 Found 4313 hits with Last Name = 'hua' and Initial = 'm'
Found 4313 hits with Last Name = 'hua' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM161571
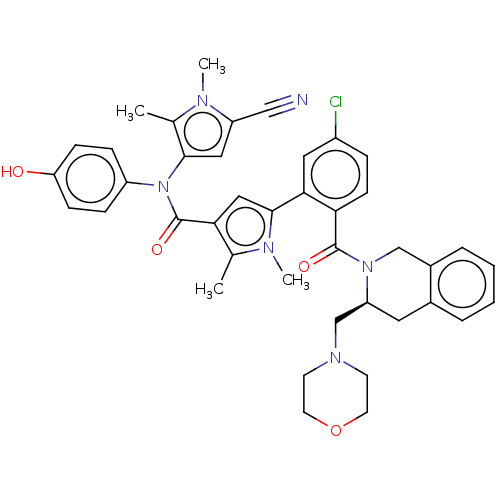
(US9108983, Example 386 | US9108983, Example 449)Show SMILES Cc1c(cc(C#N)n1C)N(C(=O)c1cc(-c2cc(Cl)ccc2C(=O)N2Cc3ccccc3C[C@H]2CN2CCOCC2)n(C)c1C)c1ccc(O)cc1 Show InChI InChI=1S/C41H41ClN6O4/c1-26-36(41(51)48(31-10-12-34(49)13-11-31)38-21-32(23-43)44(3)27(38)2)22-39(45(26)4)37-20-30(42)9-14-35(37)40(50)47-24-29-8-6-5-7-28(29)19-33(47)25-46-15-17-52-18-16-46/h5-14,20-22,33,49H,15-19,24-25H2,1-4H3/t33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203205
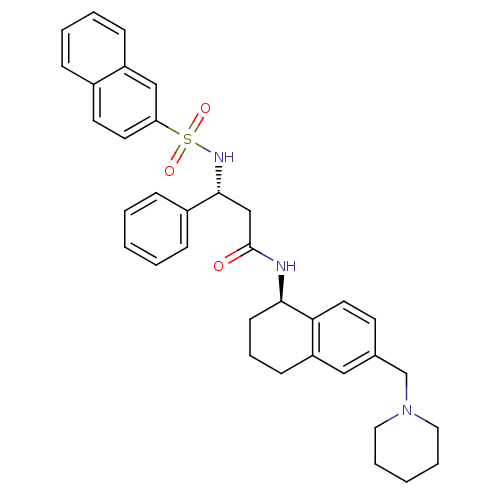
((R)-3-(naphthalene-3-sulfonamido)-3-phenyl-N-((R)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C35H39N3O3S/c39-35(36-33-15-9-14-30-22-26(16-19-32(30)33)25-38-20-7-2-8-21-38)24-34(28-11-3-1-4-12-28)37-42(40,41)31-18-17-27-10-5-6-13-29(27)23-31/h1,3-6,10-13,16-19,22-23,33-34,37H,2,7-9,14-15,20-21,24-25H2,(H,36,39)/t33-,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in clone CHO-D-/aequorin cells |
Bioorg Med Chem Lett 18: 4764-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.108
BindingDB Entry DOI: 10.7270/Q29C6X87 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM161571

(US9108983, Example 386 | US9108983, Example 449)Show SMILES Cc1c(cc(C#N)n1C)N(C(=O)c1cc(-c2cc(Cl)ccc2C(=O)N2Cc3ccccc3C[C@H]2CN2CCOCC2)n(C)c1C)c1ccc(O)cc1 Show InChI InChI=1S/C41H41ClN6O4/c1-26-36(41(51)48(31-10-12-34(49)13-11-31)38-21-32(23-43)44(3)27(38)2)22-39(45(26)4)37-20-30(42)9-14-35(37)40(50)47-24-29-8-6-5-7-28(29)19-33(47)25-46-15-17-52-18-16-46/h5-14,20-22,33,49H,15-19,24-25H2,1-4H3/t33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM161571

(US9108983, Example 386 | US9108983, Example 449)Show SMILES Cc1c(cc(C#N)n1C)N(C(=O)c1cc(-c2cc(Cl)ccc2C(=O)N2Cc3ccccc3C[C@H]2CN2CCOCC2)n(C)c1C)c1ccc(O)cc1 Show InChI InChI=1S/C41H41ClN6O4/c1-26-36(41(51)48(31-10-12-34(49)13-11-31)38-21-32(23-43)44(3)27(38)2)22-39(45(26)4)37-20-30(42)9-14-35(37)40(50)47-24-29-8-6-5-7-28(29)19-33(47)25-46-15-17-52-18-16-46/h5-14,20-22,33,49H,15-19,24-25H2,1-4H3/t33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50590323
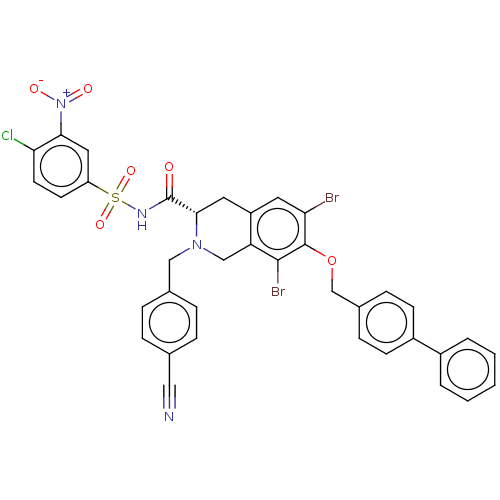
(CHEMBL5173522)Show SMILES [O-][N+](=O)c1cc(ccc1Cl)S(=O)(=O)NC(=O)[C@@H]1Cc2cc(Br)c(OCc3ccc(cc3)-c3ccccc3)c(Br)c2CN1Cc1ccc(cc1)C#N |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463294

(CHEMBL4249256)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]51C[C@H](C(=O)c4ccccc4)[C@]2(OC)C=C1)ccc3OC |r,wU:16.16,1.0,wD:17.38,28.36,7.7,19.23,c:37,THB:10:9:17:5.6.4,(9.78,-11.07,;9.03,-9.74,;7.65,-10.81,;5.94,-9.74,;6.72,-8.4,;5.95,-7.07,;6.71,-5.74,;8.25,-5.74,;9.79,-5.74,;9.03,-4.41,;9.78,-3.07,;11.32,-3.05,;12.66,-3.81,;12.64,-2.27,;7.47,-5.14,;7.41,-7.18,;8.25,-8.41,;9.02,-7.07,;10.56,-7.06,;11.34,-8.4,;12.88,-8.4,;13.65,-7.07,;13.65,-9.73,;12.87,-11.06,;13.64,-12.4,;15.18,-12.4,;15.95,-11.05,;15.18,-9.72,;10.59,-9.7,;11.34,-11.07,;10.57,-12.41,;9.25,-8.93,;10.35,-7.84,;4.41,-7.06,;3.64,-8.38,;4.4,-9.72,;3.62,-11.05,;2.08,-11.04,)| Show InChI InChI=1S/C31H33NO4/c1-34-23-11-10-21-16-24-29-12-13-31(35-2,22(17-29)26(33)20-6-4-3-5-7-20)28-30(29,25(21)27(23)36-28)14-15-32(24)18-19-8-9-19/h3-7,10-13,19,22,24,28H,8-9,14-18H2,1-2H3/t22-,24-,28-,29-,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50463297

