3-5-10-17-25-18-12-7-13-19-25)2-4-6-11-20-26-21-16-23-14-8-9-15-24(23)22-26;;/h7-9,12-16,18-19,21-22H,1-6,10-11,17,20H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50133449
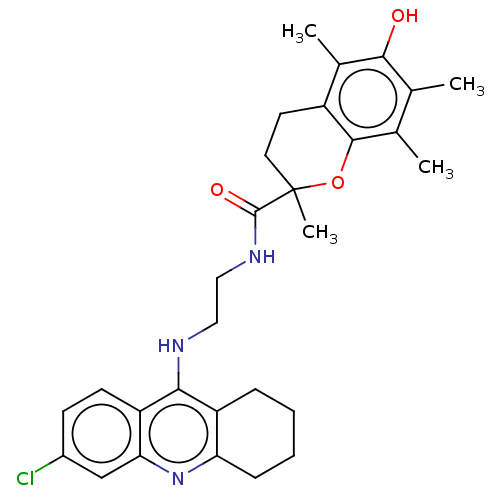
(CHEMBL3632988)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C29H34ClN3O3/c1-16-17(2)27-20(18(3)26(16)34)11-12-29(4,36-27)28(35)32-14-13-31-25-21-7-5-6-8-23(21)33-24-15-19(30)9-10-22(24)25/h9-10,15,34H,5-8,11-14H2,1-4H3,(H,31,33)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins using spectrophotometer by Ellman's method |
Bioorg Med Chem Lett 21: 6563-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.042
BindingDB Entry DOI: 10.7270/Q2HD7W2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte recombinant AChE by modified Ellman's method |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005180

(CHEMBL3093798)Show SMILES [Br-].[Br-].C(CCCCC[n+]1ccccc1)CCCCC[n+]1ccc2ccccc2c1 Show InChI InChI=1S/C25H34N2.2BrH/c1(2-4-6-11-18-26-19-13-8-14-20-26)3-5-7-12-21-27-22-17-24-15-9-10-16-25(24)23-27;;/h8-10,13-17,19-20,22-23H,1-7,11-12,18,21H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005182

(CHEMBL3093796)Show SMILES [Br-].[Br-].C(CCCC[n+]1ccccc1)CCCC[n+]1ccc2ccccc2c1 Show InChI InChI=1S/C23H30N2.2BrH/c1(2-4-9-16-24-17-11-6-12-18-24)3-5-10-19-25-20-15-22-13-7-8-14-23(22)21-25;;/h6-8,11-15,17-18,20-21H,1-5,9-10,16,19H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50327936
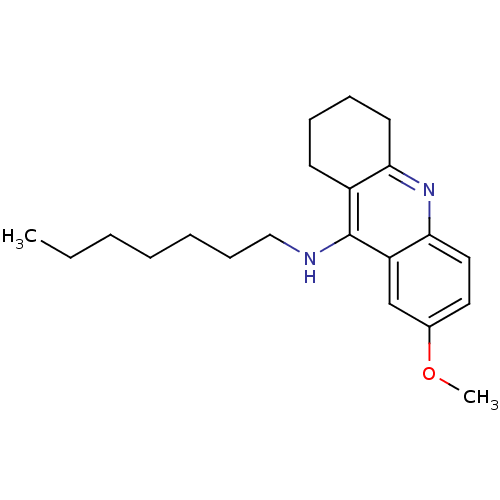
(CHEMBL1257767 | CHEMBL1618106 | N-n-heptyl-7-metho...)Show InChI InChI=1S/C21H30N2O/c1-3-4-5-6-9-14-22-21-17-10-7-8-11-19(17)23-20-13-12-16(24-2)15-18(20)21/h12-13,15H,3-11,14H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE after 5 mins using spectrophotometer by Ellman's method |
Bioorg Med Chem Lett 21: 6563-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.042
BindingDB Entry DOI: 10.7270/Q2HD7W2Q |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50073117
