

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50114673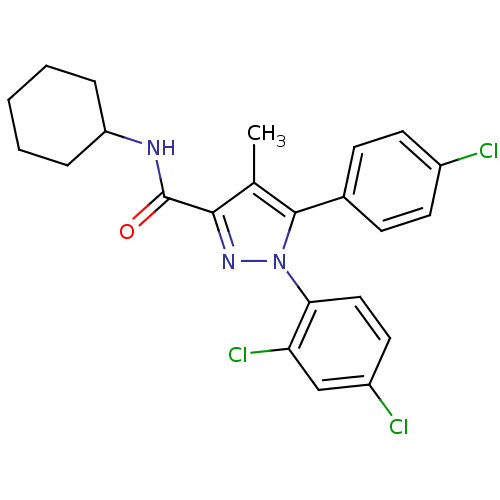 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50114673 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]WIN55,212-2 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50114673 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195532 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]WIN55,212-2 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195532 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]WIN55,212-2 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50140224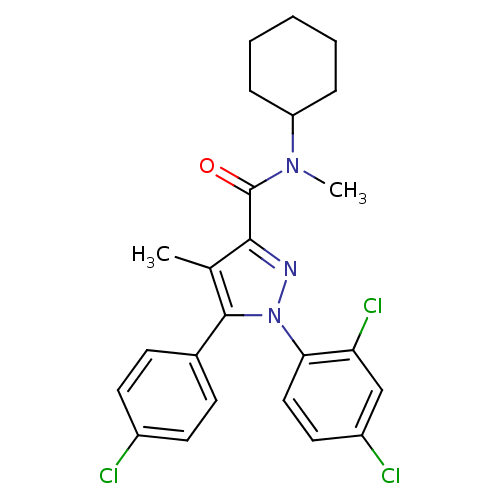 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195530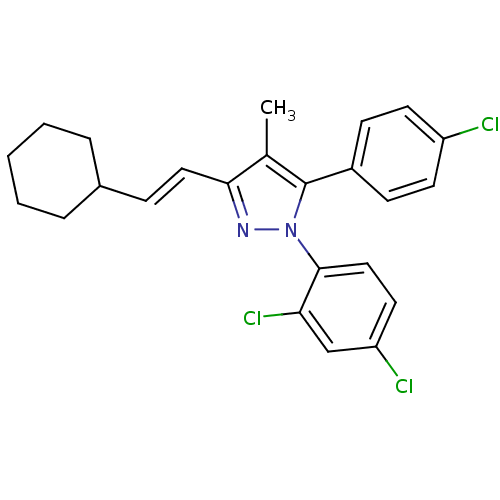 (5-(4-chlorophenyl)-3-[(E)-2-cyclohexylethenyl]-1-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50140224 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195532 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195530 (5-(4-chlorophenyl)-3-[(E)-2-cyclohexylethenyl]-1-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]WIN55,212-2 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195530 (5-(4-chlorophenyl)-3-[(E)-2-cyclohexylethenyl]-1-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50140224 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]WIN55,212-2 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195531 (1-[2-(5-(4-chlorophenyl)-1(2,4-dichlorophenyl)-4-m...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 213 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195531 (1-[2-(5-(4-chlorophenyl)-1(2,4-dichlorophenyl)-4-m...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]WIN55,212-2 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50195531 (1-[2-(5-(4-chlorophenyl)-1(2,4-dichlorophenyl)-4-m...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415825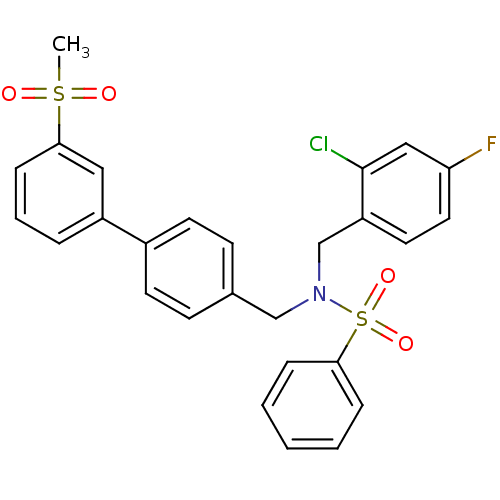 (CHEMBL1091034) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assay | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415821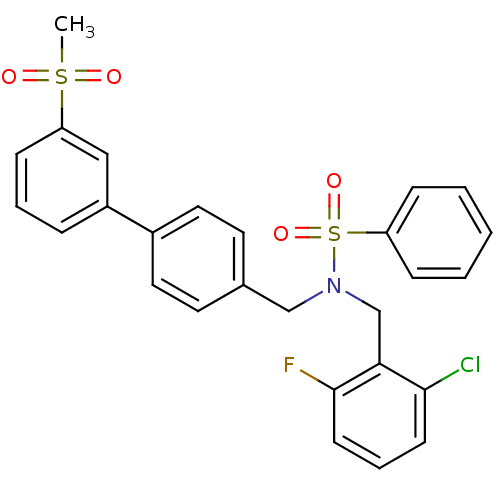 (CHEMBL1093554) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assay | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415820 (CHEMBL1093840) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assay | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assay | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415815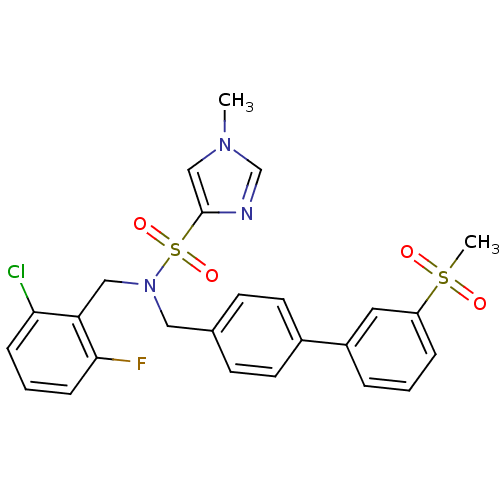 (CHEMBL1092952) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assay | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415819 (CHEMBL1091976) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assay | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415818 (CHEMBL1093523) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assay | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assay | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415816 (CHEMBL1093266 | GSK-2033) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assay | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at LXRbeta ligand binding domain assessed as inhibition of T1317-induced transcriptional activity in african green monkey CV1 cel... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415817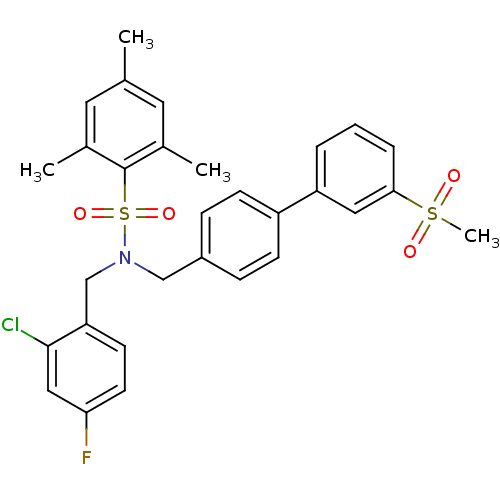 (CHEMBL1093828) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assay | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at LXRalpha ligand binding domain assessed as inhibition of T1317-induced transcriptional activity in african green monkey CV1 ce... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415826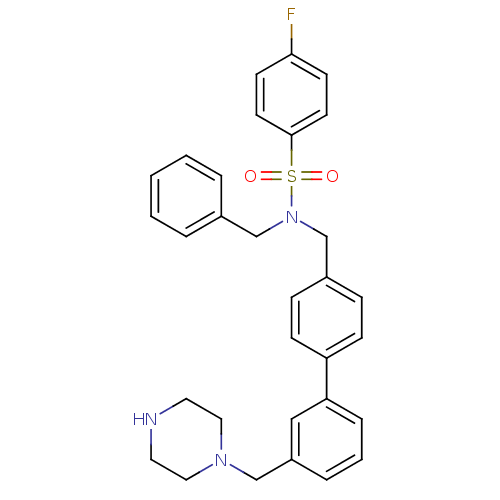 (CHEMBL1090238) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assay | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415824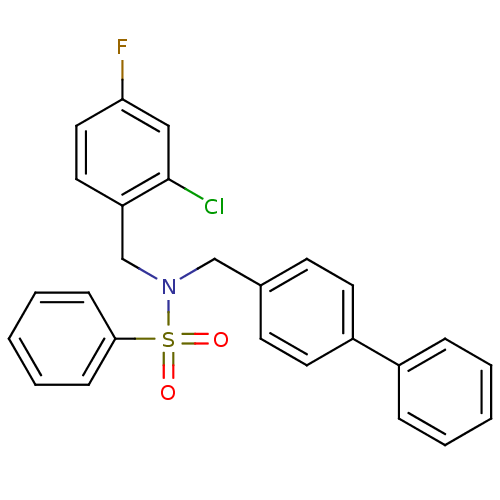 (CHEMBL1090569) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assay | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415822 (CHEMBL1091958) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assay | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415823 (CHEMBL1090570) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assay | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50415825 (CHEMBL1091034) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRalpha ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 ... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50415821 (CHEMBL1093554) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRalpha ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 ... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50415820 (CHEMBL1093840) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRalpha ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 ... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50415819 (CHEMBL1091976) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRalpha ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 ... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50415818 (CHEMBL1093523) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRalpha ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 ... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50415817 (CHEMBL1093828) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 126 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRalpha ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 ... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRalpha ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 ... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 794 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRalpha ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 ... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 501 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRbeta ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 b... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50415815 (CHEMBL1092952) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRalpha ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 ... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415815 (CHEMBL1092952) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRbeta ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 b... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRbeta ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 b... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Greensboro Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 49: 5969-87 (2006) Article DOI: 10.1021/jm060446b BindingDB Entry DOI: 10.7270/Q26H4H28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415818 (CHEMBL1093523) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRbeta ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 b... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415817 (CHEMBL1093828) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRbeta ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 b... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415819 (CHEMBL1091976) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRbeta ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 b... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415821 (CHEMBL1093554) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRbeta ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 b... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |