

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930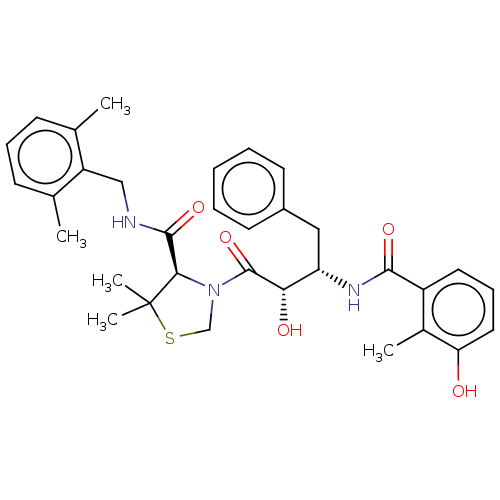 (CHEMBL584130 | KNI-814) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM50084033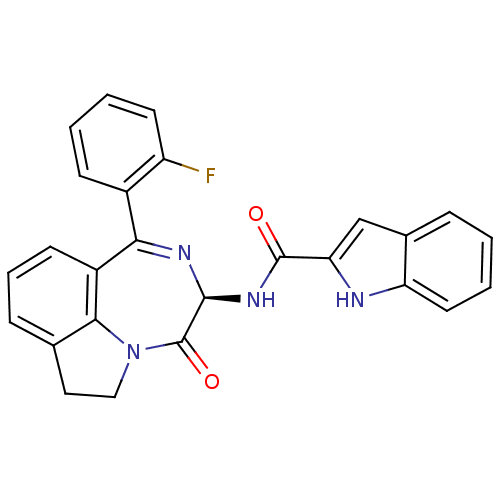 (1H-Indole-2-carboxylic acid [(R)-1-(2-fluoro-pheny...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 571-5 (1994) BindingDB Entry DOI: 10.7270/Q2WQ029S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50547388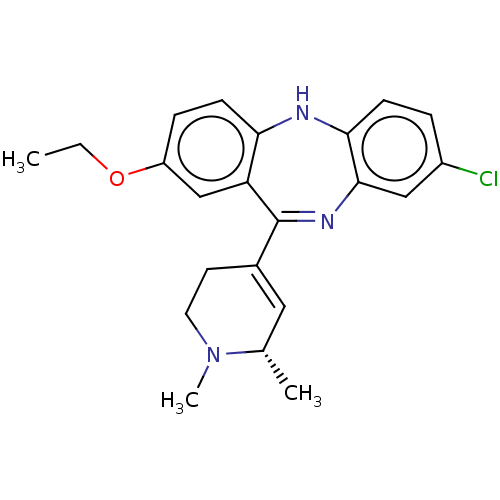 (CHEMBL4758966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human 5HT2A receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127563 BindingDB Entry DOI: 10.7270/Q26113Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50547385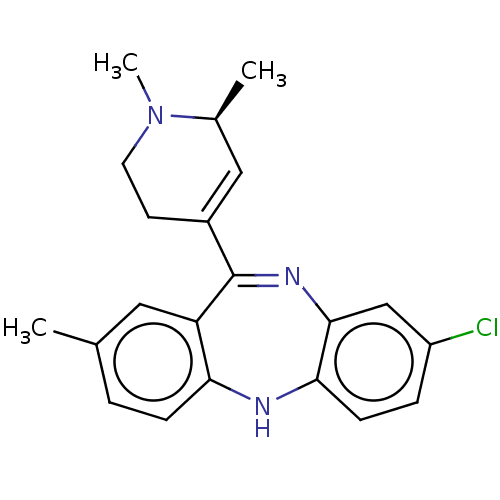 (CHEMBL4745124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human 5HT2A receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127563 BindingDB Entry DOI: 10.7270/Q26113Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323472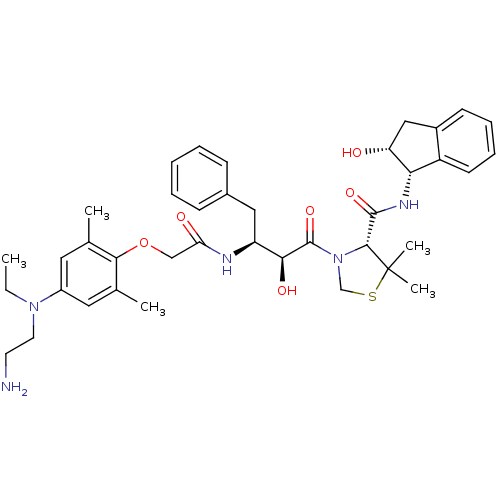 ((R)-3-((2S,3S)-3-(2-(4-((2-aminoethyl)(ethyl)amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323469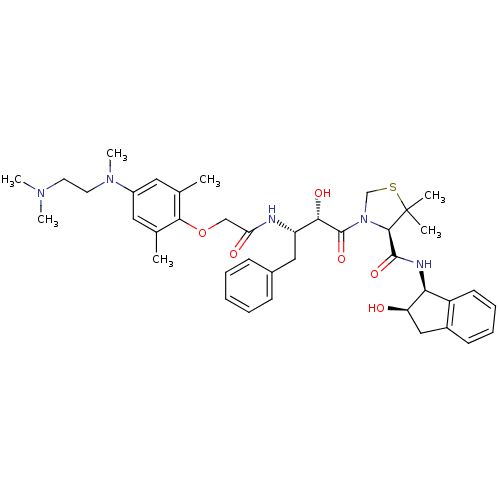 ((R)-3-((2S,3S)-3-(2-(4-((2-(dimethylamino)ethyl)(m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50253136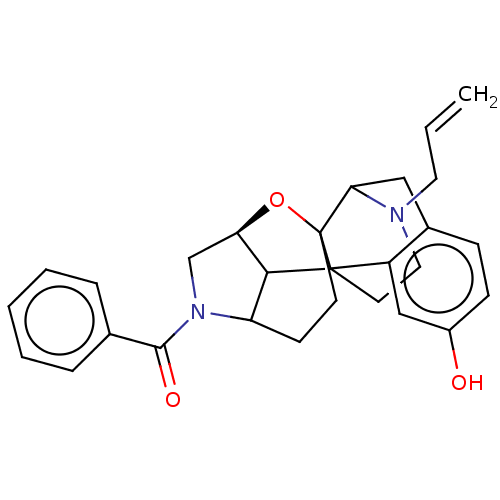 (CHEMBL4075409) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, School of Pharmacy, Kitasato University, 5-9-1, Shirokane, Minato-ku, Tokyo 108-8641, Japan; Discovery Research Laboratories, Nippon Chemiphar Co., Ltd., 1-22, Hiko Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum membrane | Bioorg Med Chem Lett 27: 2742-2745 (2017) Article DOI: 10.1016/j.bmcl.2017.04.059 BindingDB Entry DOI: 10.7270/Q2H134GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50547397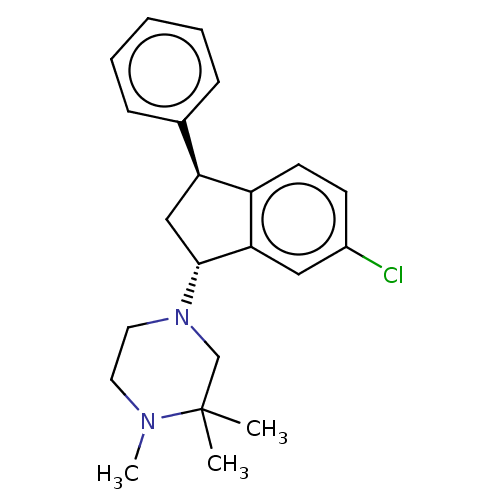 (LU-31-130 | Lu 31-130 | Zicronapine) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human