

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50363984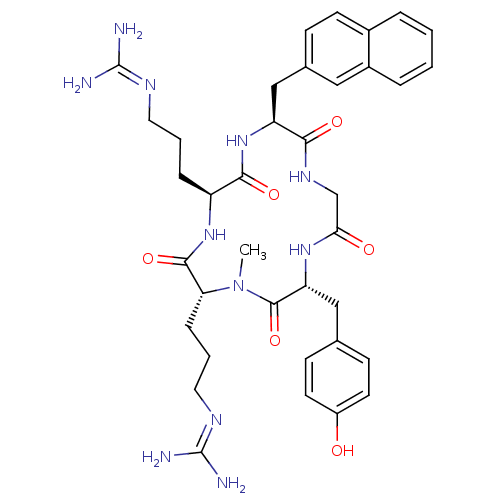 (CHEMBL1949730) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 expressed in HEK293 cell membrane after 1 hr | J Med Chem 55: 2746-57 (2012) Article DOI: 10.1021/jm2016914 BindingDB Entry DOI: 10.7270/Q2FJ2HTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50166106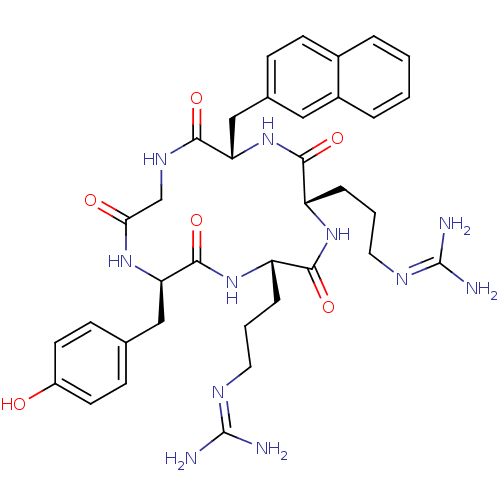 (CHEMBL436283 | N-{3-[(2S,5S,8S,14R)-5-(3-Guanidino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 expressed in HEK293 cell membrane after 1 hr | J Med Chem 55: 2746-57 (2012) Article DOI: 10.1021/jm2016914 BindingDB Entry DOI: 10.7270/Q2FJ2HTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50383301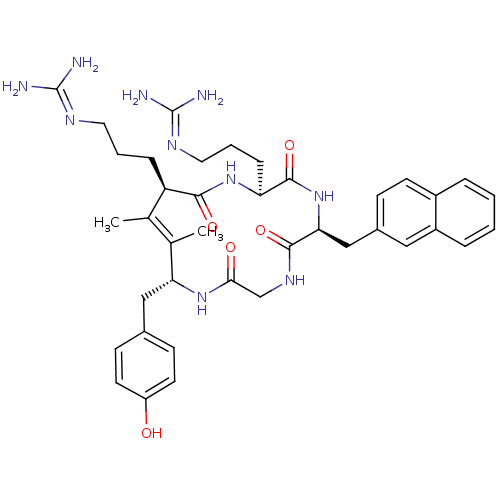 (CHEMBL2029611) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 expressed in HEK293 cell membrane after 1 hr | J Med Chem 55: 2746-57 (2012) Article DOI: 10.1021/jm2016914 BindingDB Entry DOI: 10.7270/Q2FJ2HTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50383303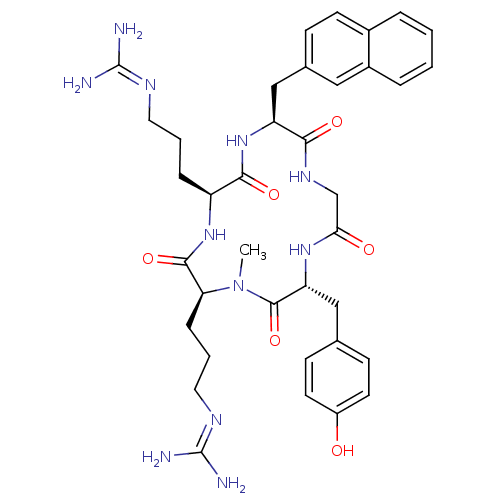 (CHEMBL2029613) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 expressed in HEK293 cell membrane after 1 hr | J Med Chem 55: 2746-57 (2012) Article DOI: 10.1021/jm2016914 BindingDB Entry DOI: 10.7270/Q2FJ2HTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50383298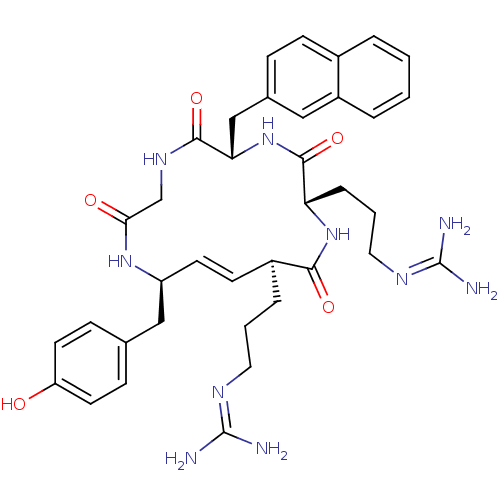 (CHEMBL2029608) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 expressed in HEK293 cell membrane after 1 hr | J Med Chem 55: 2746-57 (2012) Article DOI: 10.1021/jm2016914 BindingDB Entry DOI: 10.7270/Q2FJ2HTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50383299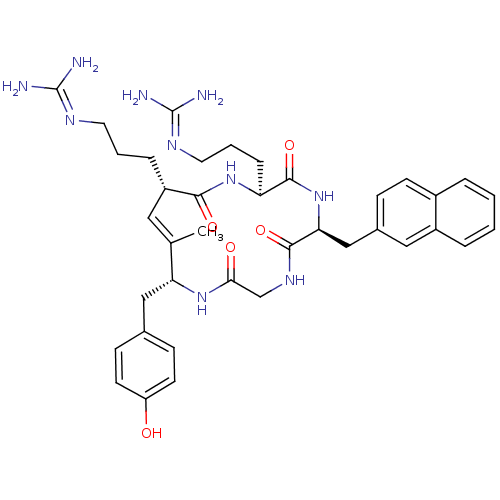 (CHEMBL2029609) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 expressed in HEK293 cell membrane after 1 hr | J Med Chem 55: 2746-57 (2012) Article DOI: 10.1021/jm2016914 BindingDB Entry DOI: 10.7270/Q2FJ2HTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50383300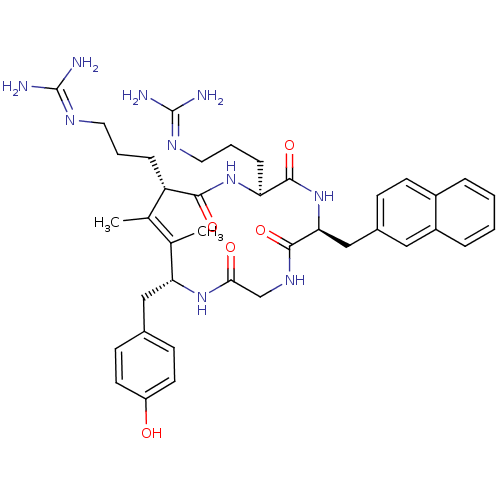 (CHEMBL2029610) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 expressed in HEK293 cell membrane after 1 hr | J Med Chem 55: 2746-57 (2012) Article DOI: 10.1021/jm2016914 BindingDB Entry DOI: 10.7270/Q2FJ2HTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50383302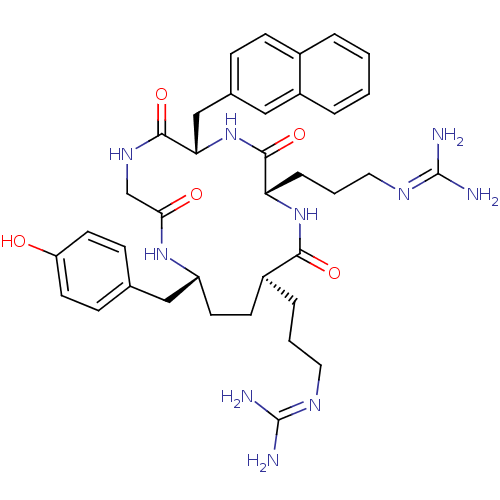 (CHEMBL2029612) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 expressed in HEK293 cell membrane after 1 hr | J Med Chem 55: 2746-57 (2012) Article DOI: 10.1021/jm2016914 BindingDB Entry DOI: 10.7270/Q2FJ2HTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50204206 (CHEMBL3896958) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using p-nitrophenyl-glycoside as substrate by spectrophotometric method | Bioorg Med Chem 25: 107-115 (2017) Article DOI: 10.1016/j.bmc.2016.10.015 BindingDB Entry DOI: 10.7270/Q26H4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50204109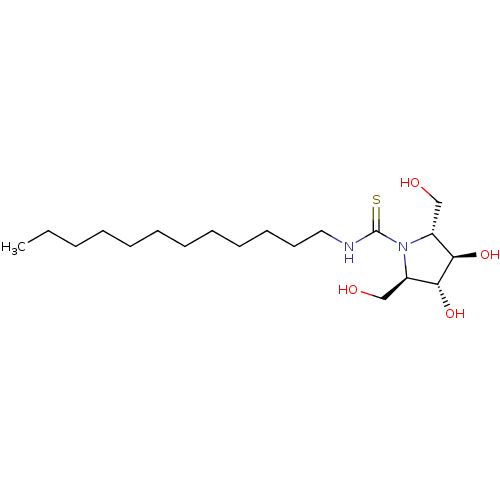 (CHEMBL3935498) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using p-nitrophenyl-glycoside as substrate by spectrophotometric method | Bioorg Med Chem 25: 107-115 (2017) Article DOI: 10.1016/j.bmc.2016.10.015 BindingDB Entry DOI: 10.7270/Q26H4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50204204 (CHEMBL3891002) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using p-nitrophenyl-glycoside as substrate by spectrophotometric method | Bioorg Med Chem 25: 107-115 (2017) Article DOI: 10.1016/j.bmc.2016.10.015 BindingDB Entry DOI: 10.7270/Q26H4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50204212 (CHEMBL3901083) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using p-nitrophenyl-glycoside as substrate by spectrophotometric method | Bioorg Med Chem 25: 107-115 (2017) Article DOI: 10.1016/j.bmc.2016.10.015 BindingDB Entry DOI: 10.7270/Q26H4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50204205 (CHEMBL3909011) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using