

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278899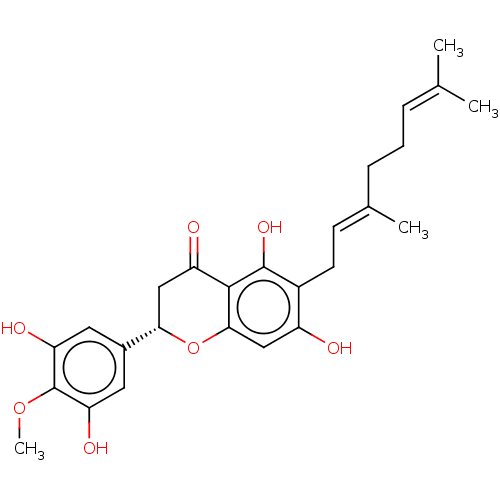 (CHEMBL4174694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50380203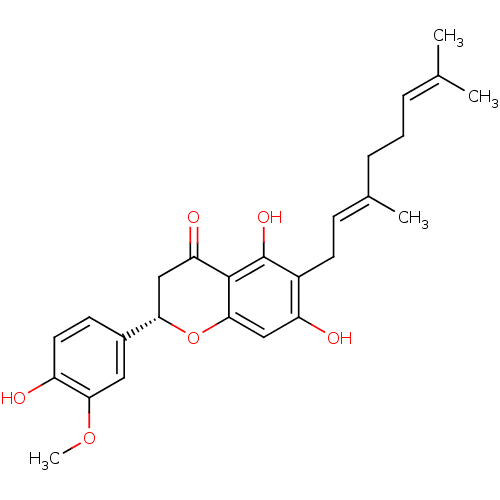 (CHEMBL253152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278908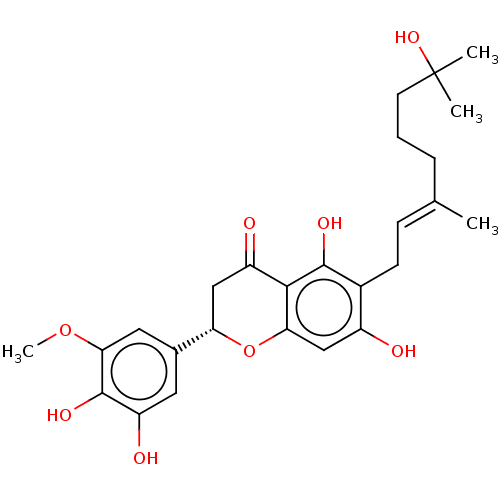 (CHEMBL4160635) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278898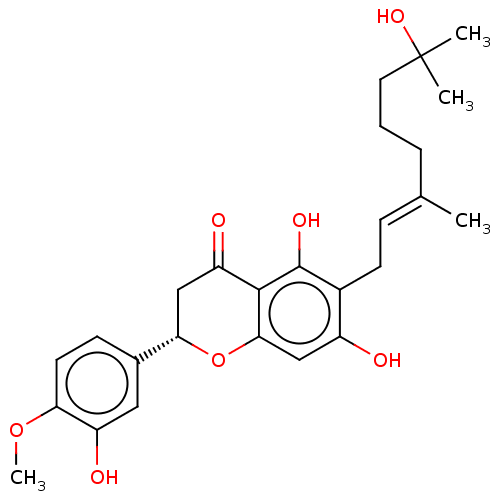 (CHEMBL4167885) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278911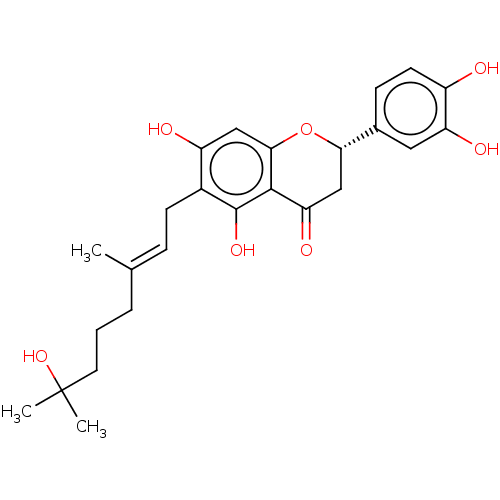 (CHEMBL4171240) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278902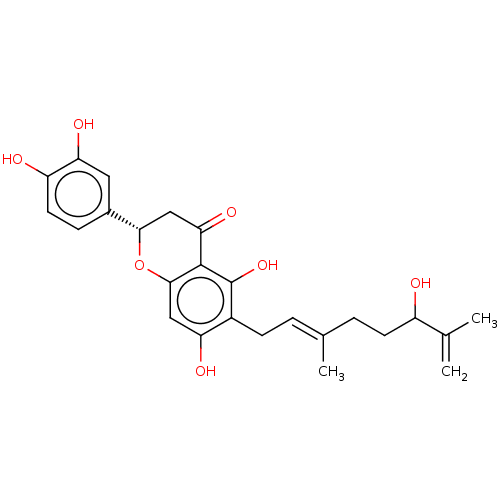 (CHEBI:66191 | tanariflavanone D) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278883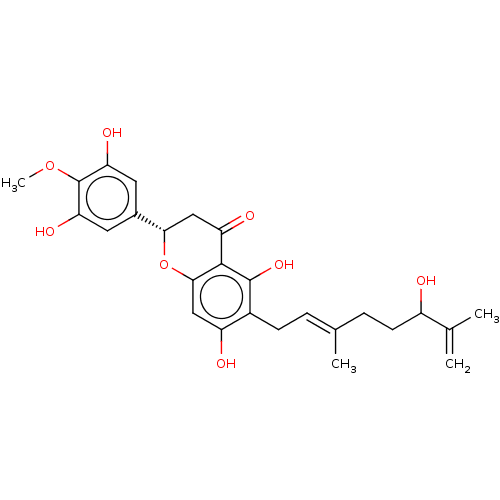 (CHEMBL4167043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278905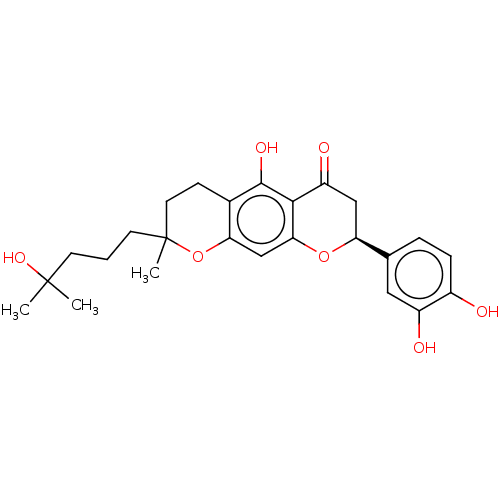 (CHEMBL2387711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278904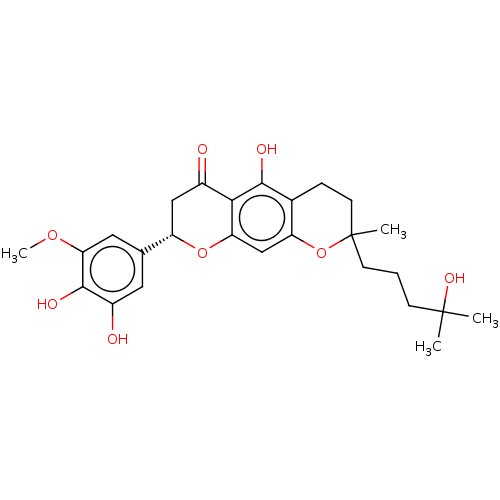 (CHEMBL4164709) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of high affinity radioligand binding to human alphaV-beta3 integrin | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50380205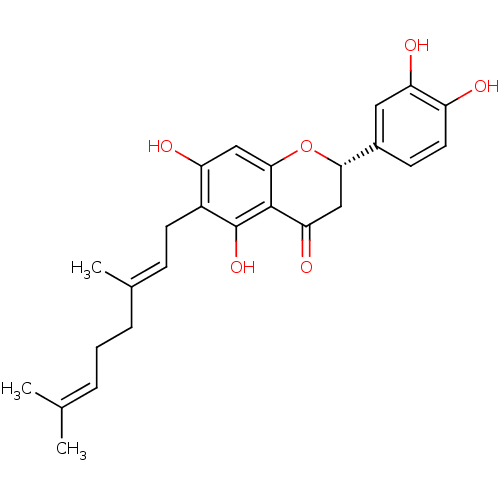 (NYMPHAEOL A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278916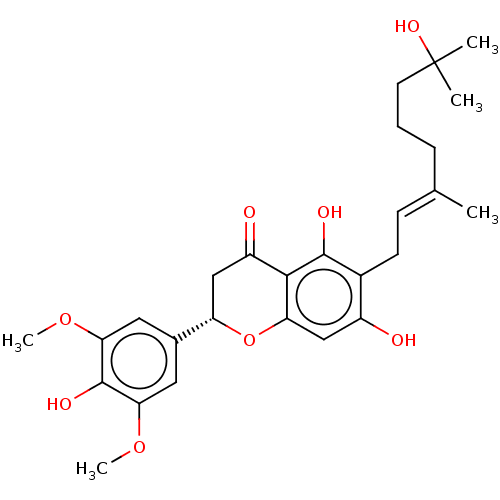 (CHEMBL4167477) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50005781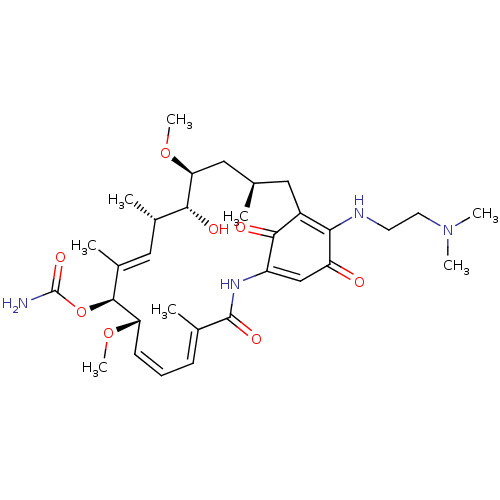 (ALVESPIMYCIN | CHEBI:65324) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of hypoxia-induced HIF1 activation in human AGS cells by reporter gene assay | Bioorg Med Chem Lett 18: 2619-23 (2008) Article DOI: 10.1016/j.bmcl.2008.03.028 BindingDB Entry DOI: 10.7270/Q2Z322FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090474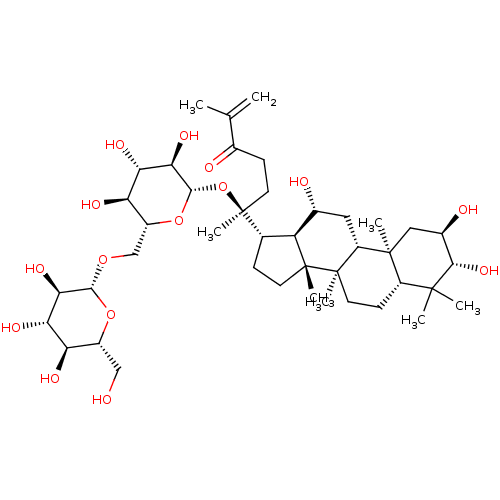 (CHEMBL3581710) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate preincubated for 15 mins prior substrate addition measured after 15 ... | Bioorg Med Chem Lett 22: 2760-3 (2012) Article DOI: 10.1016/j.bmcl.2012.02.088 BindingDB Entry DOI: 10.7270/Q2H41SFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase pre-incubated for 15 mins before p-nitrophenylbutyrate substrate addition by microplate reader based method | Bioorg Med Chem Lett 25: 3455-7 (2015) Article DOI: 10.1016/j.bmcl.2015.07.017 BindingDB Entry DOI: 10.7270/Q2H41T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090504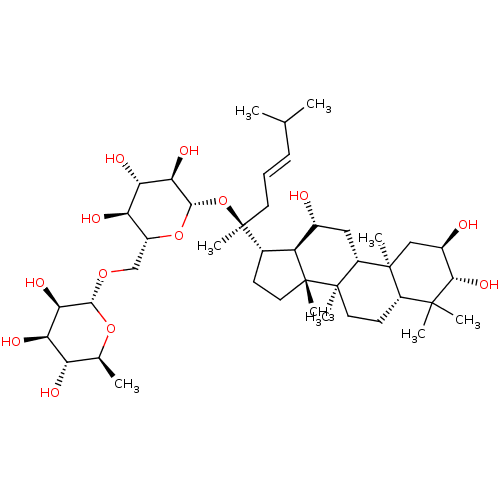 (CHEMBL3581714) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090483 (CHEMBL3581712) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50240041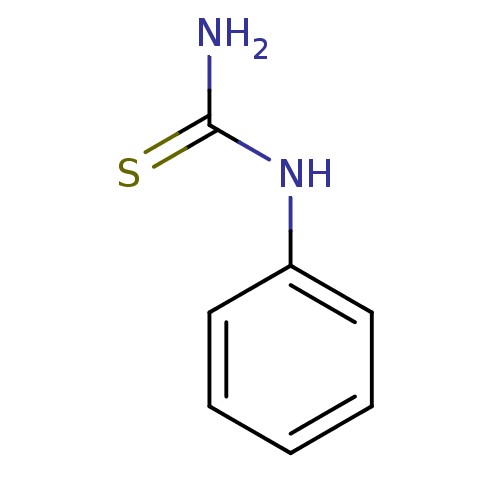 (1-phenyl-2-thiourea | 1-phenylthiourea | CHEMBL263...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of tyrosinase | Bioorg Med Chem 18: 1555-62 (2010) Article DOI: 10.1016/j.bmc.2010.01.005 BindingDB Entry DOI: 10.7270/Q23F4PQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278908 (CHEMBL4160635) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278899 (CHEMBL4174694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090486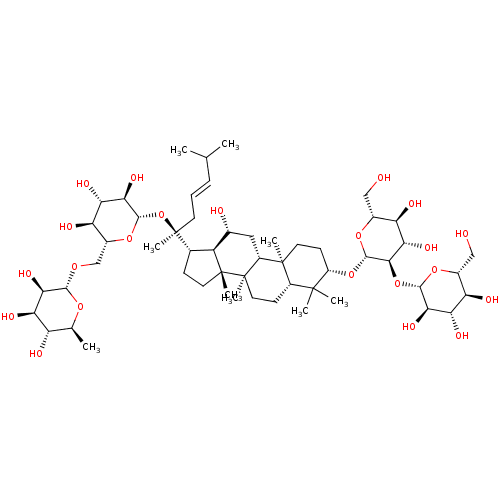 (CHEMBL3581713) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50478313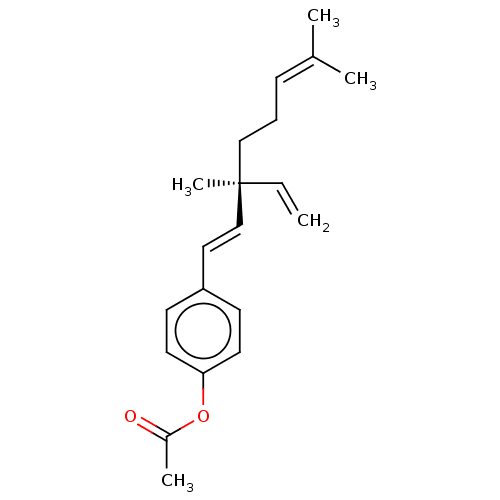 (Acetylbakuchiol) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of hypoxia-induced HIF1 activation in human AGS cells by reporter gene assay | Bioorg Med Chem Lett 18: 2619-23 (2008) Article DOI: 10.1016/j.bmcl.2008.03.028 BindingDB Entry DOI: 10.7270/Q2Z322FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50478311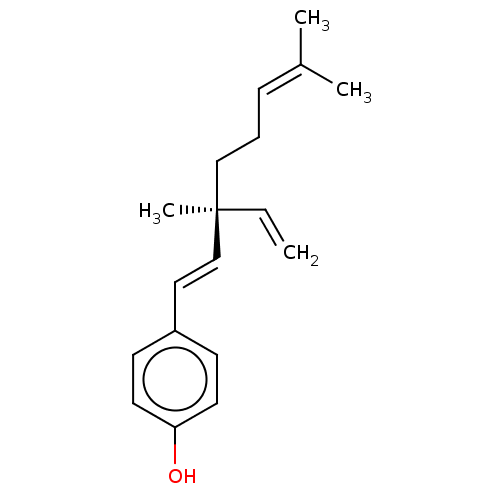 ((S)-Bakuchiol | Bakuchiol | UP 256) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of hypoxia-induced HIF1 activation in human AGS cells by reporter gene assay | Bioorg Med Chem Lett 18: 2619-23 (2008) Article DOI: 10.1016/j.bmcl.2008.03.028 BindingDB Entry DOI: 10.7270/Q2Z322FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50251013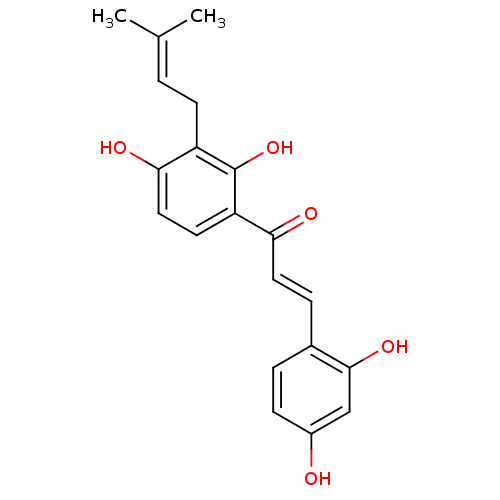 (2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278898 (CHEMBL4167885) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50193719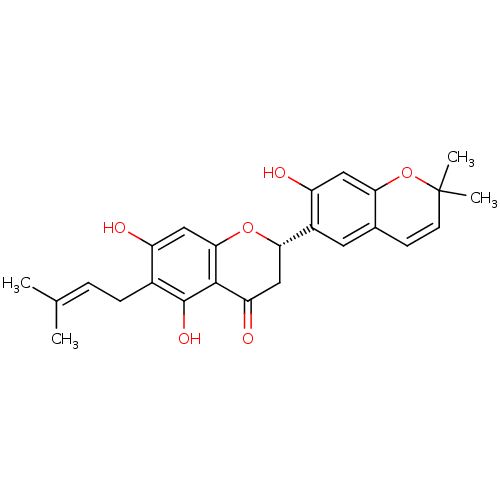 ((S)-5,7,7'-trihydroxy-2',2'-dimethyl-6-(3-methylbu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase pre-incubated for 15 mins before p-nitrophenylbutyrate substrate addition by microplate reader based method | Bioorg Med Chem Lett 25: 3455-7 (2015) Article DOI: 10.1016/j.bmcl.2015.07.017 BindingDB Entry DOI: 10.7270/Q2H41T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278911 (CHEMBL4171240) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50380203 (CHEMBL253152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278905 (CHEMBL2387711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50478315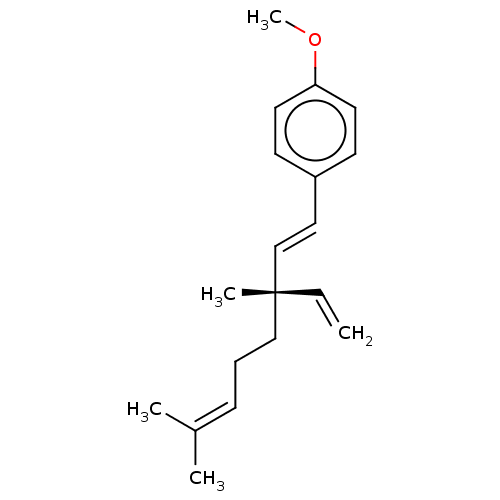 (CHEMBL262630 | O-Methylbakuchiol) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of hypoxia-induced HIF1 activation in human AGS cells by reporter gene assay | Bioorg Med Chem Lett 18: 2619-23 (2008) Article DOI: 10.1016/j.bmcl.2008.03.028 BindingDB Entry DOI: 10.7270/Q2Z322FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50193723 ((S)-2-(2,4-dihydroxy-5-(3-methylbut-2-enyl)phenyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase pre-incubated for 15 mins before p-nitrophenylbutyrate substrate addition by microplate reader based method | Bioorg Med Chem Lett 25: 3455-7 (2015) Article DOI: 10.1016/j.bmcl.2015.07.017 BindingDB Entry DOI: 10.7270/Q2H41T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278883 (CHEMBL4167043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278902 (CHEBI:66191 | tanariflavanone D) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090480 (CHEMBL3581705) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090481 (CHEMBL3581711) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278904 (CHEMBL4164709) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50242015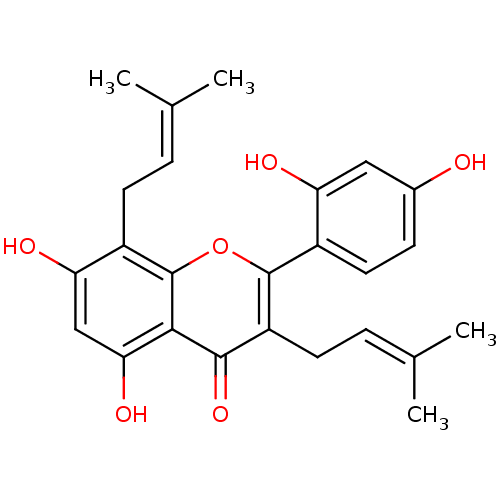 (CHEMBL518543 | Kuwanon C, 4 | kuwanon C) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090475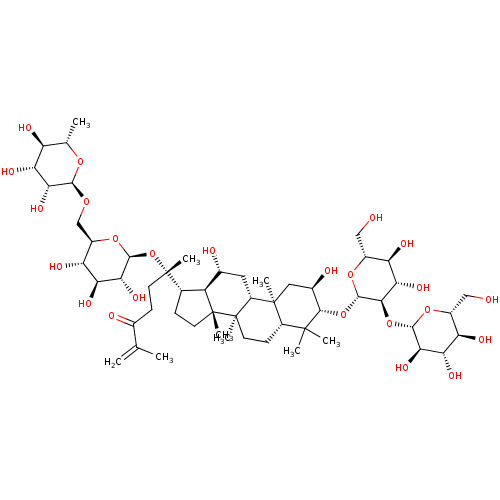 (CHEMBL3581709) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50380205 (NYMPHAEOL A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278916 (CHEMBL4167477) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50478312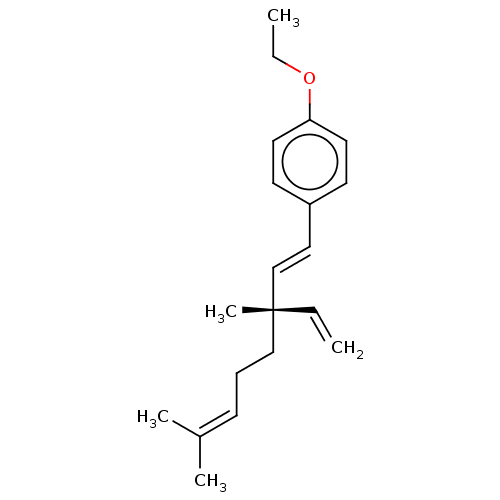 (CHEMBL260776 | O-Ethylbakuchiol) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of hypoxia-induced HIF1 activation in human AGS cells by reporter gene assay | Bioorg Med Chem Lett 18: 2619-23 (2008) Article DOI: 10.1016/j.bmcl.2008.03.028 BindingDB Entry DOI: 10.7270/Q2Z322FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50381001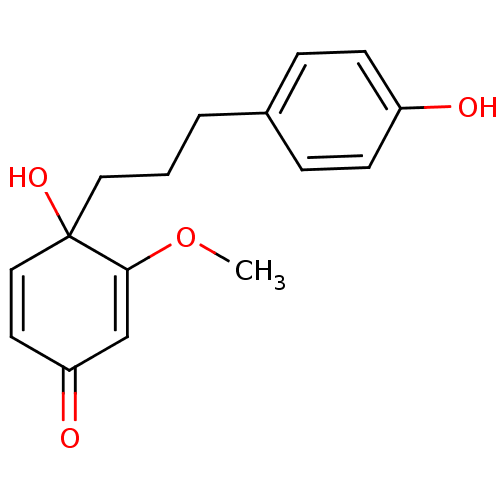 (CHEMBL2017114) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate preincubated for 15 mins prior substrate addition measured after 15 ... | Bioorg Med Chem Lett 22: 2760-3 (2012) Article DOI: 10.1016/j.bmcl.2012.02.088 BindingDB Entry DOI: 10.7270/Q2H41SFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50083074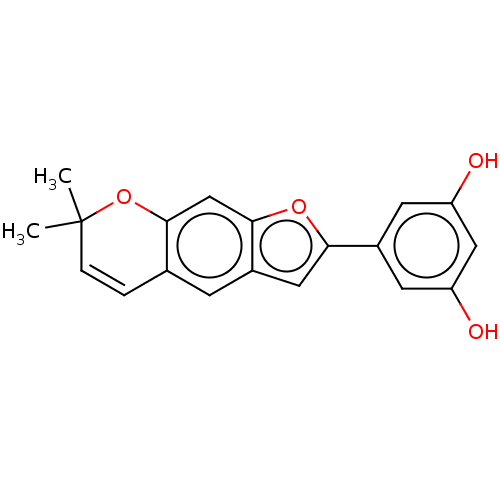 (CHEMBL3422851) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50251014 (CHEMBL465881 | moracin N) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 131 total ) | Next | Last >> |