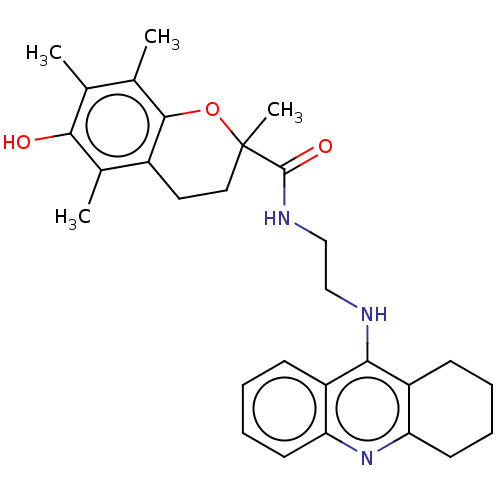
(CHEMBL3410951)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O3/c1-17-18(2)27-20(19(3)26(17)33)13-14-29(4,35-27)28(34)31-16-15-30-25-21-9-5-7-11-23(21)32-24-12-8-6-10-22(24)25/h5,7,9,11,33H,6,8,10,12-16H2,1-4H3,(H,30,32)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133472
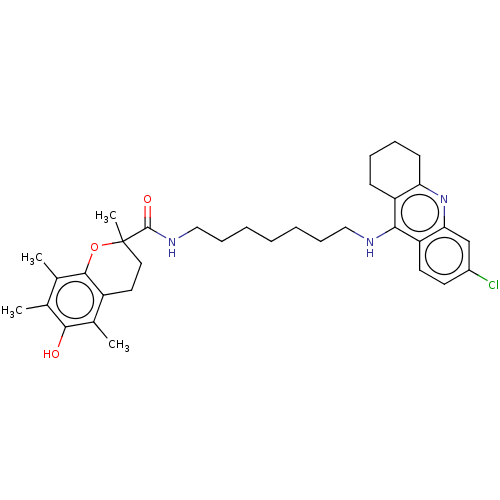
(CHEMBL3632993)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C34H44ClN3O3/c1-21-22(2)32-25(23(3)31(21)39)16-17-34(4,41-32)33(40)37-19-11-7-5-6-10-18-36-30-26-12-8-9-13-28(26)38-29-20-24(35)14-15-27(29)30/h14-15,20,39H,5-13,16-19H2,1-4H3,(H,36,38)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50133421
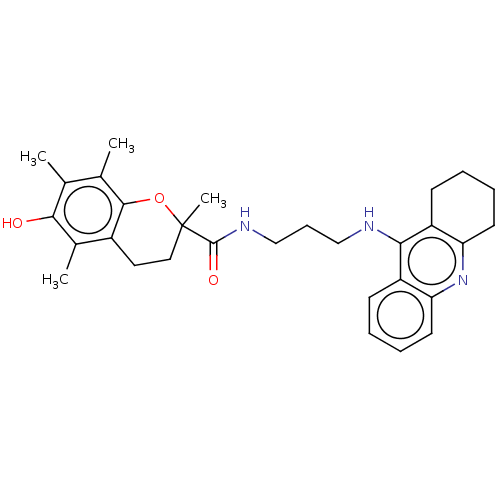
(CHEMBL3632987)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H37N3O3/c1-18-19(2)28-21(20(3)27(18)34)14-15-30(4,36-28)29(35)32-17-9-16-31-26-22-10-5-7-12-24(22)33-25-13-8-6-11-23(25)26/h5,7,10,12,34H,6,8-9,11,13-17H2,1-4H3,(H,31,33)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50073116
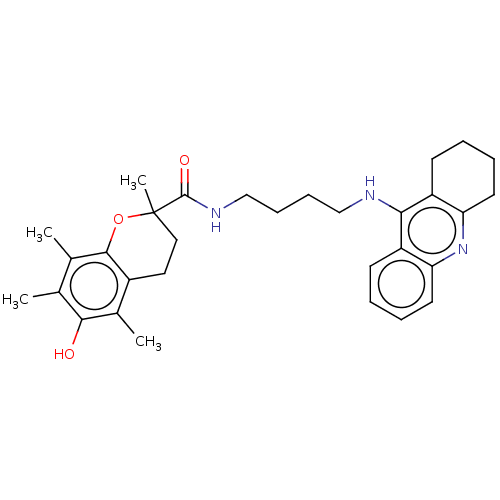
(CHEMBL3410952)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H39N3O3/c1-19-20(2)29-22(21(3)28(19)35)15-16-31(4,37-29)30(36)33-18-10-9-17-32-27-23-11-5-7-13-25(23)34-26-14-8-6-12-24(26)27/h5,7,11,13,35H,6,8-10,12,14-18H2,1-4H3,(H,32,34)(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119787
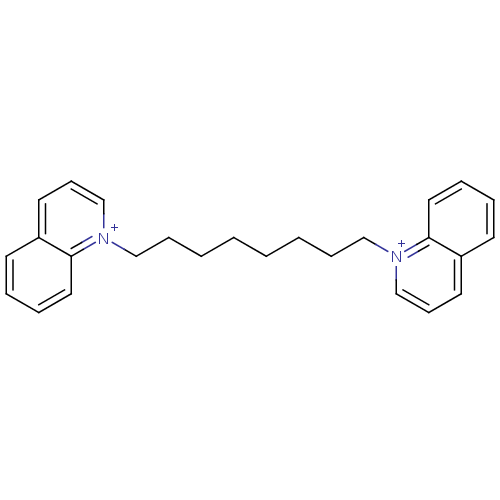
(1,1''-(octane-1,8-diyl)diquinolinium iodide | 1,8-...)Show SMILES C(CCCC[n+]1cccc2ccccc12)CCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C26H30N2/c1(3-9-19-27-21-11-15-23-13-5-7-17-25(23)27)2-4-10-20-28-22-12-16-24-14-6-8-18-26(24)28/h5-8,11-18,21-22H,1-4,9-10,19-20H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte recombinant AChE by modified Ellman's method |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133473

