

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110618 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-(2-(3,5-dimethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110626 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-[3-{(S)-2-[2-(1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110617 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-[3-[(S)-2-(2-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090552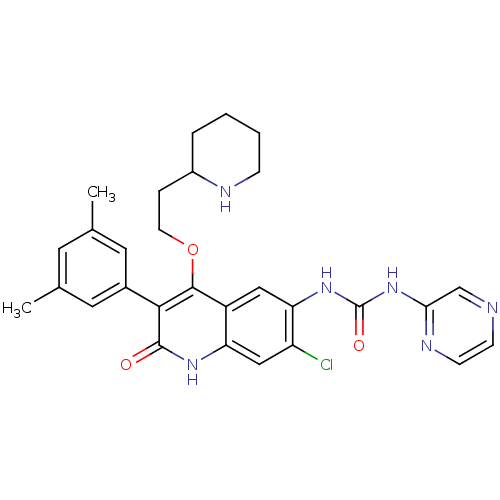 (1-[7-Chloro-3-(3,5-dimethyl-phenyl)-2-oxo-4-(2-pip...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity against Gonadotropin-releasing hormone receptor (GnRHr) in rat pituitary cells | Bioorg Med Chem Lett 10: 1723-7 (2000) BindingDB Entry DOI: 10.7270/Q26W99B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50090540 (7-Chloro-2-oxo-4-((S)-2-piperidin-2-yl-ethoxy)-3-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human Gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 10: 1723-7 (2000) BindingDB Entry DOI: 10.7270/Q26W99B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110618 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-(2-(3,5-dimethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110623 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-[3-{(S)-2-[2-(1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110597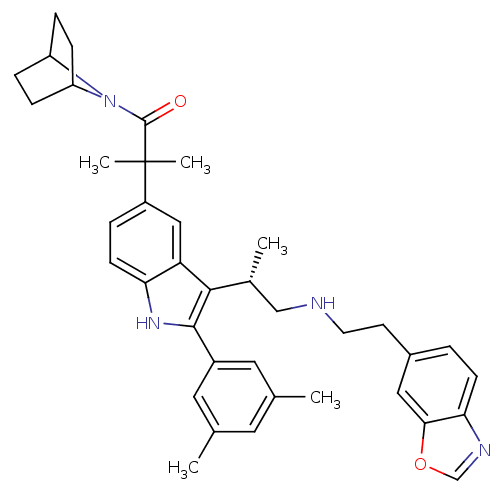 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-[3-[(S)-2-(2-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090555 (1-[7-Chloro-3-(3,5-dimethyl-phenyl)-2-oxo-4-(2-pip...