(CHEMBL4246433)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(O)c1ccc(OC)cc1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H41NO5/c1-30(28(35)21-7-10-23(36-2)11-8-21)19-31-13-14-33(30,38-4)29-32(31)15-16-34(18-20-5-6-20)25(31)17-22-9-12-24(37-3)27(39-29)26(22)32/h7-12,20,25,28-29,35H,5-6,13-19H2,1-4H3/t25-,28?,29-,30-,31-,32+,33+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50209744
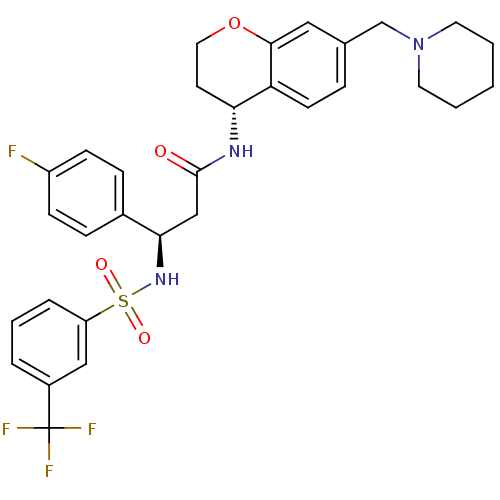
((R)-3-(4-fluorophenyl)-N-((R)-7-(piperidin-1-ylmet...)Show SMILES Fc1ccc(cc1)[C@@H](CC(=O)N[C@@H]1CCOc2cc(CN3CCCCC3)ccc12)NS(=O)(=O)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C31H33F4N3O4S/c32-24-10-8-22(9-11-24)28(37-43(40,41)25-6-4-5-23(18-25)31(33,34)35)19-30(39)36-27-13-16-42-29-17-21(7-12-26(27)29)20-38-14-2-1-3-15-38/h4-12,17-18,27-28,37H,1-3,13-16,19-20H2,(H,36,39)/t27-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHOD cells |
J Med Chem 50: 2200-12 (2007)
Article DOI: 10.1021/jm070055c
BindingDB Entry DOI: 10.7270/Q2MS3SG2 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50590323

(CHEMBL5173522)Show SMILES [O-][N+](=O)c1cc(ccc1Cl)S(=O)(=O)NC(=O)[C@@H]1Cc2cc(Br)c(OCc3ccc(cc3)-c3ccccc3)c(Br)c2CN1Cc1ccc(cc1)C#N |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM161571

(US9108983, Example 386 | US9108983, Example 449)Show SMILES Cc1c(cc(C#N)n1C)N(C(=O)c1cc(-c2cc(Cl)ccc2C(=O)N2Cc3ccccc3C[C@H]2CN2CCOCC2)n(C)c1C)c1ccc(O)cc1 Show InChI InChI=1S/C41H41ClN6O4/c1-26-36(41(51)48(31-10-12-34(49)13-11-31)38-21-32(23-43)44(3)27(38)2)22-39(45(26)4)37-20-30(42)9-14-35(37)40(50)47-24-29-8-6-5-7-28(29)19-33(47)25-46-15-17-52-18-16-46/h5-14,20-22,33,49H,15-19,24-25H2,1-4H3/t33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463297

(CHEMBL4246433)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(O)c1ccc(OC)cc1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H41NO5/c1-30(28(35)21-7-10-23(36-2)11-8-21)19-31-13-14-33(30,38-4)29-32(31)15-16-34(18-20-5-6-20)25(31)17-22-9-12-24(37-3)27(39-29)26(22)32/h7-12,20,25,28-29,35H,5-6,13-19H2,1-4H3/t25-,28?,29-,30-,31-,32+,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50266959

(CHEMBL4085804)Show SMILES CC(C)n1c(C)c(c(c1-c1ccc(Cl)cc1)-c1cc(F)cc(c1)N1CCN(CC1)c1ccc(NS(=O)(=O)c2ccc(N[C@H](CCN3CCC(O)CC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C53H59ClF4N6O7S4/c1-35(2)64-36(3)52(73(4,66)67)50(51(64)37-10-12-39(54)13-11-37)38-30-40(55)32-44(31-38)63-28-26-62(27-29-63)43-16-14-41(15-17-43)60-75(70,71)47-18-19-48(49(33-47)74(68,69)53(56,57)58)59-42(34-72-46-8-6-5-7-9-46)20-23-61-24-21-45(65)22-25-61/h5-19,30-33,35,42,45,59-60,65H,20-29,34H2,1-4H3/t42-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203200
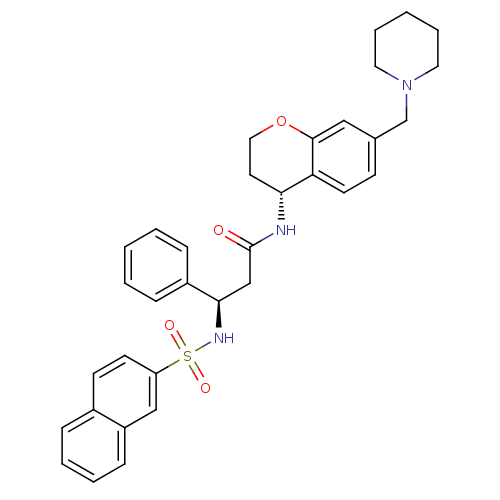
((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@@H]1CCOc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C34H37N3O4S/c38-34(35-31-17-20-41-33-21-25(13-16-30(31)33)24-37-18-7-2-8-19-37)23-32(27-10-3-1-4-11-27)36-42(39,40)29-15-14-26-9-5-6-12-28(26)22-29/h1,3-6,9-16,21-22,31-32,36H,2,7-8,17-20,23-24H2,(H,35,38)/t31-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHOD cells |
J Med Chem 50: 2200-12 (2007)
Article DOI: 10.1021/jm070055c
BindingDB Entry DOI: 10.7270/Q2MS3SG2 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50074509
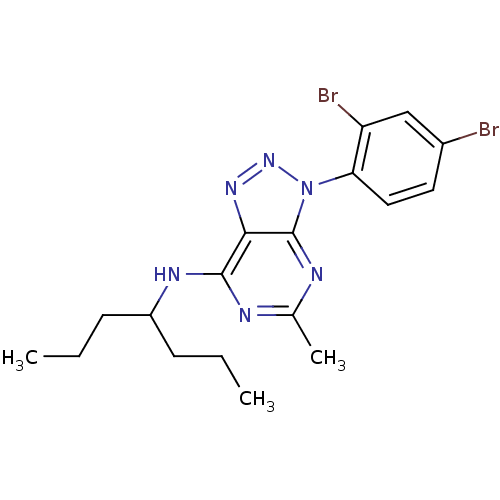
(CHEMBL354886 | [3-(2,4-Dibromo-phenyl)-5-methyl-3H...)Show SMILES CCCC(CCC)Nc1nc(C)nc2n(nnc12)-c1ccc(Br)cc1Br Show InChI InChI=1S/C18H22Br2N6/c1-4-6-13(7-5-2)23-17-16-18(22-11(3)21-17)26(25-24-16)15-9-8-12(19)10-14(15)20/h8-10,13H,4-7H2,1-3H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells |
J Med Chem 42: 833-48 (1999)
Article DOI: 10.1021/jm980224g
BindingDB Entry DOI: 10.7270/Q2610ZGP |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50074501
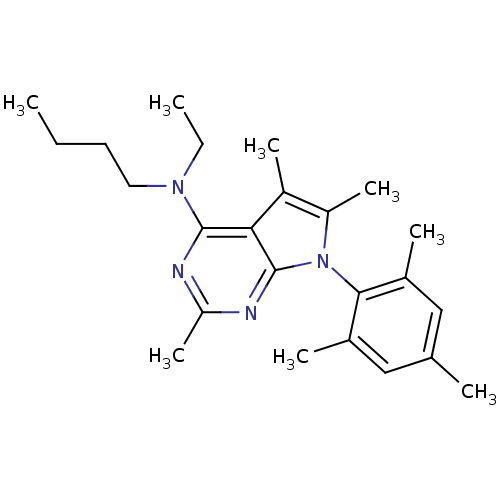
(Butyl-ethyl-[2,5,6-trimethyl-7-(2,4,6-trimethyl-ph...)Show SMILES CCCCN(CC)c1nc(C)nc2n(c(C)c(C)c12)-c1c(C)cc(C)cc1C |(4.9,1.82,;3.57,2.56,;2.24,1.79,;.91,2.55,;-.44,1.78,;-1.76,2.55,;-3.1,1.78,;-.42,.25,;-1.75,-.52,;-1.75,-2.06,;-3.08,-2.83,;-.42,-2.83,;.91,-2.06,;2.38,-2.54,;3.31,-1.29,;4.85,-1.29,;2.38,-.03,;2.86,1.43,;.91,-.52,;2.86,-4.02,;4.37,-4.33,;5.39,-3.17,;4.85,-5.78,;3.82,-6.93,;4.31,-8.4,;2.32,-6.61,;1.84,-5.14,;.33,-4.82,)| Show InChI InChI=1S/C24H34N4/c1-9-11-12-27(10-2)23-21-18(6)19(7)28(24(21)26-20(8)25-23)22-16(4)13-15(3)14-17(22)5/h13-14H,9-12H2,1-8H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells |
J Med Chem 42: 833-48 (1999)
Article DOI: 10.1021/jm980224g
BindingDB Entry DOI: 10.7270/Q2610ZGP |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50074492
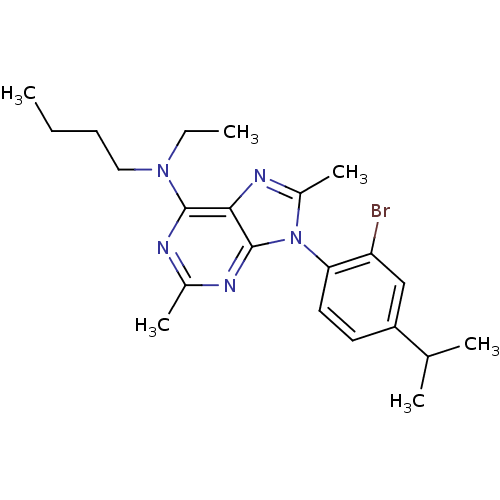
(CHEMBL168554 | [9-(2-Bromo-4-isopropyl-phenyl)-2,8...)Show SMILES CCCCN(CC)c1nc(C)nc2n(c(C)nc12)-c1ccc(cc1Br)C(C)C |(12.64,1.78,;12.64,.23,;11.31,-.54,;11.31,-2.08,;9.99,-2.85,;8.64,-2.08,;7.32,-2.85,;9.99,-4.38,;8.64,-5.15,;8.64,-6.69,;7.31,-7.46,;9.99,-7.46,;11.31,-6.69,;12.76,-7.15,;13.67,-5.92,;15.21,-5.94,;12.76,-4.68,;11.31,-5.15,;13.39,-8.57,;12.49,-9.81,;13.11,-11.22,;14.65,-11.38,;15.56,-10.13,;14.93,-8.73,;15.84,-7.48,;15.27,-12.8,;16.79,-12.97,;14.35,-14.04,)| Show InChI InChI=1S/C22H30BrN5/c1-7-9-12-27(8-2)21-20-22(25-15(5)24-21)28(16(6)26-20)19-11-10-17(14(3)4)13-18(19)23/h10-11,13-14H,7-9,12H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells |
J Med Chem 42: 833-48 (1999)
Article DOI: 10.1021/jm980224g
BindingDB Entry DOI: 10.7270/Q2610ZGP |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526945
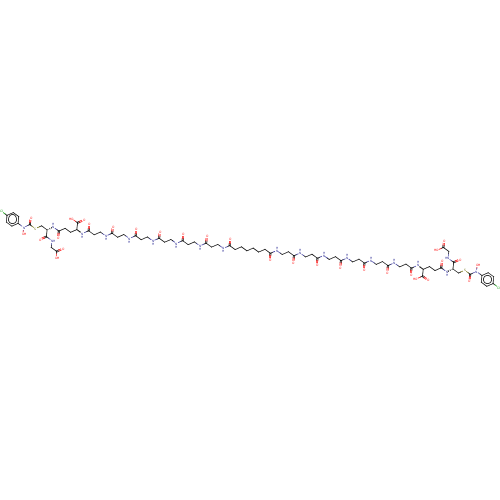
(CHEMBL4473806)Show SMILES ON(C(=O)SC[C@H](NC(=O)CC[C@H](NC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCCCCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C78H112Cl2N20O30S2/c79-47-7-11-49(12-8-47)99(129)77(127)131-45-53(73(121)93-43-71(117)118)97-67(113)17-15-51(75(123)124)95-69(115)29-41-91-65(111)27-39-89-63(109)25-37-87-61(107)23-35-85-59(105)21-33-83-57(103)19-31-81-55(101)5-3-1-2-4-6-56(102)82-32-20-58(104)84-34-22-60(106)86-36-24-62(108)88-38-26-64(110)90-40-28-66(112)92-42-30-70(116)96-52(76(125)126)16-18-68(114)98-54(74(122)94-44-72(119)120)46-132-78(128)100(130)50-13-9-48(80)10-14-50/h7-14,51-54,129-130H,1-6,15-46H2,(H,81,101)(H,82,102)(H,83,103)(H,84,104)(H,85,105)(H,86,106)(H,87,107)(H,88,108)(H,89,109)(H,90,110)(H,91,111)(H,92,112)(H,93,121)(H,94,122)(H,95,115)(H,96,116)(H,97,113)(H,98,114)(H,117,118)(H,119,120)(H,123,124)(H,125,126)/t51-,52-,53-,54-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Michaelis-Menten analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50572018
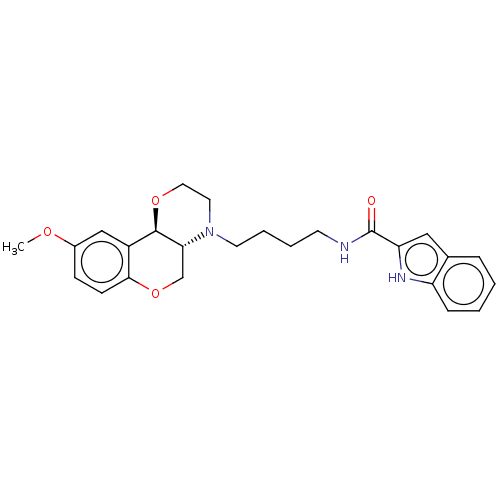
(CHEMBL4854351)Show SMILES [H][C@@]12COc3ccc(OC)cc3[C@@]1([H])OCCN2CCCCNC(=O)c1cc2ccccc2[nH]1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]spiperone from human D3 receptor transfected in HEK293 cell membrane measured after 50 mins by competitive radioligand binding an... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128269
BindingDB Entry DOI: 10.7270/Q2TB1BP0 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526943
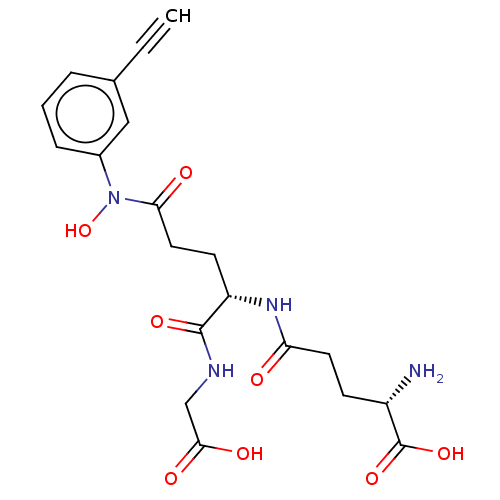
(CHEMBL4436073)Show SMILES N[C@@H](CCC(=O)N[C@@H](CCC(=O)N(O)c1cccc(c1)C#C)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H24N4O8/c1-2-12-4-3-5-13(10-12)24(32)17(26)9-7-15(19(29)22-11-18(27)28)23-16(25)8-6-14(21)20(30)31/h1,3-5,10,14-15,32H,6-9,11,21H2,(H,22,29)(H,23,25)(H,27,28)(H,30,31)/t14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Bc1-xL (unknown origin) by fluorescence polarization competition assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128215
BindingDB Entry DOI: 10.7270/Q2K35ZDS |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50568342
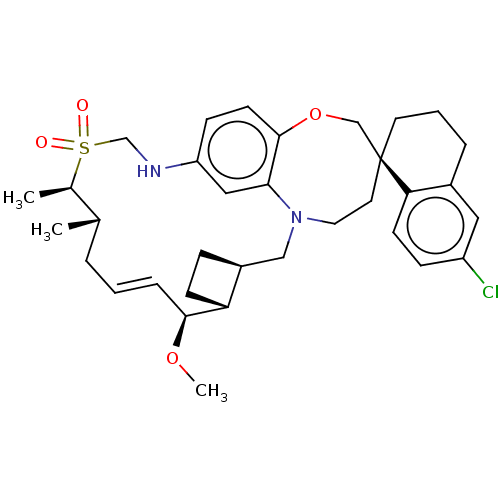
(CHEMBL4851522)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\C[C@H](C)[C@@H](C)S(=O)(=O)CNc1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CCN(C2)c3c1 |r,t:10| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Mc1-1 (unknown origin) by fluorescence polarization competition assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128215
BindingDB Entry DOI: 10.7270/Q2K35ZDS |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50590322
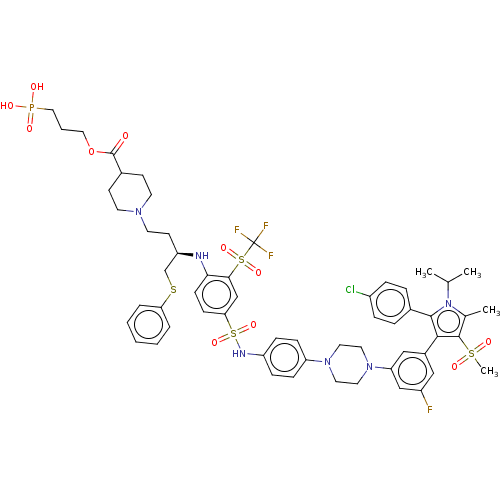
(PELCITOCLAX | Pelcitoclax)Show SMILES CC(C)n1c(C)c(c(c1-c1ccc(Cl)cc1)-c1cc(F)cc(c1)N1CCN(CC1)c1ccc(NS(=O)(=O)c2ccc(N[C@H](CCN3CCC(CC3)C(=O)OCCCP(O)(O)=O)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50590322

(PELCITOCLAX | Pelcitoclax)Show SMILES CC(C)n1c(C)c(c(c1-c1ccc(Cl)cc1)-c1cc(F)cc(c1)N1CCN(CC1)c1ccc(NS(=O)(=O)c2ccc(N[C@H](CCN3CCC(CC3)C(=O)OCCCP(O)(O)=O)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)cc1)S(C)(=O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50266959

(CHEMBL4085804)Show SMILES CC(C)n1c(C)c(c(c1-c1ccc(Cl)cc1)-c1cc(F)cc(c1)N1CCN(CC1)c1ccc(NS(=O)(=O)c2ccc(N[C@H](CCN3CCC(O)CC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C53H59ClF4N6O7S4/c1-35(2)64-36(3)52(73(4,66)67)50(51(64)37-10-12-39(54)13-11-37)38-30-40(55)32-44(31-38)63-28-26-62(27-29-63)43-16-14-41(15-17-43)60-75(70,71)47-18-19-48(49(33-47)74(68,69)53(56,57)58)59-42(34-72-46-8-6-5-7-9-46)20-23-61-24-21-45(65)22-25-61/h5-19,30-33,35,42,45,59-60,65H,20-29,34H2,1-4H3/t42-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50272598
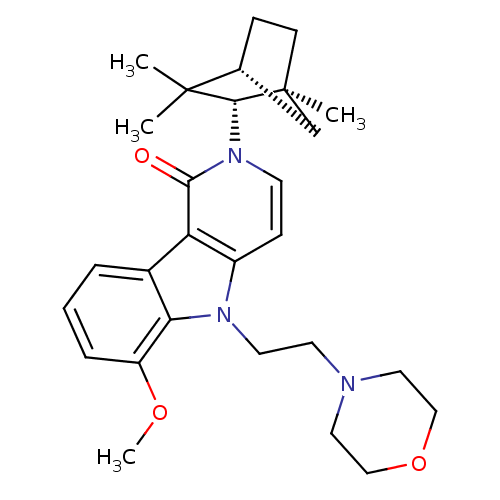
(6-Methoxy-5-(2-morpholin-4-yl-ethyl)-2-(1,3,3-trim...)Show SMILES COc1cccc2c1n(CCN1CCOCC1)c1ccn([C@H]3[C@@]4(C)CC[C@H](C4)C3(C)C)c(=O)c21 |r| Show InChI InChI=1S/C28H37N3O3/c1-27(2)19-8-10-28(3,18-19)26(27)31-11-9-21-23(25(31)32)20-6-5-7-22(33-4)24(20)30(21)13-12-29-14-16-34-17-15-29/h5-7,9,11,19,26H,8,10,12-18H2,1-4H3/t19-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50572021
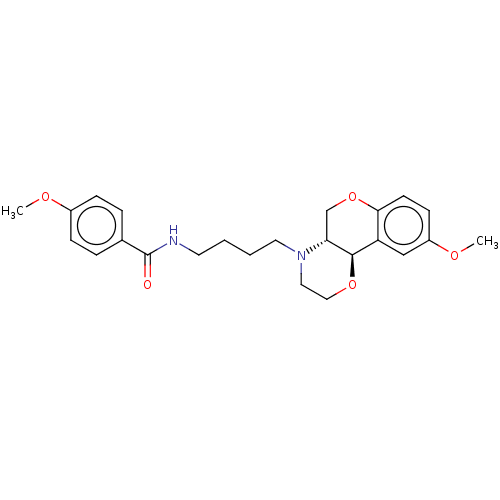
(CHEMBL4864112)Show SMILES [H][C@@]12COc3ccc(OC)cc3[C@@]1([H])OCCN2CCCCNC(=O)c1ccc(OC)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]spiperone from human D3 receptor transfected in HEK293 cell membrane measured after 50 mins by competitive radioligand binding an... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128269
BindingDB Entry DOI: 10.7270/Q2TB1BP0 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM16452

((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...)Show SMILES OC(=O)Cc1nn(Cc2nc3cc(ccc3s2)C(F)(F)F)c(=O)c2ccccc12 Show InChI InChI=1S/C19H12F3N3O3S/c20-19(21,22)10-5-6-15-14(7-10)23-16(29-15)9-25-18(28)12-4-2-1-3-11(12)13(24-25)8-17(26)27/h1-7H,8-9H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Dixon plot analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Homo sapiens) | BDBM50572019

(CHEMBL4855879)Show SMILES [H][C@@]12COc3ccc(OC)cc3[C@@]1([H])OCCN2CCCCNC(=O)c1cc(OC)c(OC)c(OC)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]spiperone from human D3 receptor transfected in HEK293 cell membrane measured after 50 mins by competitive radioligand binding an... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128269
BindingDB Entry DOI: 10.7270/Q2TB1BP0 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50119380
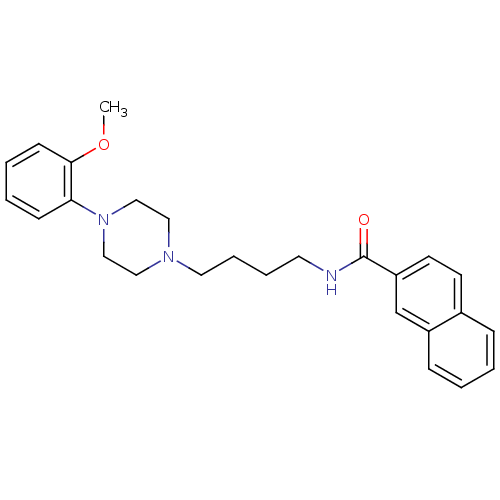
(CHEMBL25236 | CHEMBL540612 | N-(4-(4-(2-methoxyphe...)Show SMILES COc1ccccc1N1CCN(CCCCNC(=O)c2ccc3ccccc3c2)CC1 Show InChI InChI=1S/C26H31N3O2/c1-31-25-11-5-4-10-24(25)29-18-16-28(17-19-29)15-7-6-14-27-26(30)23-13-12-21-8-2-3-9-22(21)20-23/h2-5,8-13,20H,6-7,14-19H2,1H3,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]spiperone from human D3 receptor transfected in HEK293 cell membrane measured after 50 mins by competitive radioligand binding an... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128269
BindingDB Entry DOI: 10.7270/Q2TB1BP0 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM177786
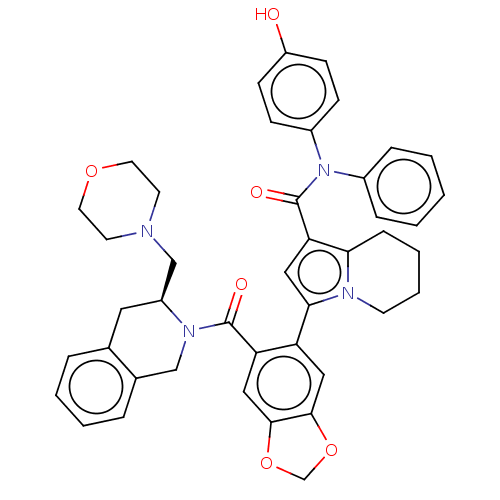
(US9120791, Example 1 | US9120791, Example 38 | US9...)Show SMILES Oc1ccc(cc1)N(C(=O)c1cc(-c2cc3OCOc3cc2C(=O)N2Cc3ccccc3C[C@H]2CN2CCOCC2)n2CCCCc12)c1ccccc1 Show InChI InChI=1S/C43H42N4O6/c48-34-15-13-32(14-16-34)47(31-10-2-1-3-11-31)43(50)37-23-39(45-17-7-6-12-38(37)45)35-24-40-41(53-28-52-40)25-36(35)42(49)46-26-30-9-5-4-8-29(30)22-33(46)27-44-18-20-51-21-19-44/h1-5,8-11,13-16,23-25,33,48H,6-7,12,17-22,26-28H2/t33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50074465
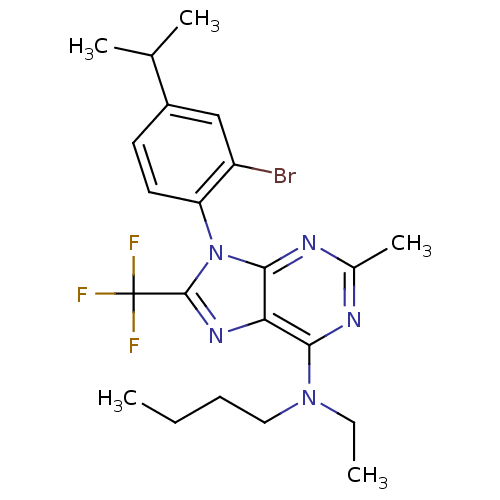
(CHEMBL354982 | [9-(2-Bromo-4-isopropyl-phenyl)-2-m...)Show SMILES CCCCN(CC)c1nc(C)nc2n(c(nc12)C(F)(F)F)-c1ccc(cc1Br)C(C)C |(12.23,1.79,;12.23,.23,;10.9,-.54,;10.9,-2.08,;9.57,-2.85,;8.22,-2.08,;6.89,-2.85,;9.57,-4.39,;8.22,-5.16,;8.22,-6.7,;6.88,-7.47,;9.57,-7.47,;10.9,-6.7,;12.35,-7.17,;13.26,-5.93,;12.35,-4.69,;10.9,-5.16,;14.8,-5.95,;16.34,-5.96,;14.79,-7.47,;14.8,-4.41,;12.68,-8.68,;11.55,-9.71,;11.88,-11.21,;13.33,-11.69,;14.47,-10.65,;14.15,-9.15,;15.41,-8.29,;13.66,-13.21,;12.51,-14.24,;15.13,-13.7,)| Show InChI InChI=1S/C22H27BrF3N5/c1-6-8-11-30(7-2)19-18-20(28-14(5)27-19)31(21(29-18)22(24,25)26)17-10-9-15(13(3)4)12-16(17)23/h9-10,12-13H,6-8,11H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells |
J Med Chem 42: 833-48 (1999)
Article DOI: 10.1021/jm980224g
BindingDB Entry DOI: 10.7270/Q2610ZGP |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50244564
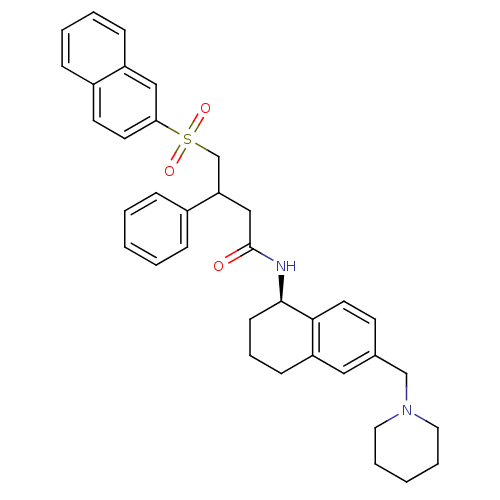
(4-(naphthalen-2-ylsulfonyl)-3-phenyl-N-((R)-6-(pip...)Show SMILES O=C(CC(CS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C36H40N2O3S/c39-36(37-35-15-9-14-31-22-27(16-19-34(31)35)25-38-20-7-2-8-21-38)24-32(28-10-3-1-4-11-28)26-42(40,41)33-18-17-29-12-5-6-13-30(29)23-33/h1,3-6,10-13,16-19,22-23,32,35H,2,7-9,14-15,20-21,24-26H2,(H,37,39)/t32?,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in clone CHO-D-/aequorin cells |
Bioorg Med Chem Lett 18: 4764-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.108
BindingDB Entry DOI: 10.7270/Q29C6X87 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50074500
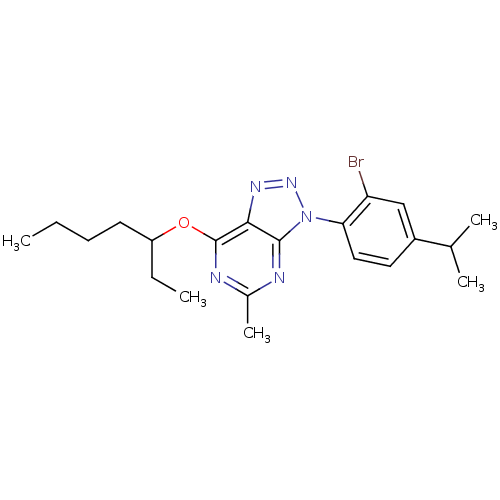
(3-(2-Bromo-4-isopropyl-phenyl)-7-(1-ethyl-pentylox...)Show SMILES CCCCC(CC)Oc1nc(C)nc2n(nnc12)-c1ccc(cc1Br)C(C)C Show InChI InChI=1S/C21H28BrN5O/c1-6-8-9-16(7-2)28-21-19-20(23-14(5)24-21)27(26-25-19)18-11-10-15(13(3)4)12-17(18)22/h10-13,16H,6-9H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells |
J Med Chem 42: 833-48 (1999)
Article DOI: 10.1021/jm980224g
BindingDB Entry DOI: 10.7270/Q2610ZGP |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50058163
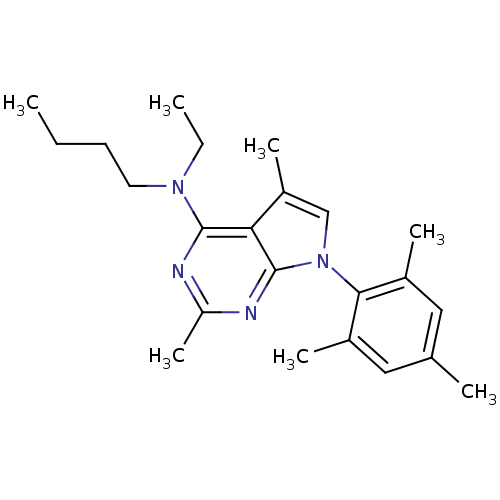
(Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...)Show SMILES CCCCN(CC)c1nc(C)nc2n(cc(C)c12)-c1c(C)cc(C)cc1C |(-10.62,2.69,;-9.29,3.46,;-7.95,2.7,;-6.62,3.48,;-5.28,2.71,;-3.95,3.49,;-3.96,5.03,;-5.27,1.17,;-6.6,.4,;-6.6,-1.14,;-7.94,-1.91,;-5.27,-1.91,;-3.92,-1.14,;-2.44,-1.61,;-1.54,-.35,;-2.46,.9,;-1.99,2.37,;-3.93,.41,;-1.96,-3.07,;-2.99,-4.22,;-4.49,-3.91,;-2.5,-5.68,;-.99,-5.99,;-.51,-7.46,;.03,-4.83,;-.46,-3.38,;.56,-2.22,)| Show InChI InChI=1S/C23H32N4/c1-8-10-11-26(9-2)22-20-18(6)14-27(23(20)25-19(7)24-22)21-16(4)12-15(3)13-17(21)5/h12-14H,8-11H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells |
J Med Chem 42: 833-48 (1999)
Article DOI: 10.1021/jm980224g
BindingDB Entry DOI: 10.7270/Q2610ZGP |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50244566
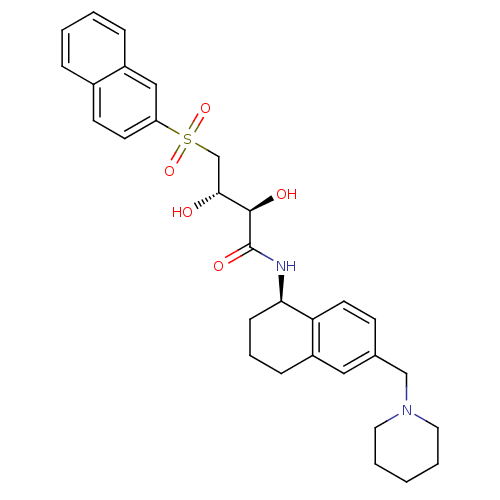
((2R,3S)-2,3-dihydroxy-4-(naphthalen-2-ylsulfonyl)-...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2ccccc2c1)[C@@H](O)C(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C30H36N2O5S/c33-28(20-38(36,37)25-13-12-22-7-2-3-8-23(22)18-25)29(34)30(35)31-27-10-6-9-24-17-21(11-14-26(24)27)19-32-15-4-1-5-16-32/h2-3,7-8,11-14,17-18,27-29,33-34H,1,4-6,9-10,15-16,19-20H2,(H,31,35)/t27-,28-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in clone CHO-D-/aequorin cells |
Bioorg Med Chem Lett 18: 4764-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.108
BindingDB Entry DOI: 10.7270/Q29C6X87 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50010586