dopamine D1 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127563 BindingDB Entry DOI: 10.7270/Q26113Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50547386 (CHEMBL4760903) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human 5HT2A receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127563 BindingDB Entry DOI: 10.7270/Q26113Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50547389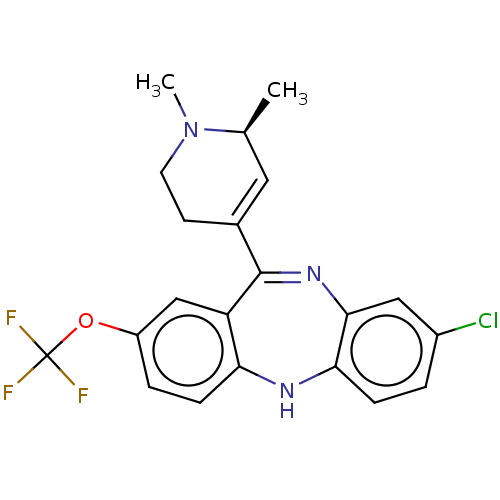 (CHEMBL4745489) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human 5HT2A receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127563 BindingDB Entry DOI: 10.7270/Q26113Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50253129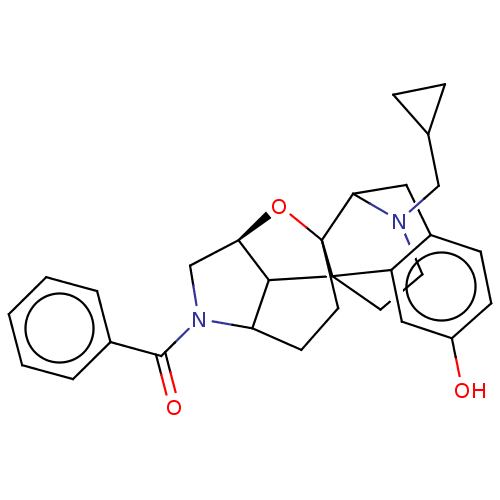 (CHEMBL4066752) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, School of Pharmacy, Kitasato University, 5-9-1, Shirokane, Minato-ku, Tokyo 108-8641, Japan; Discovery Research Laboratories, Nippon Chemiphar Co., Ltd., 1-22, Hiko Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 27: 2742-2745 (2017) Article DOI: 10.1016/j.bmcl.2017.04.059 BindingDB Entry DOI: 10.7270/Q2H134GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50454212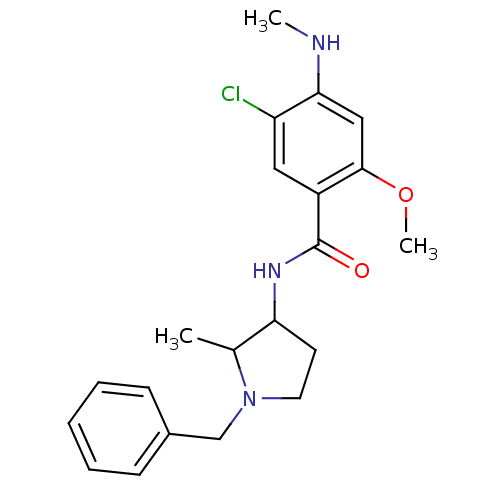 (CHEBI:64217 | Emonapride | Nemonapride) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50454212 (CHEBI:64217 | Emonapride | Nemonapride) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121729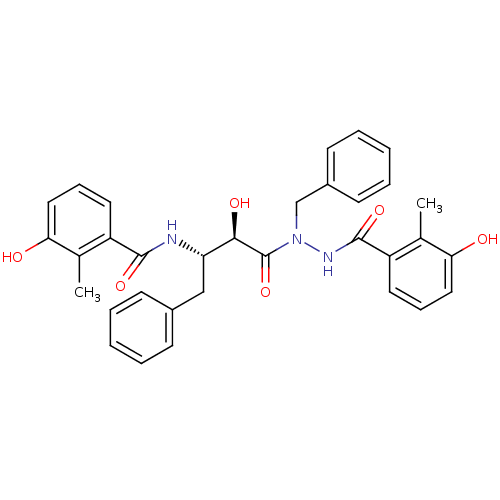 (CHEMBL368169 | KNI-1167 | N-[(S)-3-[N-Benzyl-N'-(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50355491 (CHEMBL1835870) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Inhibition of human recombinant FLT3 by radiometric assay | J Med Chem 55: 725-34 (2012) Article DOI: 10.1021/jm201198w BindingDB Entry DOI: 10.7270/Q2GQ6Z6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480931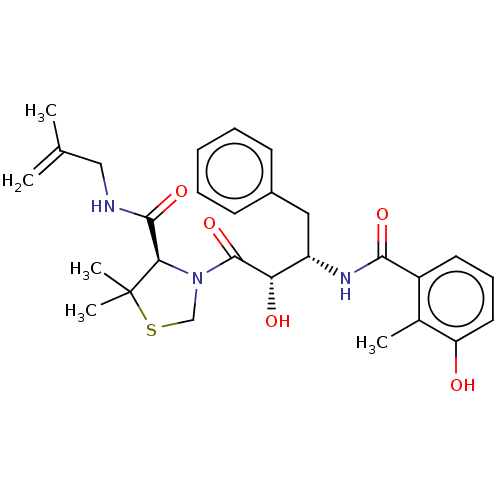 (CHEMBL575512 | KNI-1614) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50547390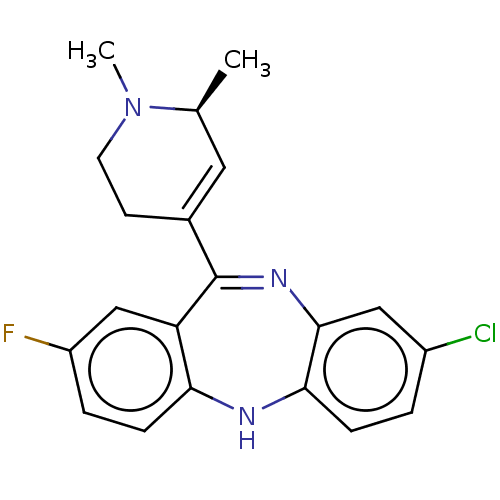 (CHEMBL4758723) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human dopamine D1 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127563 BindingDB Entry DOI: 10.7270/Q26113Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209553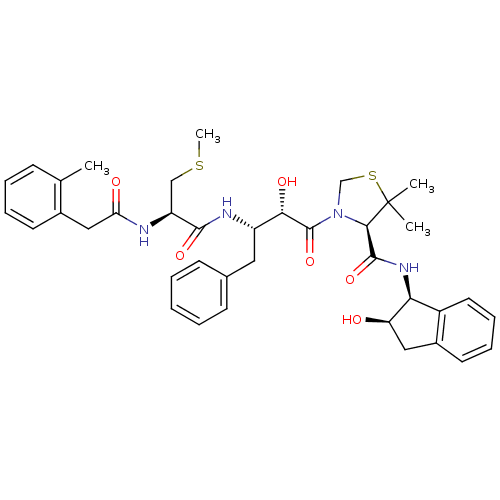 ((R)-N-((1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 17: 3048-52 (2007) Article DOI: 10.1016/j.bmcl.2007.03.052 BindingDB Entry DOI: 10.7270/Q2J102VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50254132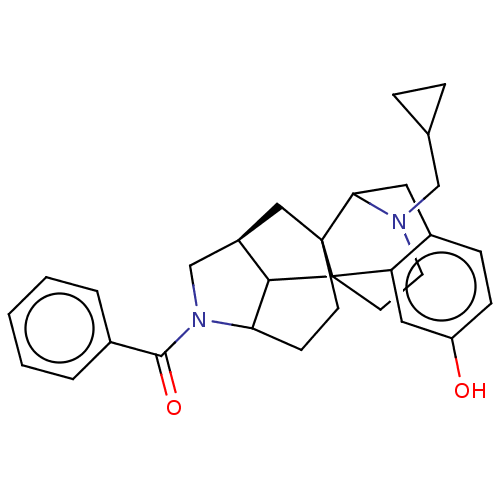 (CHEMBL4083940) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.211 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Chemiphar Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human recombinant DOR expressed in CHO cell membranes | Bioorg Med Chem Lett 27: 3495-3498 (2017) Article DOI: 10.1016/j.bmcl.2017.05.072 BindingDB Entry DOI: 10.7270/Q25H7JPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50253140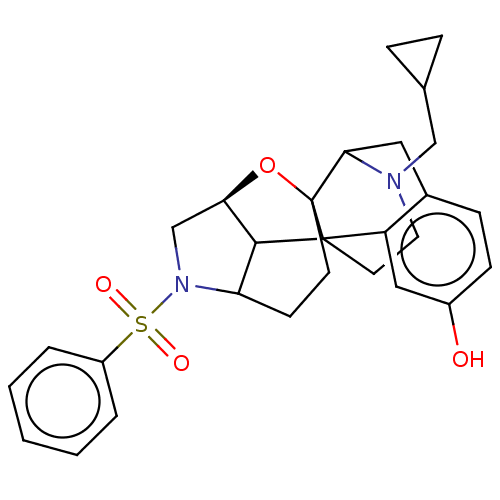 (CHEMBL4081856) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, School of Pharmacy, Kitasato University, 5-9-1, Shirokane, Minato-ku, Tokyo 108-8641, Japan; Discovery Research Laboratories, Nippon Chemiphar Co., Ltd., 1-22, Hiko Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 27: 2742-2745 (2017) Article DOI: 10.1016/j.bmcl.2017.04.059 BindingDB Entry DOI: 10.7270/Q2H134GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50254124 (CHEMBL4103044) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Chemiphar Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from human recombinant KOR expressed in CHO cell membranes | Bioorg Med Chem Lett 27: 3495-3498 (2017) Article DOI: 10.1016/j.bmcl.2017.05.072 BindingDB Entry DOI: 