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against rat pituitary Gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 10: 1723-7 (2000) BindingDB Entry DOI: 10.7270/Q26W99B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50327846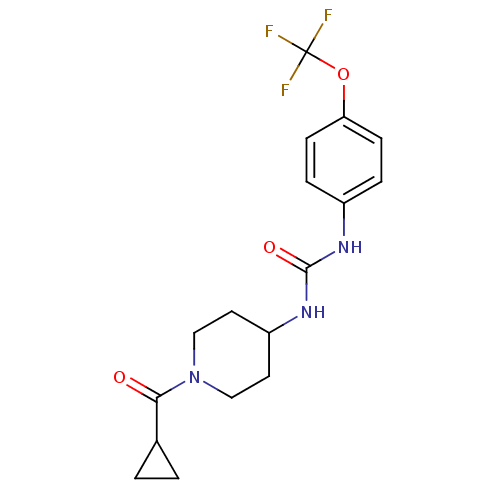 (1-(1-(Cyclopropanecarbonyl)piperidin-4-yl)-3-(4-(t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of soluble epoxide hydrolase (unknown origin) | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496759 (CHEMBL3222130) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110613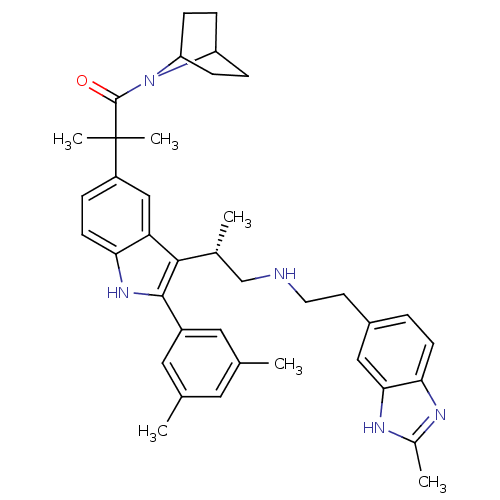 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-(2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110611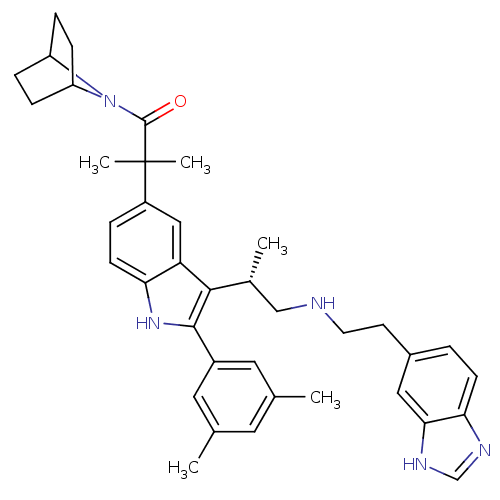 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-[3-{(S)-2-[2-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110619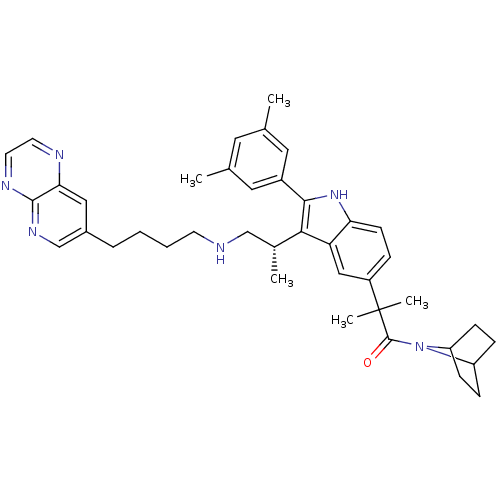 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-{2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110627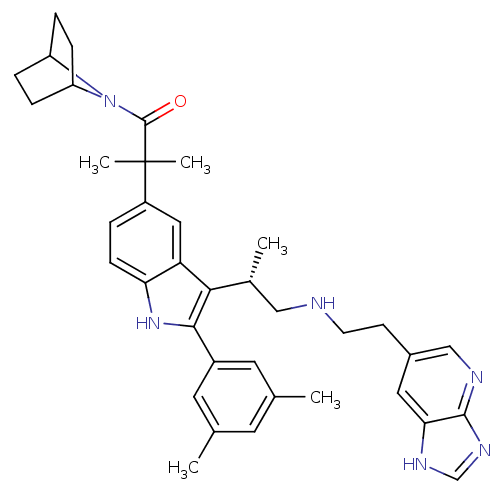 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-(2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110600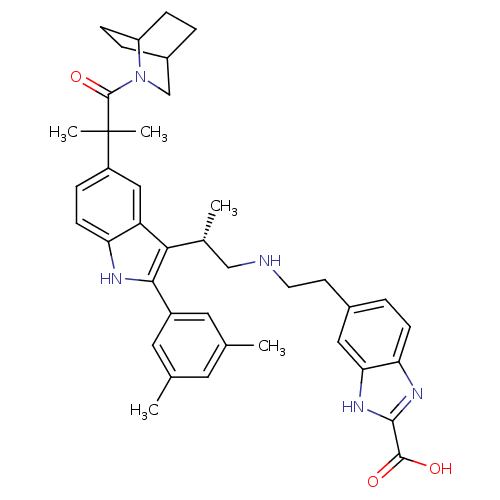 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110599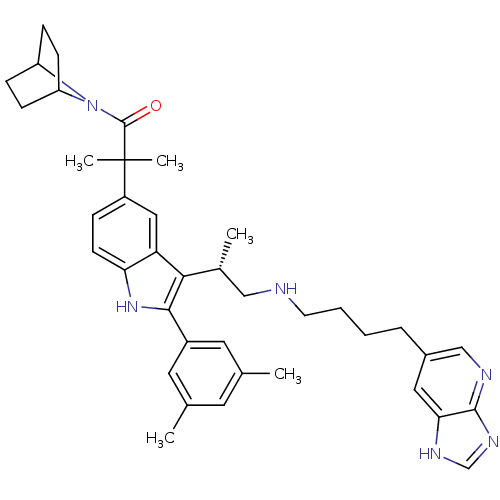 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-(2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110627 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-(2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50098391 ((S)-4-(2-(azetidin-2-yl)ethoxy)-7-chloro-2-oxo-N-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of the compound to GnRH receptor in human | J Med Chem 44: 917-22 (2001) BindingDB Entry DOI: 10.7270/Q2H70F3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110614 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-[3-[(S)-2-(2-be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496754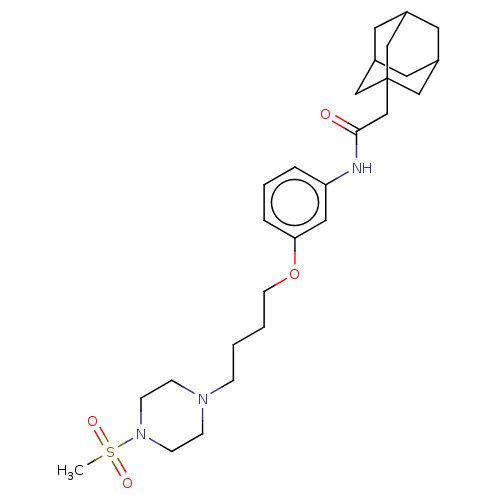 (CHEMBL3222131) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50090542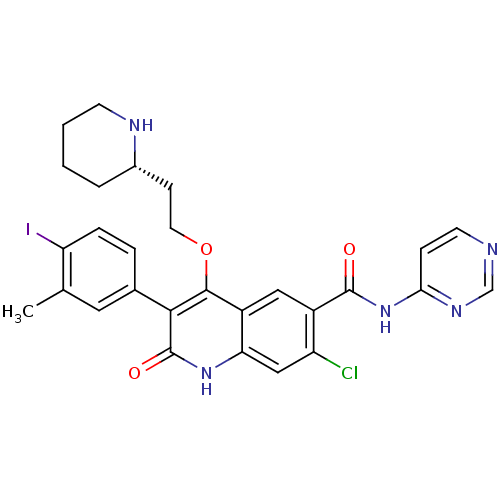 (7-Chloro-3-(4-iodo-3-methyl-phenyl)-2-oxo-4-((S)-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human Gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 10: 1723-7 (2000) BindingDB Entry DOI: 10.7270/Q26W99B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50098391 ((S)-4-(2-(azetidin-2-yl)ethoxy)-7-chloro-2-oxo-N-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of gonadotropin-releasing hormone receptor in rhesus monkey | J Med Chem 44: 917-22 (2001) BindingDB Entry DOI: 