

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50238182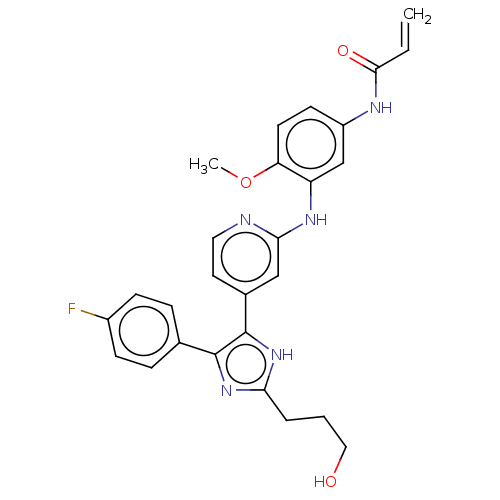 (CHEMBL4100860) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen Curated by ChEMBL | Assay Description Inhibition of binding of [3H][D-Ala2,D-Leu5]enkephalin to Opioid receptor delta 1 in the rat brain homogenate | J Med Chem 60: 5613-5637 (2017) Article DOI: 10.1021/acs.jmedchem.7b00316 BindingDB Entry DOI: 10.7270/Q2V98BCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50238177 (CHEMBL4098072) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-fused EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus using TK-substrate-biotin incubated ... | J Med Chem 60: 5613-5637 (2017) Article DOI: 10.1021/acs.jmedchem.7b00316 BindingDB Entry DOI: 10.7270/Q2V98BCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50238183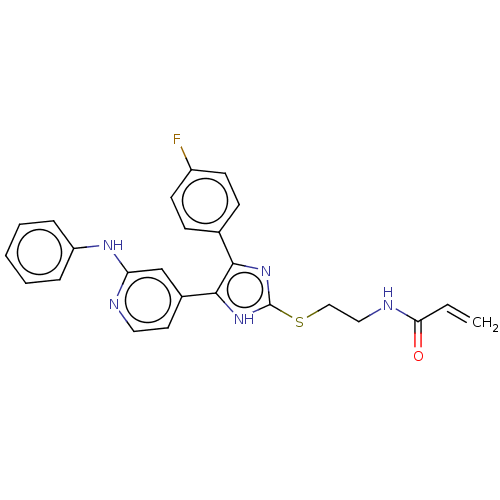 (CHEMBL4071012) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-fused EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus using TK-substrate-biotin incubated ... | J Med Chem 60: 5613-5637 (2017) Article DOI: 10.1021/acs.jmedchem.7b00316 BindingDB Entry DOI: 10.7270/Q2V98BCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14768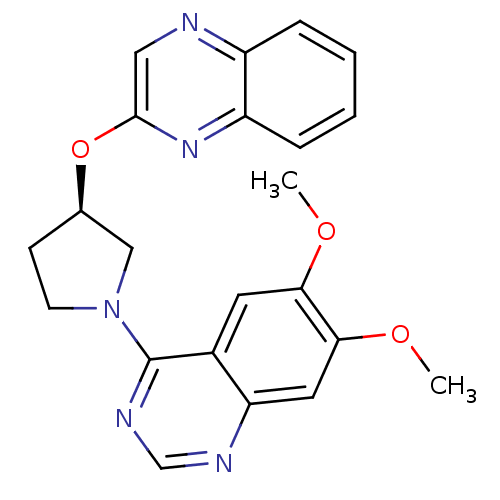 ((R)-6,7-Dimethoxy-4-[3-(quinoxalin-2-yloxy)-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM107767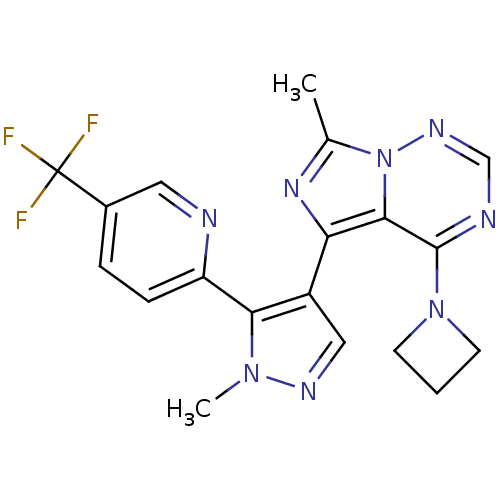 (US11419874, PF-05180999 | US8598155, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of radiolabeled 4-(azetidin-1-yl)-3-[5-[4-(trifluoromethyl)phenyl]-1H-pyrazol-4-yl]-1-(tritritiomethyl)pyrazolo[3,4-d]pyrimidine from PD... | J Med Chem 61: 1001-1018 (2018) Article DOI: 10.1021/acs.jmedchem.7b01466 BindingDB Entry DOI: 10.7270/Q2ZS2ZZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50440293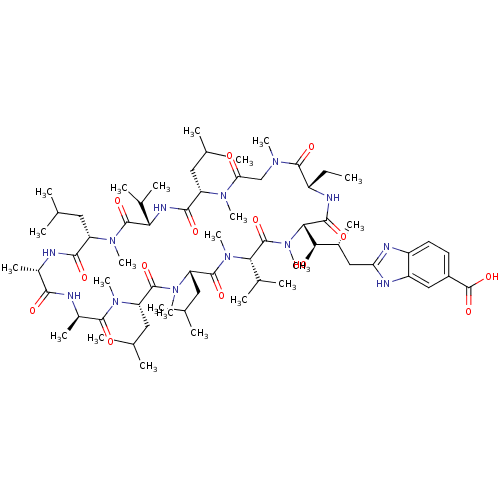 (CHEMBL2424822) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14760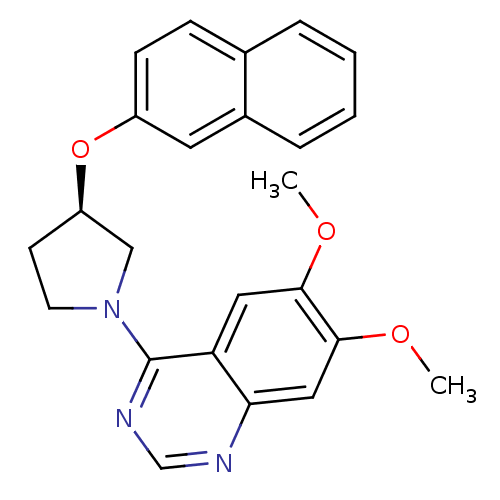 ((R)-6,7-Dimethoxy-4-[3-(naphthalen-2-yloxy)-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50440299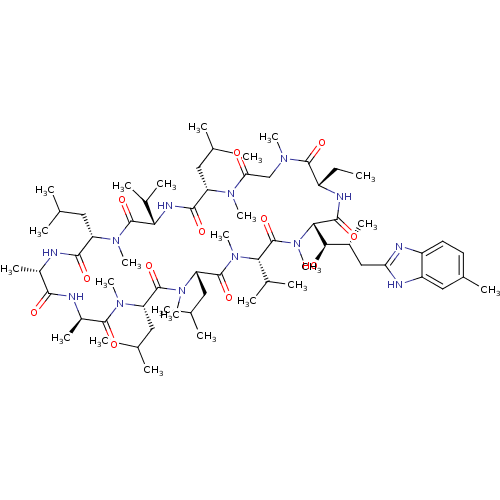 (CHEMBL2424823) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14764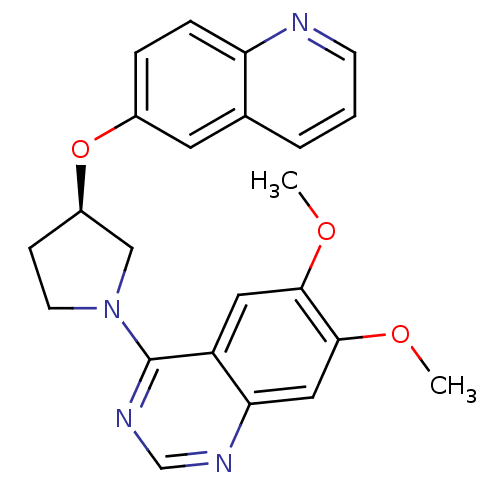 ((R)-6,7-Dimethoxy-4-[3-(quinolin-6-yloxy)-pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14766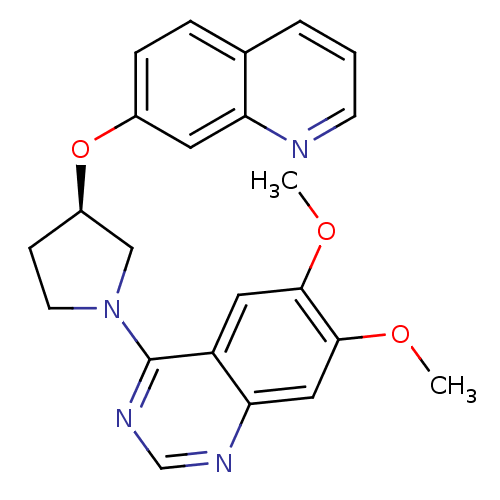 ((R)-6,7-Dimethoxy-4-[3-(quinolin-7-yloxy)-pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50440297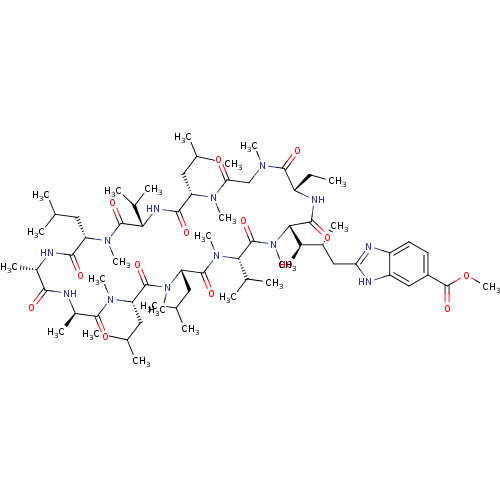 (CHEMBL2424817) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246998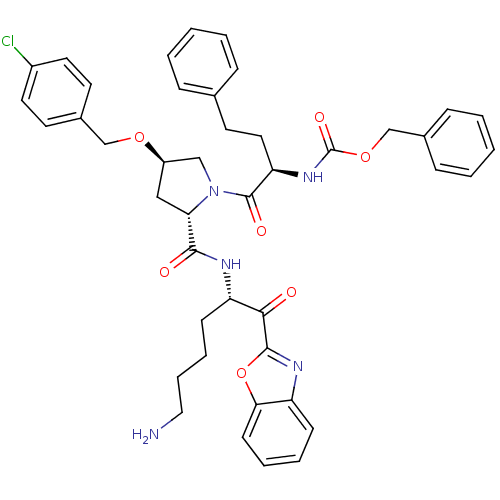 (CHEMBL505558 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-fused EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus using TK-substrate-biotin incubated ... | J Med Chem 60: 5613-5637 (2017) Article DOI: 10.1021/acs.jmedchem.7b00316 BindingDB Entry DOI: 10.7270/Q2V98BCK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14763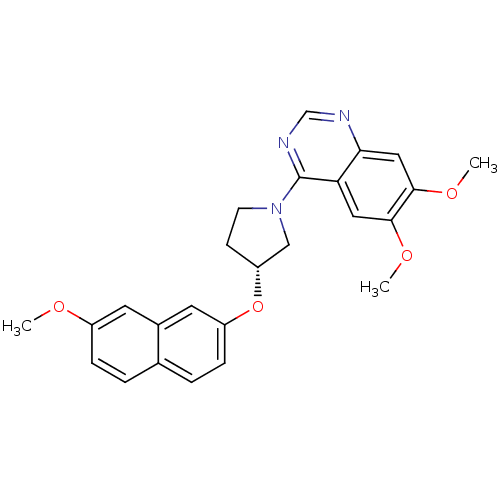 ((R)-6,7-Dimethoxy-4-[3-(7-methoxy-naphthalen-2-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14754 (1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-isoq...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14765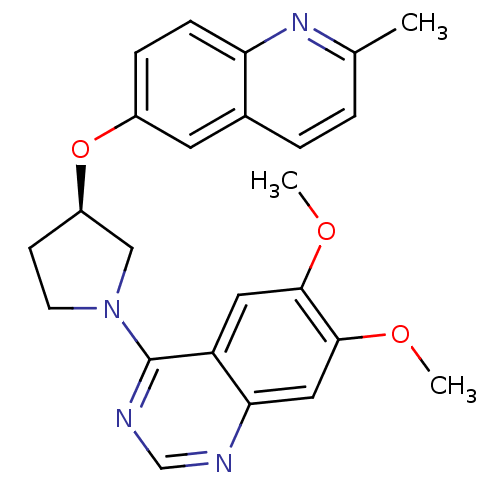 ((R)-6,7-Dimethoxy-4-[3-(2-methyl-quinolin-6-yloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14762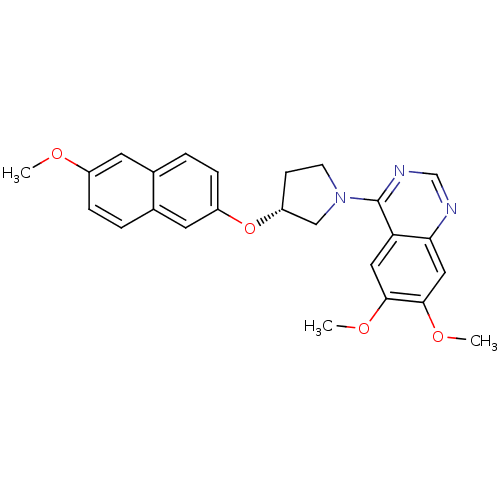 ((R)-6,7-Dimethoxy-4-[3-(6-methoxy-naphthalen-2-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246999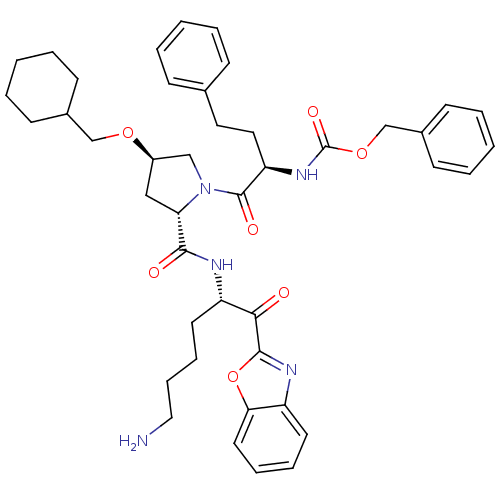 (CHEMBL500474 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14767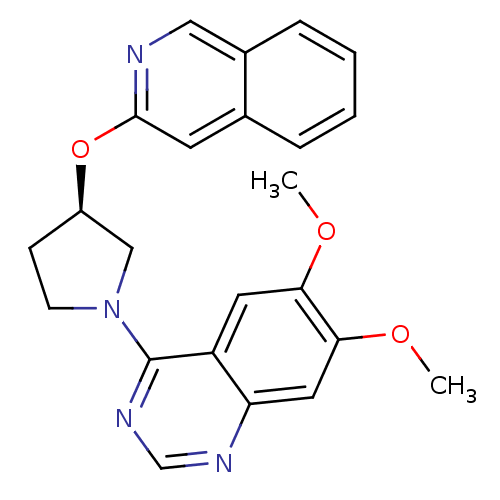 ((R)-4-[3-(Isoquinolin-3-yloxy)-pyrrolidin-1-yl]-6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50440300 (CHEMBL2424821) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14755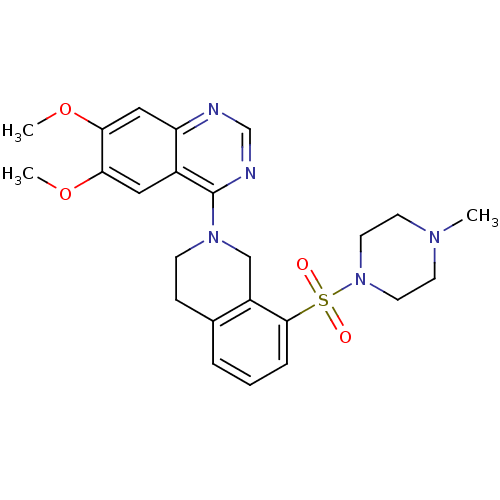 (6,7-dimethoxy-4-[8-(4-methylpiperazin-1-yl)sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | 25 