10.7270/Q25H7JPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50274257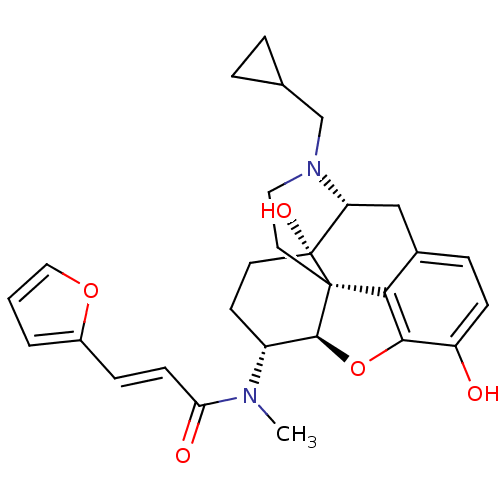 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from guinea pig cerebellum kappa opioid receptor | Bioorg Med Chem Lett 20: 121-4 (2010) Article DOI: 10.1016/j.bmcl.2009.11.027 BindingDB Entry DOI: 10.7270/Q2GX4CJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 571-5 (1994) BindingDB Entry DOI: 10.7270/Q2WQ029S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052196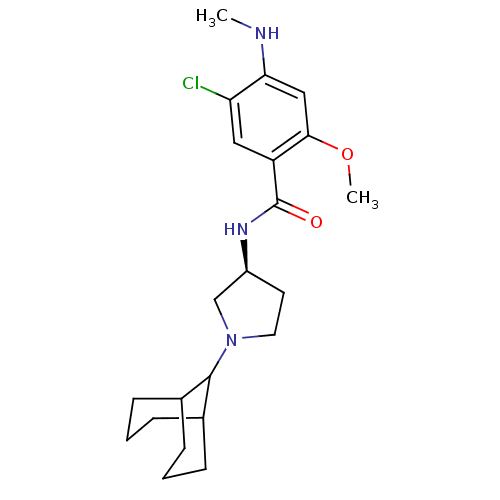 (CHEMBL319597 | N-((S)-1-Bicyclo[3.3.1]non-9-yl-pyr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121730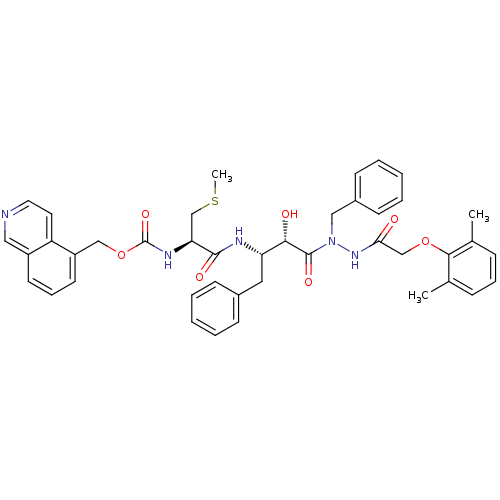 (CHEMBL366433 | {1-[(S)-3-{N-Benzyl-N'-[2-(2,6-dime...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50254133 (CHEMBL4076632) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Chemiphar Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human recombinant DOR expressed in CHO cell membranes | Bioorg Med Chem Lett 27: 3495-3498 (2017) Article DOI: 10.1016/j.bmcl.2017.05.072 BindingDB Entry DOI: 10.7270/Q25H7JPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50253129 (CHEMBL4066752) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.274 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, School of Pharmacy, Kitasato University, 5-9-1, Shirokane, Minato-ku, Tokyo 108-8641, Japan; Discovery Research Laboratories, Nippon Chemiphar Co., Ltd., 1-22, Hiko Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum membrane | Bioorg Med Chem Lett 27: 2742-2745 (2017) Article DOI: 10.1016/j.bmcl.2017.04.059 BindingDB Entry DOI: 10.7270/Q2H134GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Adenosine A1 receptor binding using [3H]DPCPX in rat cortical membranes | J Med Chem 42: 779-83 (1999) Article DOI: 10.1021/jm980671w BindingDB Entry DOI: 10.7270/Q2J105WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50253138 (CHEMBL4076303) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.301 