10.7270/Q2H70F3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496748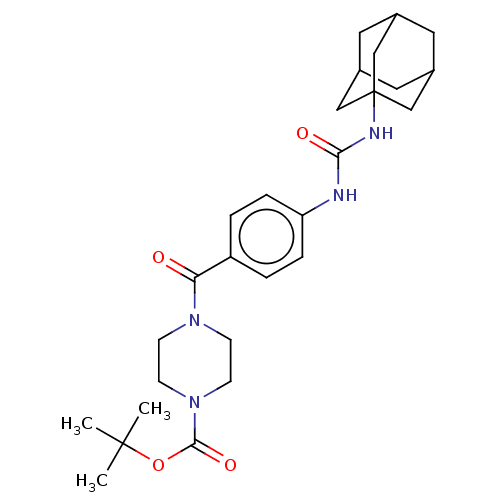 (CHEMBL3222118) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110593 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110593 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496753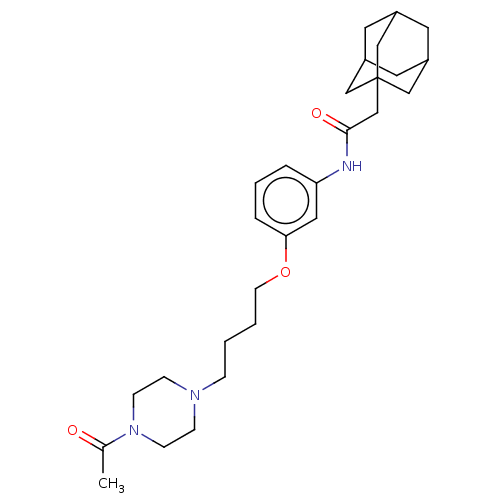 (CHEMBL3222129) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110594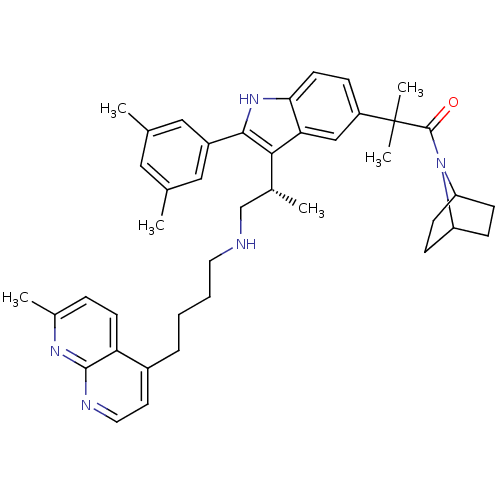 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-(2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110606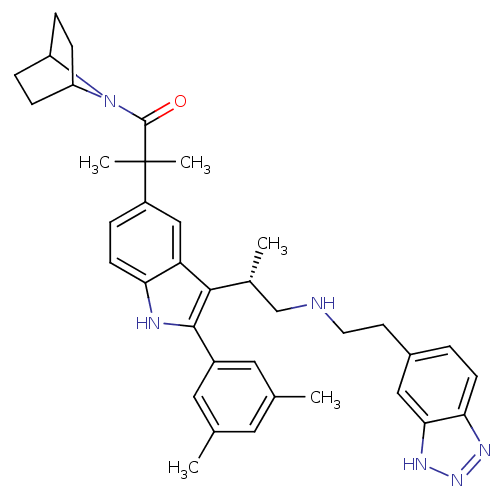 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-[3-{(S)-2-[2-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110596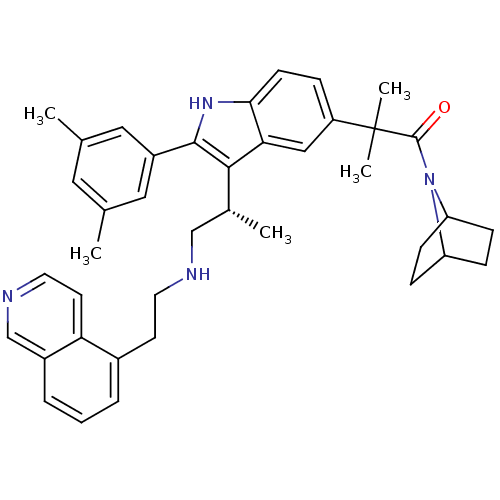 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-{2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090555 (1-[7-Chloro-3-(3,5-dimethyl-phenyl)-2-oxo-4-(2-pip...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its antagonism in rat primary pituitary cell assay (rLH) | Bioorg Med Chem Lett 10: 1723-7 (2000) BindingDB Entry DOI: 10.7270/Q26W99B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110595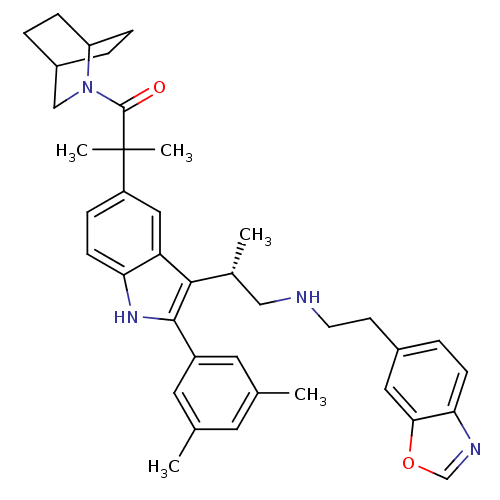 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-[3-[(S)-2-(2-be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110620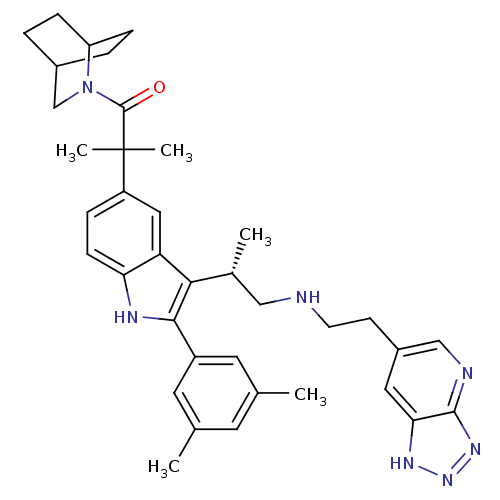 