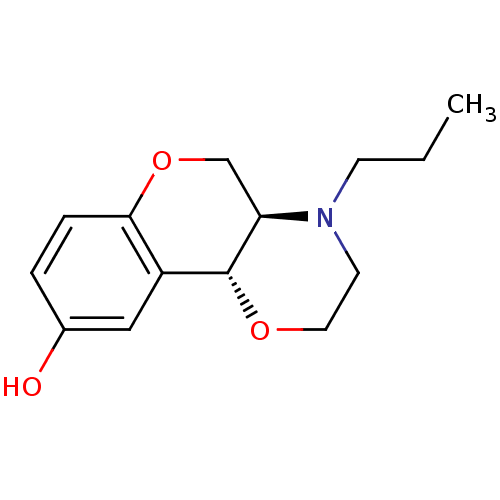
((+)-1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4,9-diox...)Show InChI InChI=1S/C14H19NO3/c1-2-5-15-6-7-17-14-11-8-10(16)3-4-13(11)18-9-12(14)15/h3-4,8,12,14,16H,2,5-7,9H2,1H3/t12-,14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]spiperone from human D3 receptor transfected in HEK293 cell membrane measured after 50 mins by competitive radioligand binding an... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128269
BindingDB Entry DOI: 10.7270/Q2TB1BP0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
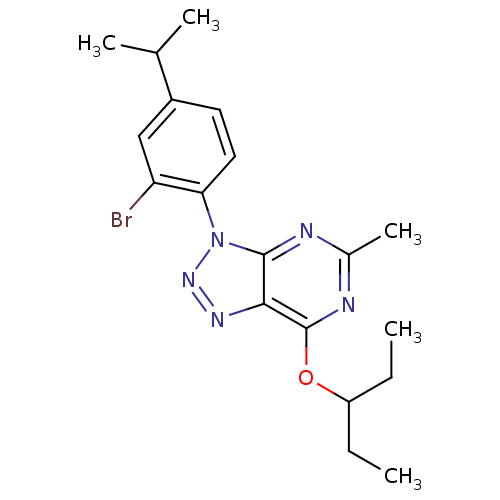
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50074470
(3-(2-Bromo-4-isopropyl-phenyl)-7-(1-ethyl-propoxy)...)Show SMILES CCC(CC)Oc1nc(C)nc2n(nnc12)-c1ccc(cc1Br)C(C)C Show InChI InChI=1S/C19H24BrN5O/c1-6-14(7-2)26-19-17-18(21-12(5)22-19)25(24-23-17)16-9-8-13(11(3)4)10-15(16)20/h8-11,14H,6-7H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells |
J Med Chem 42: 833-48 (1999)
Article DOI: 10.1021/jm980224g
BindingDB Entry DOI: 10.7270/Q2610ZGP |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50074482
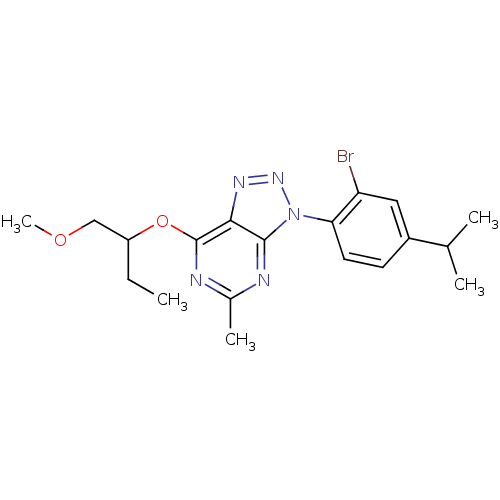
(3-(2-Bromo-4-isopropyl-phenyl)-7-(1-methoxymethyl-...)Show SMILES CCC(COC)Oc1nc(C)nc2n(nnc12)-c1ccc(cc1Br)C(C)C Show InChI InChI=1S/C19H24BrN5O2/c1-6-14(10-26-5)27-19-17-18(21-12(4)22-19)25(24-23-17)16-8-7-13(11(2)3)9-15(16)20/h7-9,11,14H,6,10H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells |
J Med Chem 42: 833-48 (1999)
Article DOI: 10.1021/jm980224g
BindingDB Entry DOI: 10.7270/Q2610ZGP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50008224
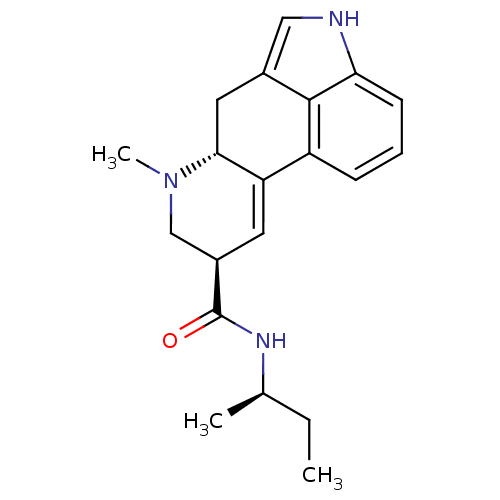
((6aR,9R)-7-Methyl-4,6,6a,7,8,9-hexahydro-indolo[4,...)Show SMILES CC[C@@H](C)NC(=O)[C@H]1CN(C)[C@@H]2Cc3c[nH]c4cccc(C2=C1)c34 |c:22| Show InChI InChI=1S/C20H25N3O/c1-4-12(2)22-20(24)14-8-16-15-6-5-7-17-19(15)13(10-21-17)9-18(16)23(3)11-14/h5-8,10,12,14,18,21H,4,9,11H2,1-3H3,(H,22,24)/t12-,14-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Affinity of the compound in displacing [3H]-8-OH-DPAT from rat hippocampal 5-HT1A receptor. |
J Med Chem 35: 203-11 (1992)
BindingDB Entry DOI: 10.7270/Q2571B03 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463293
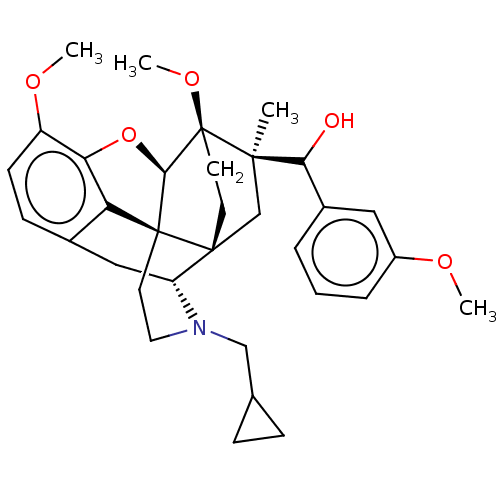
(CHEMBL4242847)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(O)c1cccc(OC)c1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H41NO5/c1-30(28(35)22-6-5-7-23(16-22)36-2)19-31-12-13-33(30,38-4)29-32(31)14-15-34(18-20-8-9-20)25(31)17-21-10-11-24(37-3)27(39-29)26(21)32/h5-7,10-11,16,20,25,28-29,35H,8-9,12-15,17-19H2,1-4H3/t25-,28?,29-,30-,31-,32+,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50074468
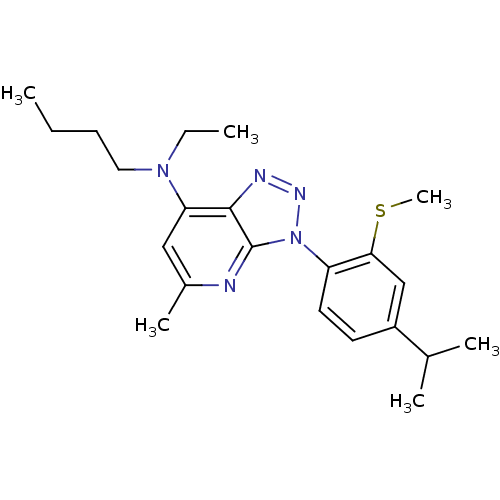
(Butyl-ethyl-[3-(4-isopropyl-2-methylsulfanyl-pheny...)Show SMILES CCCCN(CC)c1cc(C)nc2n(nnc12)-c1ccc(cc1SC)C(C)C Show InChI InChI=1S/C22H31N5S/c1-7-9-12-26(8-2)19-13-16(5)23-22-21(19)24-25-27(22)18-11-10-17(15(3)4)14-20(18)28-6/h10-11,13-15H,7-9,12H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells |
J Med Chem 42: 833-48 (1999)
Article DOI: 10.1021/jm980224g
BindingDB Entry DOI: 10.7270/Q2610ZGP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM177786

