

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM350085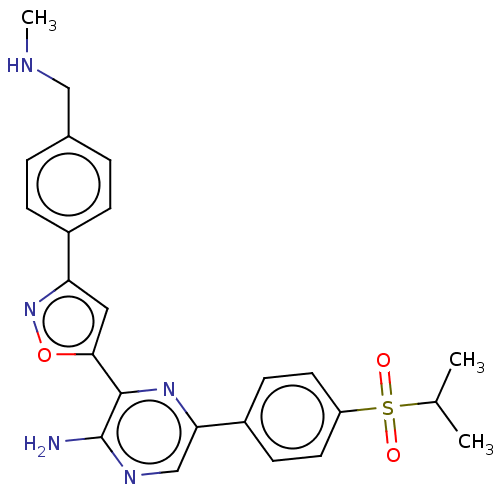 (3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VERTEX PHARMACEUTICALS INCORPORATED US Patent | Assay Description Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... | US Patent US10822331 (2020) BindingDB Entry DOI: 10.7270/Q2V98C59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM350085 (3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... | US Patent US10208027 (2019) BindingDB Entry DOI: 10.7270/Q27S7QX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM350085 (3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... | US Patent US10208027 (2019) BindingDB Entry DOI: 10.7270/Q27S7QX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM350085 (3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VERTEX PHARMACEUTICALS INCORPORATED US Patent | Assay Description Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... | US Patent US10822331 (2020) BindingDB Entry DOI: 10.7270/Q2V98C59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406713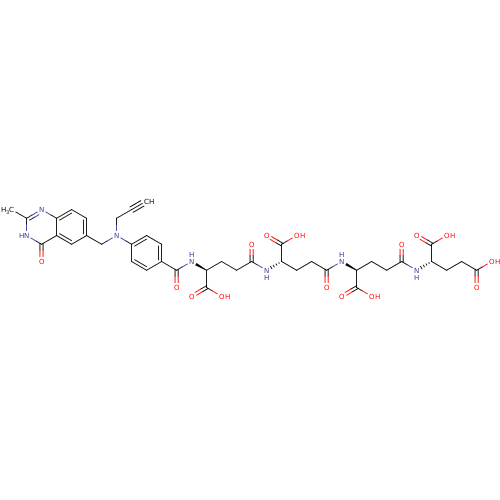 (CHEMBL1202139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406714 (CHEMBL264807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406717 (CHEMBL1202137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406721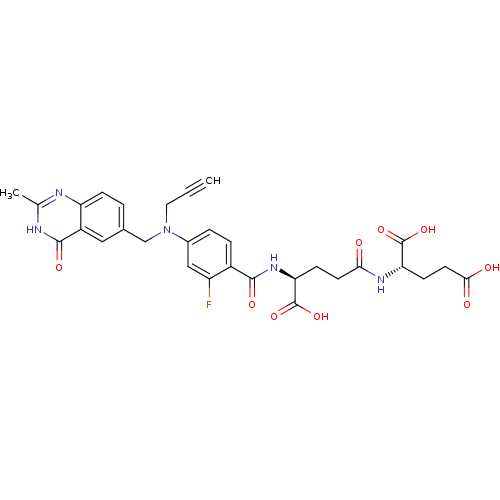 (CHEMBL171226) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406716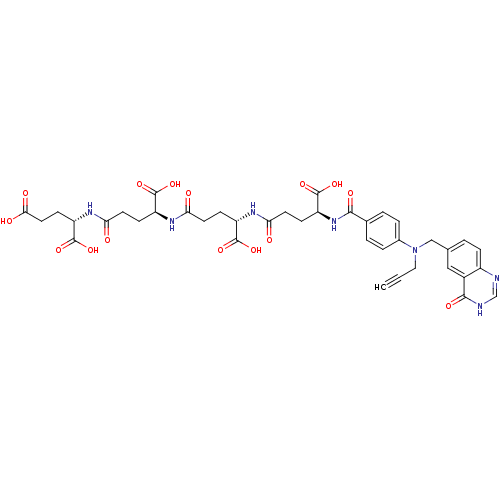 (CHEMBL1202138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069559 (Biphalin Analogue | CHEMBL2371057) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069560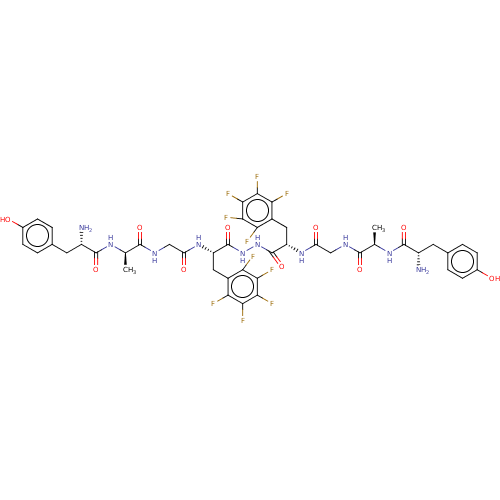 (Biphalin Analogue | CHEMBL2371080) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406718 (CHEMBL1202140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM350085 (3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VERTEX PHARMACEUTICALS INCORPORATED US Patent | Assay Description Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... | US Patent US10822331 (2020) BindingDB Entry DOI: 10.7270/Q2V98C59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM350085 (3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... | US Patent US10208027 (2019) BindingDB Entry DOI: 10.7270/Q27S7QX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM350085 (3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VERTEX PHARMACEUTICALS INCORPORATED US Patent | Assay Description Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... | US Patent US10822331 (2020) BindingDB Entry DOI: 10.7270/Q2V98C59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM350085 (3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... | US Patent US10208027 (2019) BindingDB Entry DOI: 10.7270/Q27S7QX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406719 (CHEMBL436448) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14776 (2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 5 | J Med Chem 48: 3449-62 (2005) Article DOI: 10.1021/jm040217u BindingDB Entry DOI: 10.7270/Q21G0N2H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50049164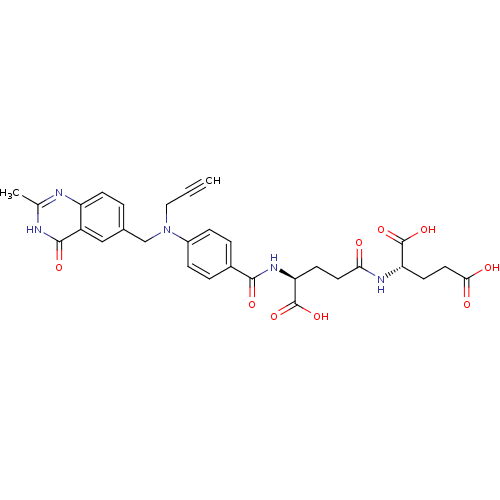 ((S)-2-((S)-4-Carboxy-4-{4-[(2-methyl-4-oxo-3,4-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069562 (2-Amino-N-((S)-1-{[((R)-1-{N'-[(R)-2-(2-{(S)-2-[2-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin-3 receptor 2 (Homo sapiens (Human)) | BDBM50558038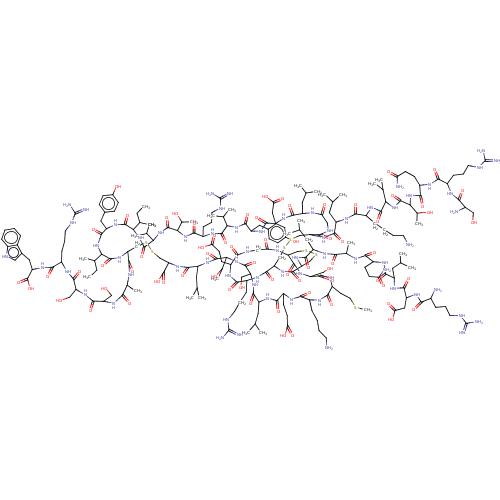 (CHEMBL4798311) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of europium-labeled Eu(A)-mINSL5 from human RXFP4 expressed in CHO-K1 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01786 BindingDB Entry DOI: 10.7270/Q2PC3624 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin-3 receptor 2 (Homo sapiens (Human)) | BDBM50558038 (CHEMBL4798311) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of europium-labeled Eu(A)-mINSL5 from human RXFP4 expressed in CHO-K1 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01786 BindingDB Entry DOI: 10.7270/Q2PC3624 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069563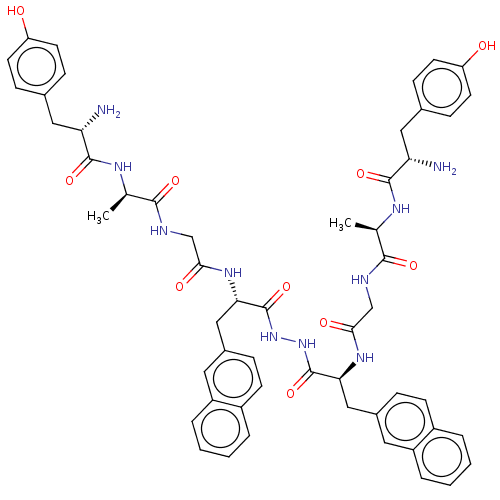 (Biphalin Analogue | CHEMBL2371079) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069558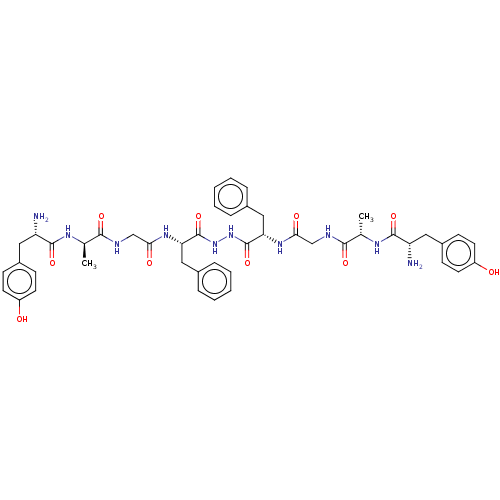 (2-Amino-N-((S)-1-{[(2-{N'-[2-(2-{(S)-2-[2-amino-3-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069561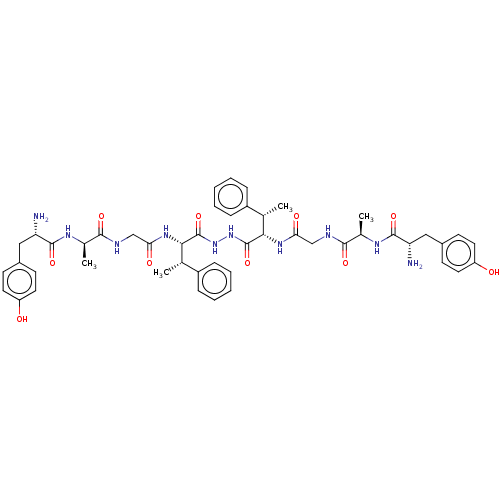 (2-Amino-N-((S)-1-{[((S)-1-{N'-[(S)-2-(2-{(S)-2-[2-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50028408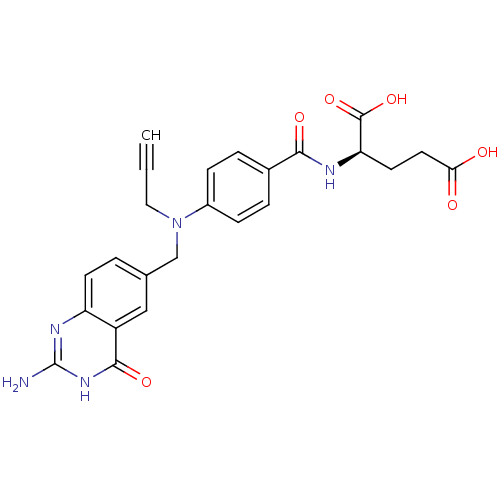 ((R)-2-{4-[(2-Amino-4-oxo-1,4-dihydro-quinazolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for binding affinity against thymidylate synthase(TS) | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Binding affinity against Thymidylate synthase was measured in vitro | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406711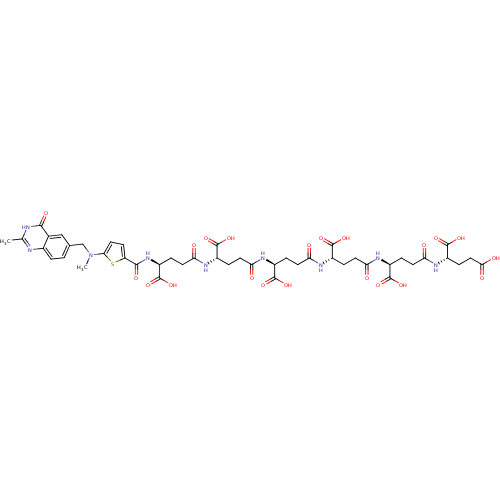 (CHEMBL405513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406712 (CHEMBL268593) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406720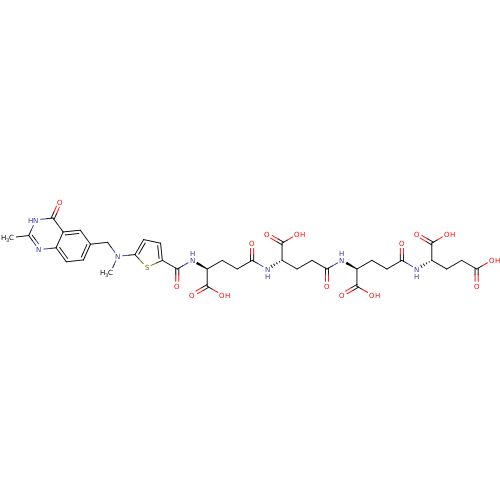 (CHEMBL172160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069558 (2-Amino-N-((S)-1-{[(2-{N'-[2-(2-{(S)-2-[2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin-3 receptor 2 (Homo sapiens (Human)) | BDBM50558042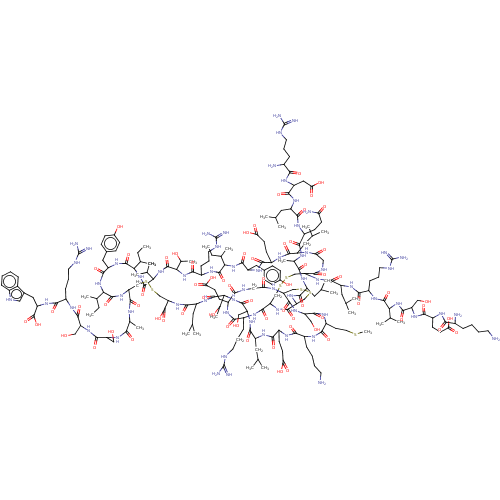 (CHEMBL4760798) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of europium-labeled Eu(A)-mINSL5 from human RXFP4 expressed in CHO-K1 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01786 BindingDB Entry DOI: 10.7270/Q2PC3624 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin-3 receptor 2 (Homo sapiens (Human)) | BDBM50558042 (CHEMBL4760798) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of europium-labeled Eu(A)-mINSL5 from human RXFP4 expressed in CHO-K1 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01786 BindingDB Entry DOI: 10.7270/Q2PC3624 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069559 (Biphalin Analogue | CHEMBL2371057) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069563 (Biphalin Analogue | CHEMBL2371079) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50406715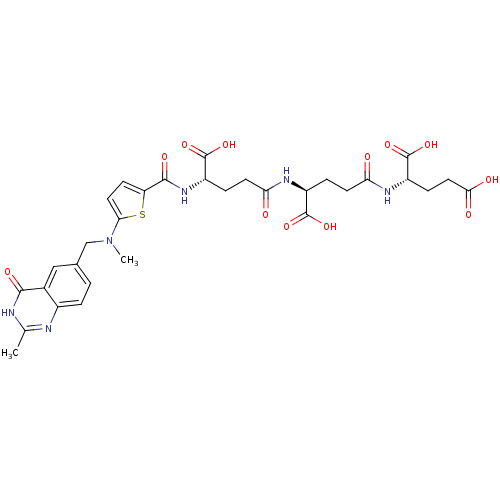 (CHEMBL355321) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069560 (Biphalin Analogue | CHEMBL2371080) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Binding affinity against Thymidylate synthase was measured in vitro | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14390 (5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 5 | J Med Chem 48: 3449-62 (2005) Article DOI: 