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, School of Pharmacy, Kitasato University, 5-9-1, Shirokane, Minato-ku, Tokyo 108-8641, Japan; Discovery Research Laboratories, Nippon Chemiphar Co., Ltd., 1-22, Hiko Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum membrane | Bioorg Med Chem Lett 27: 2742-2745 (2017) Article DOI: 10.1016/j.bmcl.2017.04.059 BindingDB Entry DOI: 10.7270/Q2H134GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50253140 (CHEMBL4081856) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.308 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, School of Pharmacy, Kitasato University, 5-9-1, Shirokane, Minato-ku, Tokyo 108-8641, Japan; Discovery Research Laboratories, Nippon Chemiphar Co., Ltd., 1-22, Hiko Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 27: 2742-2745 (2017) Article DOI: 10.1016/j.bmcl.2017.04.059 BindingDB Entry DOI: 10.7270/Q2H134GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50010652 (CHEMBL3264742) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain cerebellum membrane | ACS Med Chem Lett 5: 368-72 (2014) Article DOI: 10.1021/ml400491k BindingDB Entry DOI: 10.7270/Q2GF0W23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50254124 (CHEMBL4103044) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Chemiphar Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human recombinant DOR expressed in CHO cell membranes | Bioorg Med Chem Lett 27: 3495-3498 (2017) Article DOI: 10.1016/j.bmcl.2017.05.072 BindingDB Entry DOI: 10.7270/Q25H7JPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50253129 (CHEMBL4066752) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, School of Pharmacy, Kitasato University, 5-9-1, Shirokane, Minato-ku, Tokyo 108-8641, Japan; Discovery Research Laboratories, Nippon Chemiphar Co., Ltd., 1-22, Hiko Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain without cerebellum membrane | Bioorg Med Chem Lett 27: 2742-2745 (2017) Article DOI: 10.1016/j.bmcl.2017.04.059 BindingDB Entry DOI: 10.7270/Q2H134GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50254133 (CHEMBL4076632) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Chemiphar Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from human recombinant KOR expressed in CHO cell membranes | Bioorg Med Chem Lett 27: 3495-3498 (2017) Article DOI: 10.1016/j.bmcl.2017.05.072 BindingDB Entry DOI: 10.7270/Q25H7JPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50254131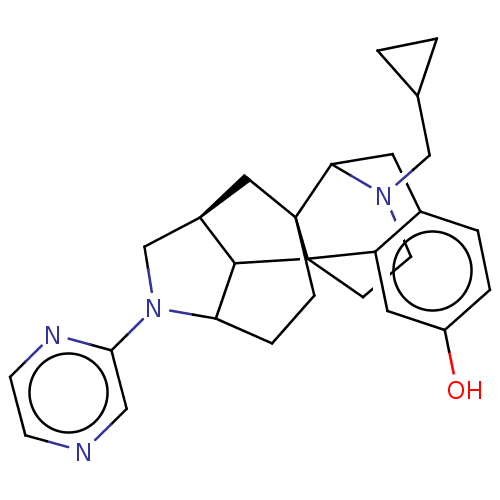 (CHEMBL4094845) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.356 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Chemiphar Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human recombinant DOR expressed in CHO cell membranes | Bioorg Med Chem Lett 27: 3495-3498 (2017) Article DOI: 10.1016/j.bmcl.2017.05.072 BindingDB Entry DOI: 10.7270/Q25H7JPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50254132 (CHEMBL4083940) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.362 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Chemiphar Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from human recombinant KOR expressed in CHO cell membranes | Bioorg Med Chem Lett 27: 3495-3498 (2017) Article DOI: 10.1016/j.bmcl.2017.05.072 BindingDB Entry DOI: 10.7270/Q25H7JPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50253137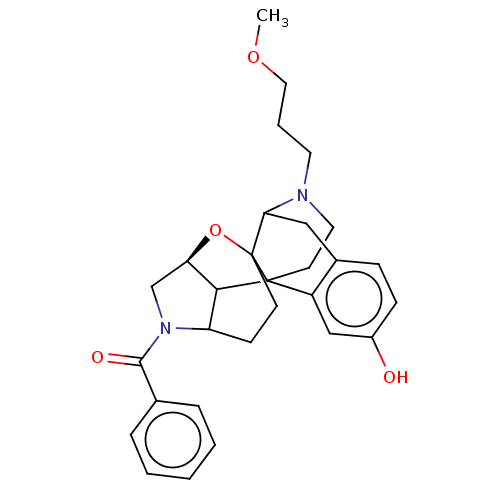 (CHEMBL4076673) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 0.368 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, School of Pharmacy, Kitasato University, 5-9-1, Shirokane, Minato-ku, Tokyo 108-8641, Japan; Discovery Research Laboratories, Nippon Chemiphar Co., Ltd., 1-22, Hiko Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum membrane | Bioorg Med Chem Lett 27: 2742-2745 (2017) Article DOI: 