p-nitrophenyl-glycoside as substrate by spectrophotometric method | Bioorg Med Chem 25: 107-115 (2017) Article DOI: 10.1016/j.bmc.2016.10.015 BindingDB Entry DOI: 10.7270/Q26H4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50508732 (CHEMBL4453990) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibition of human lysosomal alpha-glucosidase | J Nat Prod 82: 358-367 (2019) Article DOI: 10.1021/acs.jnatprod.8b00879 BindingDB Entry DOI: 10.7270/Q2J67M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50204210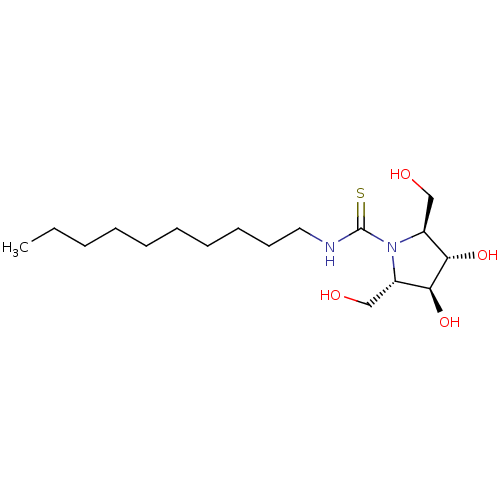 (CHEMBL3970812) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using p-nitrophenyl-glycoside as substrate by spectrophotometric method | Bioorg Med Chem 25: 107-115 (2017) Article DOI: 10.1016/j.bmc.2016.10.015 BindingDB Entry DOI: 10.7270/Q26H4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50204113 (CHEMBL3897971) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using p-nitrophenyl-glycoside as substrate by spectrophotometric method | Bioorg Med Chem 25: 107-115 (2017) Article DOI: 10.1016/j.bmc.2016.10.015 BindingDB Entry DOI: 10.7270/Q26H4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50204209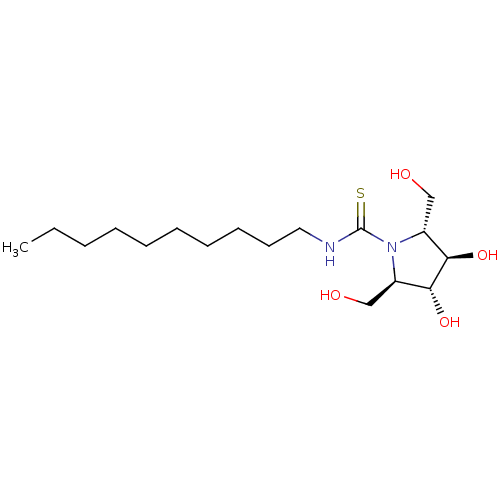 (CHEMBL3907555) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using p-nitrophenyl-glycoside as substrate by spectrophotometric method | Bioorg Med Chem 25: 107-115 (2017) Article DOI: 10.1016/j.bmc.2016.10.015 BindingDB Entry DOI: 10.7270/Q26H4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50204111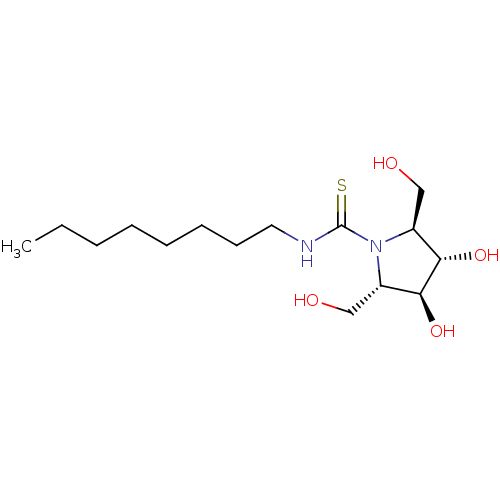 (CHEMBL3961105) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using p-nitrophenyl-glycoside as substrate by spectrophotometric method | Bioorg Med Chem 25: 107-115 (2017) Article DOI: 10.1016/j.bmc.2016.10.015 BindingDB Entry DOI: 10.7270/Q26H4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50204211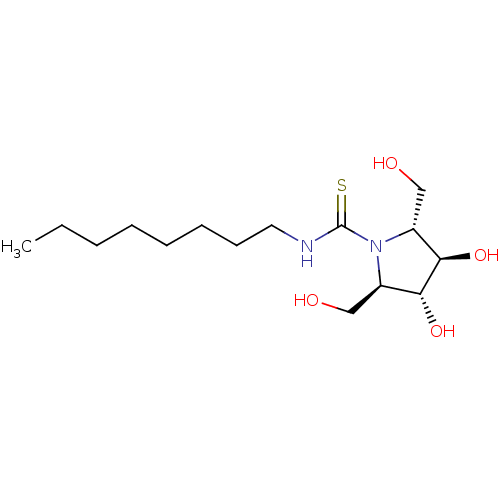 (CHEMBL3944457) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using p-nitrophenyl-glycoside as substrate by spectrophotometric method | Bioorg Med Chem 25: 107-115 (2017) Article DOI: 10.1016/j.bmc.2016.10.015 BindingDB Entry DOI: 10.7270/Q26H4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50204114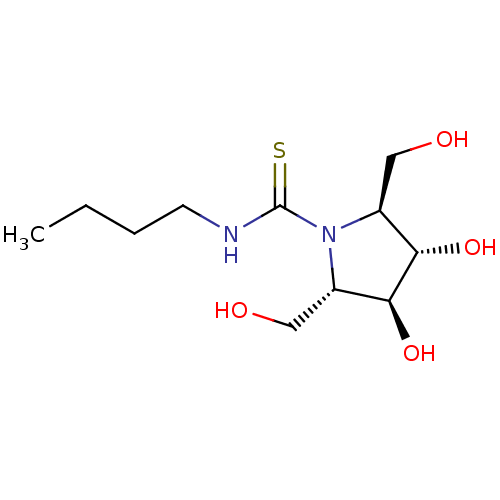 (CHEMBL3972215) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using p-nitrophenyl-glycoside as substrate by spectrophotometric method | Bioorg Med Chem 25: 107-115 (2017) Article DOI: 10.1016/j.bmc.2016.10.015 BindingDB Entry DOI: 10.7270/Q26H4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50204110 (CHEMBL3905255) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.72E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using p-nitrophenyl-glycoside as substrate by spectrophotometric method | Bioorg Med Chem 25: 107-115 (2017) Article DOI: 10.1016/j.bmc.2016.10.015 BindingDB Entry DOI: 10.7270/Q26H4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50204207 (CHEMBL3980259) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using p-nitrophenyl-glycoside as substrate by spectrophotometric method | Bioorg Med Chem 25: 107-115 (2017) Article DOI: 10.1016/j.bmc.2016.10.015 BindingDB Entry DOI: 10.7270/Q26H4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50204208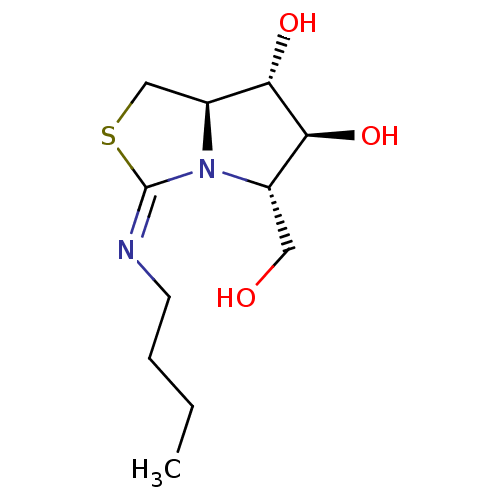 (CHEMBL3910143) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using p-nitrophenyl-glycoside as substrate by spectrophotometric method | Bioorg Med Chem 25: 107-115 (2017) Article DOI: 10.1016/j.bmc.2016.10.015 BindingDB Entry DOI: 10.7270/Q26H4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50204112 (CHEMBL3916518) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using p-nitrophenyl-glycoside as substrate by spectrophotometric method | Bioorg Med Chem 25: 107-115 (2017) Article DOI: 10.1016/j.bmc.2016.10.015 BindingDB Entry DOI: 10.7270/Q26H4KC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||