(CHEMBL3632994)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H46ClN3O3/c1-22-23(2)33-26(24(3)32(22)40)17-18-35(4,42-33)34(41)38-20-12-8-6-5-7-11-19-37-31-27-13-9-10-14-29(27)39-30-21-25(36)15-16-28(30)31/h15-16,21,40H,5-14,17-20H2,1-4H3,(H,37,39)(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133450
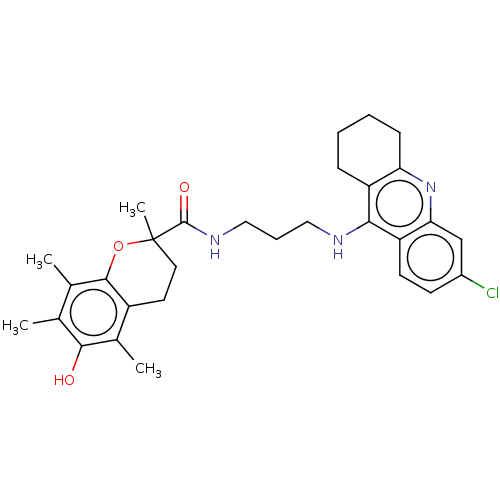
(CHEMBL3632989)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C30H36ClN3O3/c1-17-18(2)28-21(19(3)27(17)35)12-13-30(4,37-28)29(36)33-15-7-14-32-26-22-8-5-6-9-24(22)34-25-16-20(31)10-11-23(25)26/h10-11,16,35H,5-9,12-15H2,1-4H3,(H,32,34)(H,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119772

(1,1'-(nonane-1,9-diyl)diquinolinium bromide | 1,9-...)Show SMILES C(CCCC[n+]1cccc2ccccc12)CCCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C27H32N2/c1(2-4-10-20-28-22-12-16-24-14-6-8-18-26(24)28)3-5-11-21-29-23-13-17-25-15-7-9-19-27(25)29/h6-9,12-19,22-23H,1-5,10-11,20-21H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte recombinant AChE by modified Ellman's method |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50133469
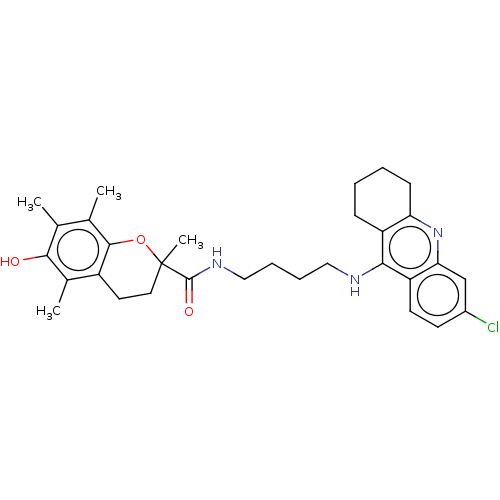
(CHEMBL3632990)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C31H38ClN3O3/c1-18-19(2)29-22(20(3)28(18)36)13-14-31(4,38-29)30(37)34-16-8-7-15-33-27-23-9-5-6-10-25(23)35-26-17-21(32)11-12-24(26)27/h11-12,17,36H,5-10,13-16H2,1-4H3,(H,33,35)(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50339948
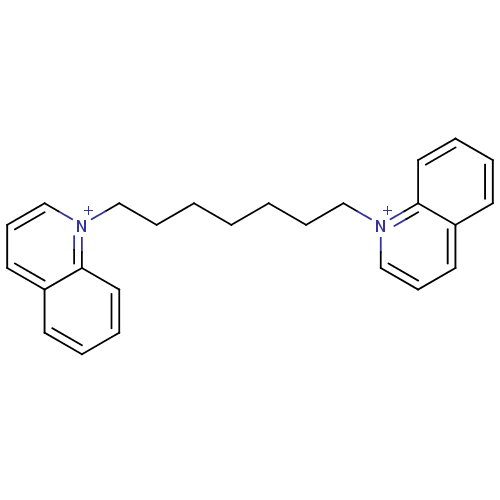
(1,1'-hept-1,7-diyl-bis(quinolinium)dibromide | CHE...)Show SMILES C(CCC[n+]1cccc2ccccc12)CCC[n+]1cccc2ccccc12 Show InChI InChI=1S/C25H28N2/c1(2-8-18-26-20-10-14-22-12-4-6-16-24(22)26)3-9-19-27-21-11-15-23-13-5-7-17-25(23)27/h4-7,10-17,20-21H,1-3,8-9,18-19H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte recombinant AChE by modified Ellman's method |
Bioorg Med Chem Lett 21: 2505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.047
BindingDB Entry DOI: 10.7270/Q2JW8F6N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data