10.1021/jm040217u BindingDB Entry DOI: 10.7270/Q21G0N2H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006689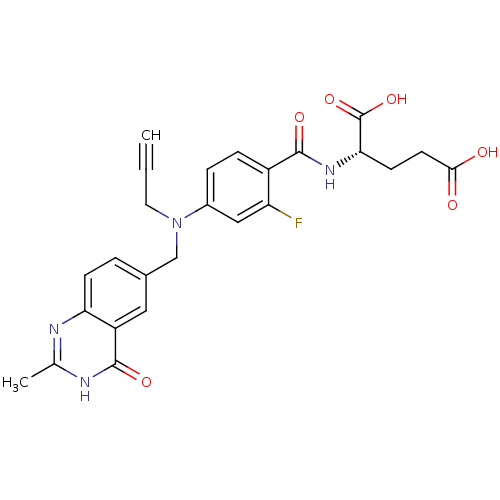 ((S)-2-(2-fluoro-4-(((2-methyl-4-oxo-3,4-dihydroqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50033900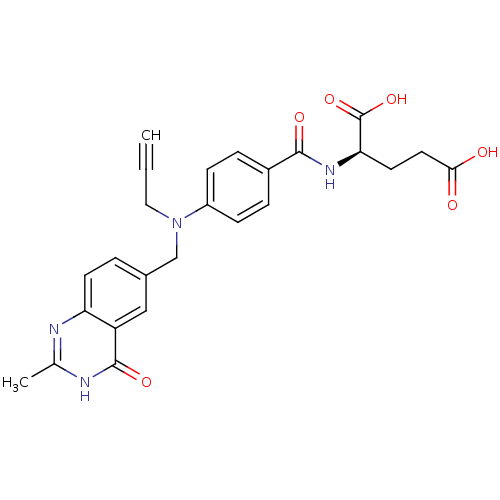 ((R)-2-{4-[(2-Methyl-4-oxo-3,4-dihydro-quinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Tested for binding affinity against thymidylate synthase(TS) | J Med Chem 37: 3294-302 (1994) BindingDB Entry DOI: 10.7270/Q2QV3N50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14777 ((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 5 | J Med Chem 48: 3449-62 (2005) Article DOI: 10.1021/jm040217u BindingDB Entry DOI: 10.7270/Q21G0N2H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069561 (2-Amino-N-((S)-1-{[((S)-1-{N'-[(S)-2-(2-{(S)-2-[2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin-3 receptor 2 (Homo sapiens (Human)) | BDBM50558040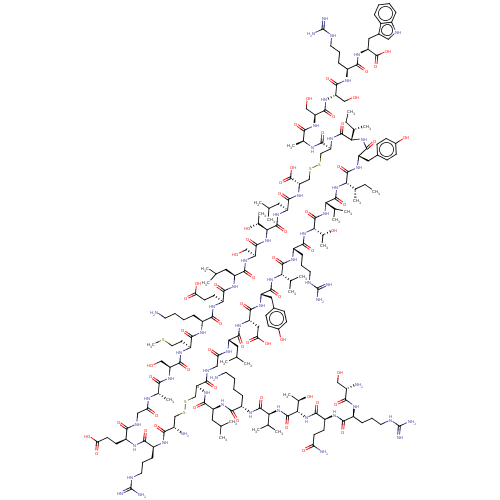 (CHEMBL4791094) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of europium-labeled Eu(A)-mINSL5 from human RXFP4 expressed in CHO-K1 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01786 BindingDB Entry DOI: 10.7270/Q2PC3624 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin-3 receptor 2 (Homo sapiens (Human)) | BDBM50558040 (CHEMBL4791094) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of europium-labeled Eu(A)-mINSL5 from human RXFP4 expressed in CHO-K1 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01786 BindingDB Entry DOI: 10.7270/Q2PC3624 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM50027177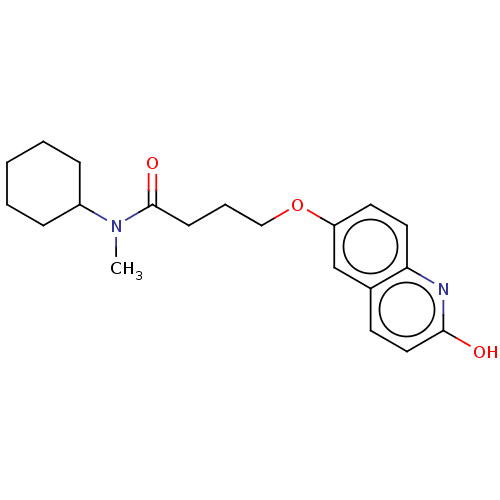 (Cilostamide) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 3 | J Med Chem 48: 3449-62 (2005) Article DOI: 10.1021/jm040217u BindingDB Entry DOI: 10.7270/Q21G0N2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin-3 receptor 2 (Homo sapiens (Human)) | BDBM50558043 (CHEMBL4742346) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of europium-labeled Eu(A)-mINSL5 from human RXFP4 expressed in CHO-K1 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01786 BindingDB Entry DOI: 10.7270/Q2PC3624 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin-3 receptor 2 (Homo sapiens (Human)) | BDBM50558043 (CHEMBL4742346) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of europium-labeled Eu(A)-mINSL5 from human RXFP4 expressed in CHO-K1 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01786 BindingDB Entry DOI: 10.7270/Q2PC3624 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin-3 receptor 2 (Homo sapiens (Human)) | BDBM50558044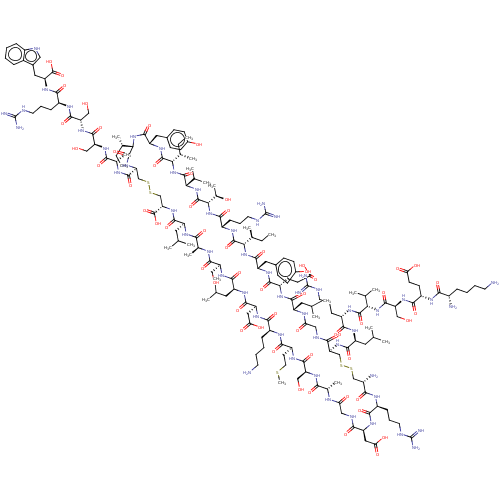 (CHEMBL4796474) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of europium-labeled Eu(A)-mINSL5 from human RXFP4 expressed in CHO-K1 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01786 BindingDB Entry DOI: 10.7270/Q2PC3624 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin-3 receptor 2 (Homo sapiens (Human)) | BDBM50558044 (CHEMBL4796474) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of europium-labeled Eu(A)-mINSL5 from human RXFP4 expressed in CHO-K1 cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01786 BindingDB Entry DOI: 10.7270/Q2PC3624 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2280 total ) | Next | Last >> |