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246997 (CHEMBL505738 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246995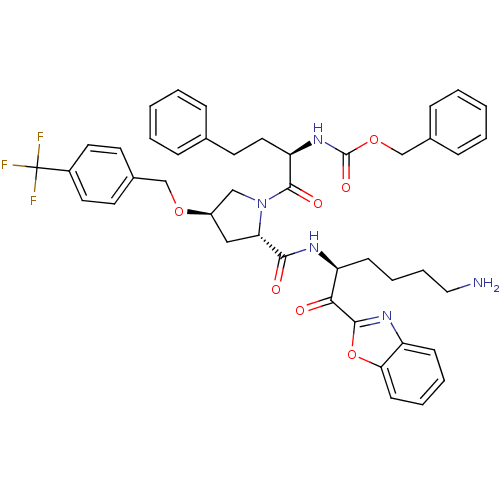 (CHEMBL505048 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50247004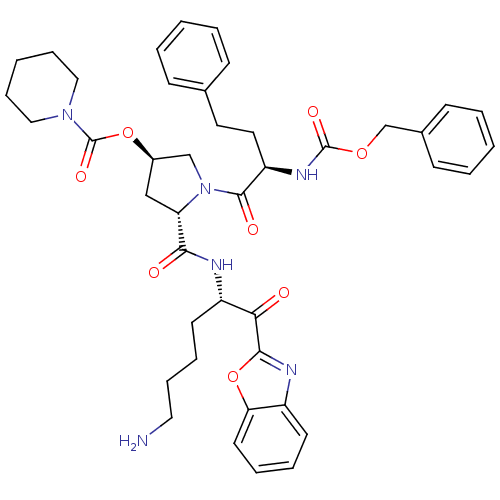 ((3R,5S)-5-(((S)-6-amino-1-(benzo[d]oxazol-2-yl)-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM50440293 (CHEMBL2424822) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypB PPIase activity (unknown origin) | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50440296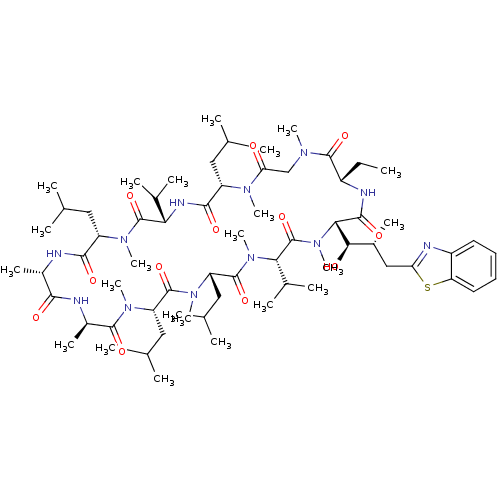 (CHEMBL2424818) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246992 (CHEMBL498914 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246994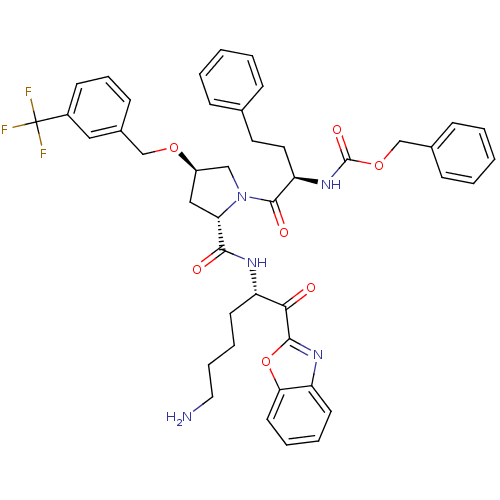 (CHEMBL443101 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14756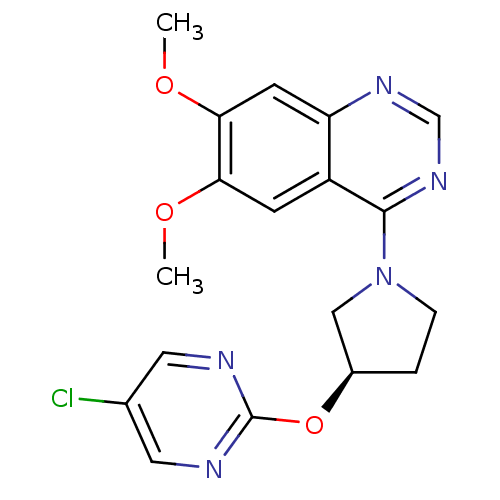 ((R)-4-[3-(5-Chloro-pyrimidin-2-yloxy)-pyrrolidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50440298 (CHEMBL2424824) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246996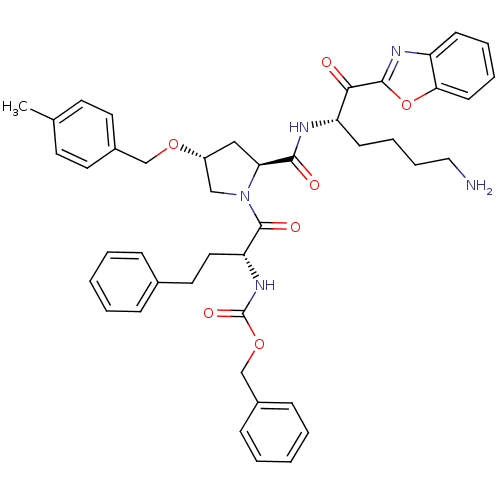 (CHEMBL506226 | {(R)-1-[(2S,4R)-2-[(S)-5-Amino-1-(b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM14761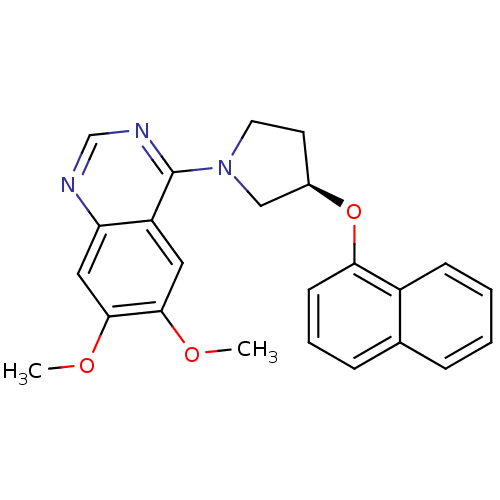 ((R)-6,7-Dimethoxy-4-[3-(naphthalen-1-yloxy)-pyrrol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246991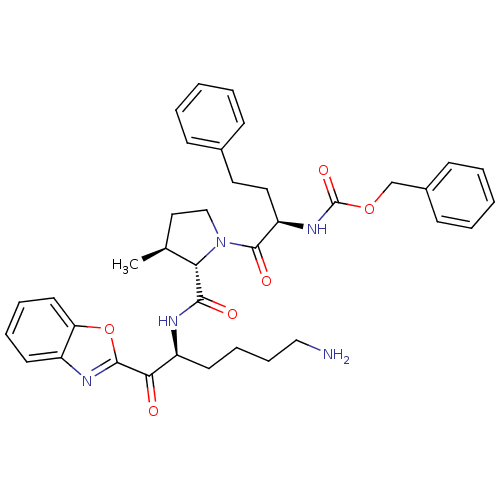 (CHEMBL509770 | benzyl (R)-1-((2S,3S)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14748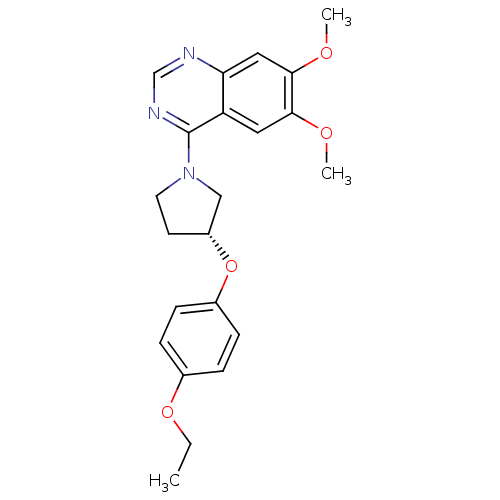 ((R)-4-[3-(4-Ethoxy-phenoxy)-pyrrolidin-1-yl]-6,7-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 54 | -41.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14745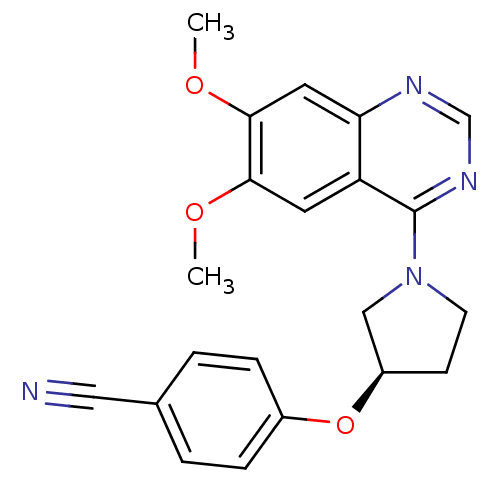 ((R)-4-[1-(6,7-Dimethoxy-quinazolin-4-yl)-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 56 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM14761 ((R)-6,7-Dimethoxy-4-[3-(naphthalen-1-yloxy)-pyrrol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50440294 (CHEMBL2424820) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50247002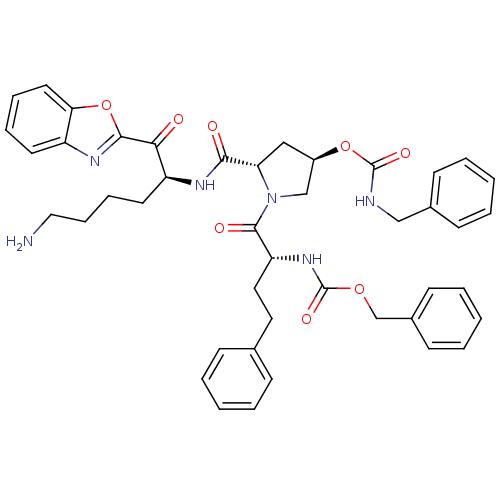 (((R)-1-{(2S,4R)-2-[(S)-5-Amino-1-(benzooxazole-2-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM14751 ((R)-4-[3-(3-Ethoxy-phenoxy)-pyrrolidin-1-yl]-6,7-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50440295 (CHEMBL2424819) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of CypA PPIase activity (unknown origin) using Glt-(Ala)n-Pro-Phe-4-nitroanilides as substrate | J Med Chem 56: 7302-11 (2013) Article DOI: 10.1021/jm4007577 BindingDB Entry DOI: 10.7270/Q2NK3GGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50247000 (CHEMBL446259 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14753 ((R)-4-[3-(3,4-Dimethoxy-phenoxy)-pyrrolidin-1-yl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 67 | -40.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14752 ((R)-4-[3-(Indan-5-yloxy)-pyrrolidin-1-yl]-6,7-dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 68 | -40.