(US9120791, Example 1 | US9120791, Example 38 | US9...)Show SMILES Oc1ccc(cc1)N(C(=O)c1cc(-c2cc3OCOc3cc2C(=O)N2Cc3ccccc3C[C@H]2CN2CCOCC2)n2CCCCc12)c1ccccc1 Show InChI InChI=1S/C43H42N4O6/c48-34-15-13-32(14-16-34)47(31-10-2-1-3-11-31)43(50)37-23-39(45-17-7-6-12-38(37)45)35-24-40-41(53-28-52-40)25-36(35)42(49)46-26-30-9-5-4-8-29(30)22-33(46)27-44-18-20-51-21-19-44/h1-5,8-11,13-16,23-25,33,48H,6-7,12,17-22,26-28H2/t33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114184
BindingDB Entry DOI: 10.7270/Q2M049DT |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50005257
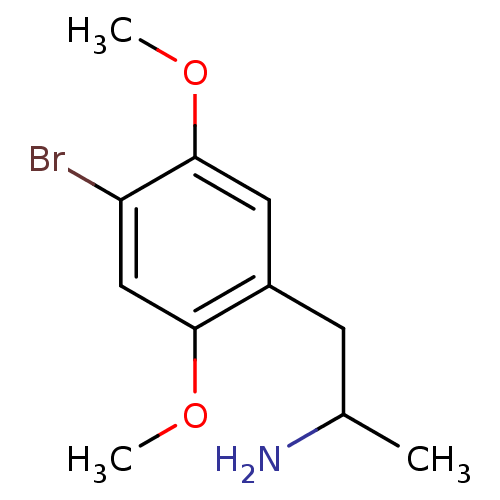
((+)-2-(4-Bromo-2,5-dimethoxy-phenyl)-1-methyl-ethy...)Show InChI InChI=1S/C11H16BrNO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to displace 0.25 nM [125I](R)-DOI from binding sites in rat frontal cortex. |
J Med Chem 34: 276-81 (1991)
BindingDB Entry DOI: 10.7270/Q2SN0C6J |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463284
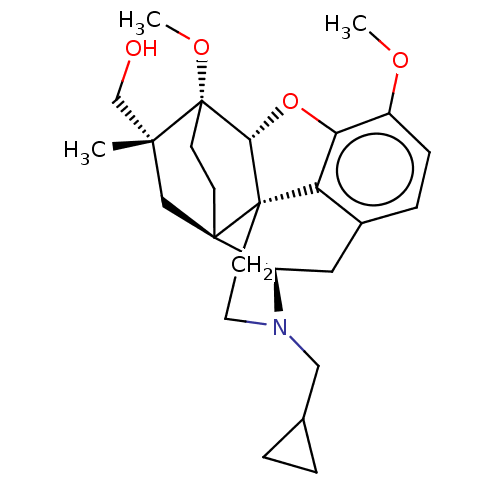
(CHEMBL4245818)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(CO)C1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C26H35NO4/c1-23(15-28)14-24-8-9-26(23,30-3)22-25(24)10-11-27(13-16-4-5-16)19(24)12-17-6-7-18(29-2)21(31-22)20(17)25/h6-7,16,19,22,28H,4-5,8-15H2,1-3H3/t19-,22-,23-,24-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50074474
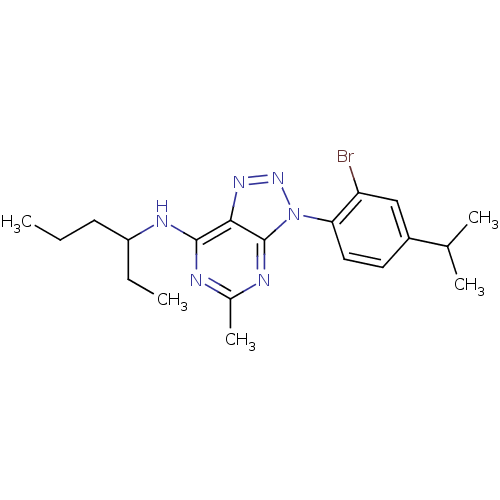
(CHEMBL166669 | [3-(2-Bromo-4-isopropyl-phenyl)-5-m...)Show SMILES CCCC(CC)Nc1nc(C)nc2n(nnc12)-c1ccc(cc1Br)C(C)C Show InChI InChI=1S/C20H27BrN6/c1-6-8-15(7-2)24-19-18-20(23-13(5)22-19)27(26-25-18)17-10-9-14(12(3)4)11-16(17)21/h9-12,15H,6-8H2,1-5H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells |
J Med Chem 42: 833-48 (1999)
Article DOI: 10.1021/jm980224g
BindingDB Entry DOI: 10.7270/Q2610ZGP |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50074459
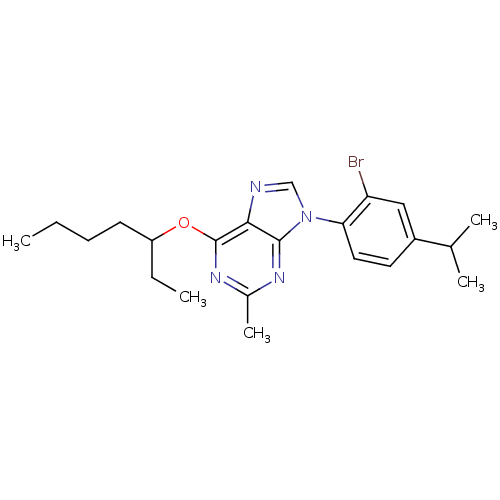
(9-(2-Bromo-4-isopropyl-phenyl)-6-(1-ethyl-pentylox...)Show SMILES CCCCC(CC)Oc1nc(C)nc2n(cnc12)-c1ccc(cc1Br)C(C)C Show InChI InChI=1S/C22H29BrN4O/c1-6-8-9-17(7-2)28-22-20-21(25-15(5)26-22)27(13-24-20)19-11-10-16(14(3)4)12-18(19)23/h10-14,17H,6-9H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells |
J Med Chem 42: 833-48 (1999)
Article DOI: 10.1021/jm980224g
BindingDB Entry DOI: 10.7270/Q2610ZGP |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50074458
(CHEMBL9633 | [3-(2-Bromo-4,6-dimethoxy-phenyl)-5-m...)Show SMILES CCCCN(CC)c1nc(C)nc2n(nnc12)-c1c(Br)cc(OC)cc1OC |(13.26,-.73,;11.93,.04,;10.59,-.73,;9.26,.02,;7.91,-.75,;6.58,.02,;5.24,-.76,;7.92,-2.29,;6.59,-3.06,;6.59,-4.61,;5.24,-5.38,;7.92,-5.38,;9.26,-4.6,;10.73,-5.08,;11.64,-3.83,;10.73,-2.58,;9.26,-3.06,;11.2,-6.55,;12.7,-6.86,;13.73,-5.71,;13.19,-8.31,;12.16,-9.47,;12.63,-10.95,;11.6,-12.09,;10.64,-9.15,;10.17,-7.69,;8.66,-7.37,;7.63,-8.52,)| Show InChI InChI=1S/C19H25BrN6O2/c1-6-8-9-25(7-2)18-16-19(22-12(3)21-18)26(24-23-16)17-14(20)10-13(27-4)11-15(17)28-5/h10-11H,6-9H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells |
J Med Chem 42: 833-48 (1999)
Article DOI: 10.1021/jm980224g
BindingDB Entry DOI: 10.7270/Q2610ZGP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data