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-(2-(3,5-dimethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104543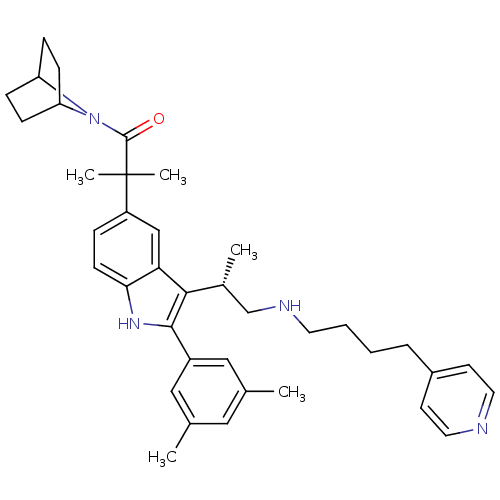 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-{2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110602 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50090588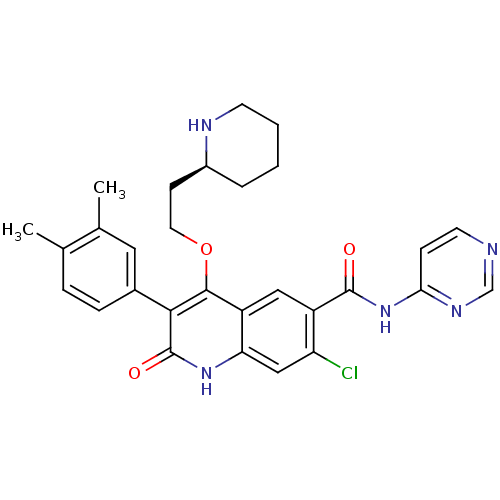 (7-Chloro-3-(3,4-dimethyl-phenyl)-2-oxo-4-((S)-2-pi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human Gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 10: 1723-7 (2000) BindingDB Entry DOI: 10.7270/Q26W99B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50090576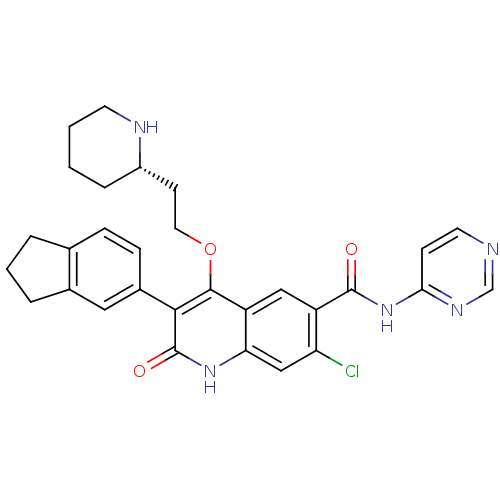 (7-Chloro-3-indan-5-yl-2-oxo-4-((S)-2-piperidin-2-y...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human Gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 10: 1723-7 (2000) BindingDB Entry DOI: 10.7270/Q26W99B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110592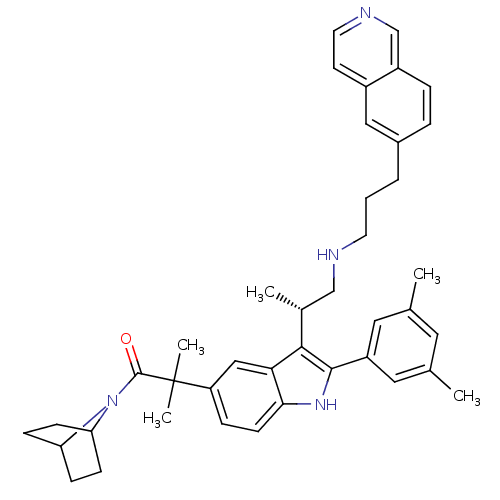 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-{2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110607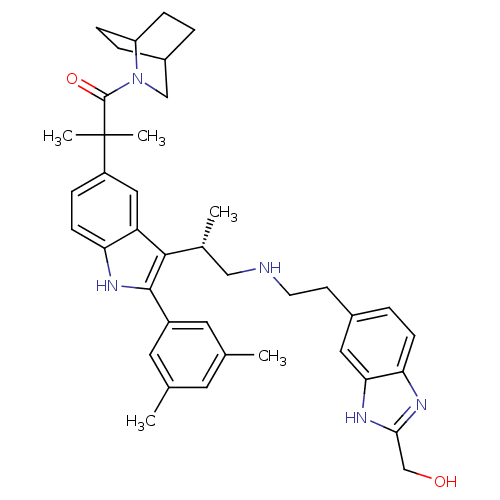 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-(2-(3,5-dimethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090538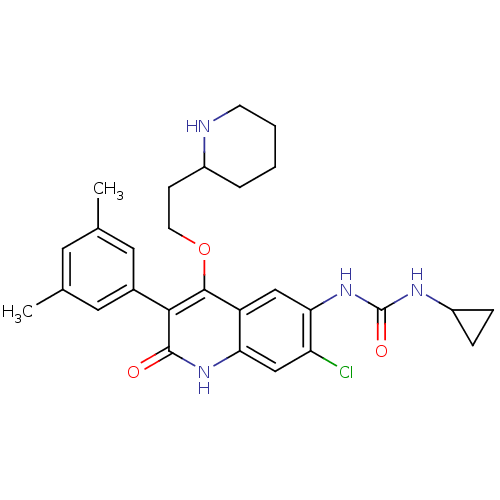 (1-[7-Chloro-3-(3,5-dimethyl-phenyl)-2-oxo-4-(2-pip...