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM14757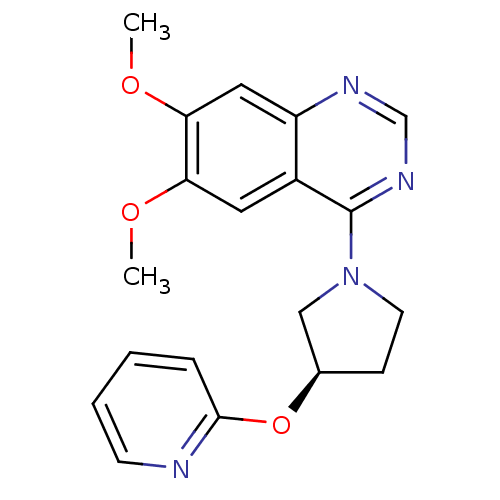 ((R)-6,7-Dimethoxy-4-[3-(pyridin-2-yloxy)-pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM14751 ((R)-4-[3-(3-Ethoxy-phenoxy)-pyrrolidin-1-yl]-6,7-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14751 ((R)-4-[3-(3-Ethoxy-phenoxy)-pyrrolidin-1-yl]-6,7-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 94 | -39.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM14739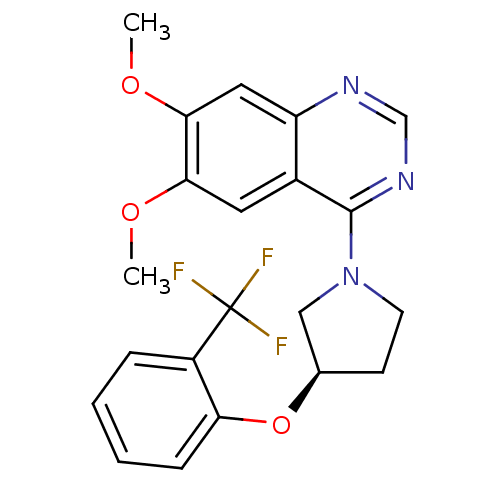 ((R)-6,7-Dimethoxy-4-[3-(2-trifluoromethyl-phenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM14743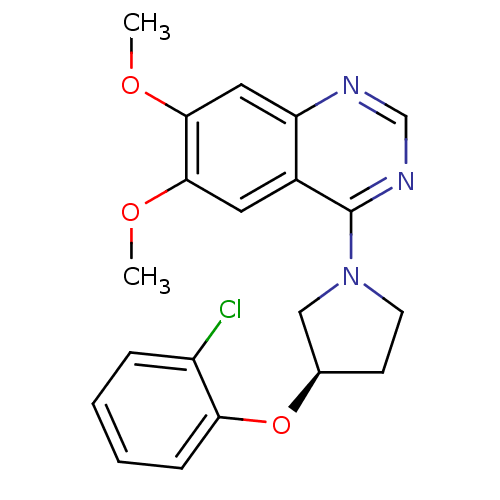 ((R)-4-[3-(2-Chloro-phenoxy)-pyrrolidin-1-yl]-6,7-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM14742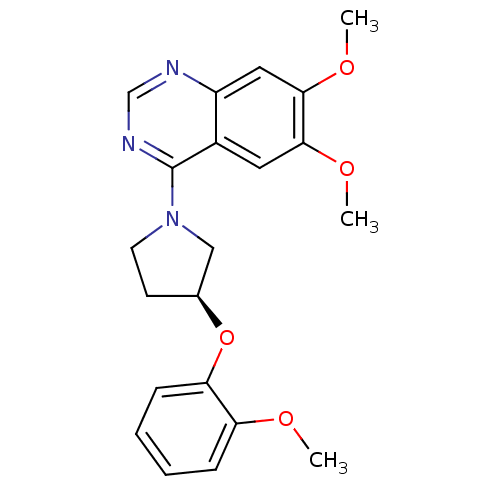 ((S)-6,7-Dimethoxy-4-[3-(2-methoxy-phenoxy)-pyrroli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1409 total ) | Next | Last >> |