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its antagonism in rat primary pituitary cell assay (rLH) | Bioorg Med Chem Lett 10: 1723-7 (2000) BindingDB Entry DOI: 10.7270/Q26W99B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110622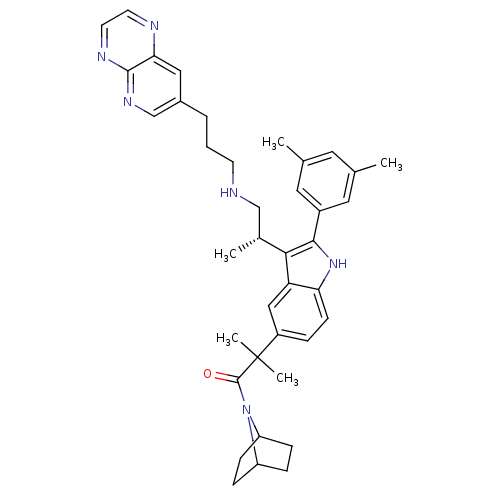 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-{2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110612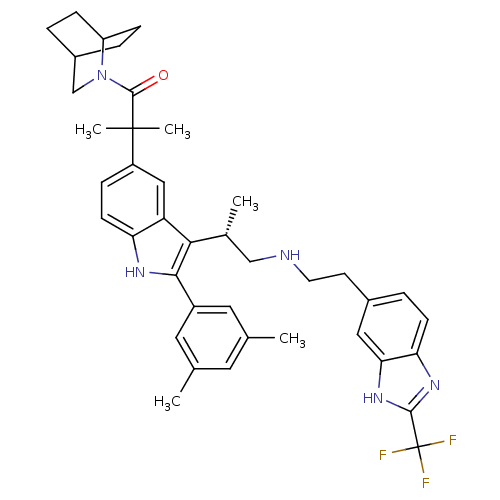 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-(2-(3,5-dimethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110603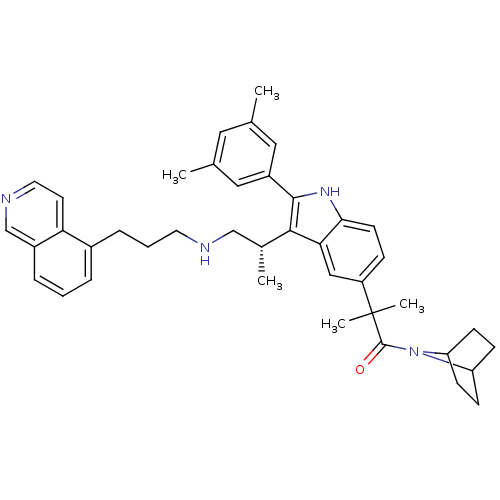 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-{2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50090554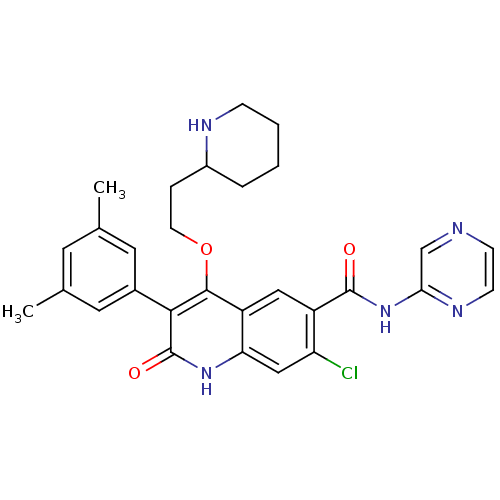 (7-Chloro-3-(3,5-dimethyl-phenyl)-2-oxo-4-(2-piperi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human Gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 10: 1723-7 (2000) BindingDB Entry DOI: 10.7270/Q26W99B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50204183 (5-(4-chlorophenyl)-6-(2,4-dichlorophenyl)-2-(3,5-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity at CB1 receptor | Bioorg Med Chem Lett 17: 2031-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.005 BindingDB Entry DOI: 10.7270/Q27944BJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50496760 (CHEMBL3222119) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a... | Medchemcomm 379-384 (2012) Article DOI: 10.1039/c2md00288d BindingDB Entry DOI: 10.7270/Q2JD50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50333051 (1-Adamantan-1-yl-3-{3-[4-(4-methanesulfonyl-pipera...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase expressed in baculovirus expression system by fluorimetric assay | J Med Chem 53: 8376-8386 (2010) Article DOI: 10.1021/jm101087u BindingDB Entry DOI: 10.7270/Q2D79BNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50098391 ((S)-4-(2-(azetidin-2-yl)ethoxy)-7-chloro-2-oxo-N-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Functional antagonism at the human GnRH receptor (PI turnover) | J Med Chem 44: 917-22 (2001) BindingDB Entry DOI: 10.7270/Q2H70F3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50090583 (7-Chloro-3-(3,4-dichloro-phenyl)-2-oxo-4-((S)-2-pi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human Gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 10: 1723-7 (2000) BindingDB Entry DOI: 10.7270/Q26W99B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090538 (1-[7-Chloro-3-(3,5-dimethyl-phenyl)-2-oxo-4-(2-pip...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against rat pituitary Gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 10: 1723-7 (2000) BindingDB Entry DOI: 10.7270/Q26W99B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 456 total ) | Next | Last >> |