10.1016/j.bmcl.2017.04.059 BindingDB Entry DOI: 10.7270/Q2H134GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50253138 (CHEMBL4076303) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.383 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, School of Pharmacy, Kitasato University, 5-9-1, Shirokane, Minato-ku, Tokyo 108-8641, Japan; Discovery Research Laboratories, Nippon Chemiphar Co., Ltd., 1-22, Hiko Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain without cerebellum membrane | Bioorg Med Chem Lett 27: 2742-2745 (2017) Article DOI: 10.1016/j.bmcl.2017.04.059 BindingDB Entry DOI: 10.7270/Q2H134GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50084033 (1H-Indole-2-carboxylic acid [(R)-1-(2-fluoro-pheny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 571-5 (1994) BindingDB Entry DOI: 10.7270/Q2WQ029S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM35254 (2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human 5HT2A receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127563 BindingDB Entry DOI: 10.7270/Q26113Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50254124 (CHEMBL4103044) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Chemiphar Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant MOR expressed in CHO cell membranes | Bioorg Med Chem Lett 27: 3495-3498 (2017) Article DOI: 10.1016/j.bmcl.2017.05.072 BindingDB Entry DOI: 10.7270/Q25H7JPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50253139 (CHEMBL4096314) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Patents | Article PubMed | 0.458 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, School of Pharmacy, Kitasato University, 5-9-1, Shirokane, Minato-ku, Tokyo 108-8641, Japan; Discovery Research Laboratories, Nippon Chemiphar Co., Ltd., 1-22, Hiko Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum membrane | Bioorg Med Chem Lett 27: 2742-2745 (2017) Article DOI: 10.1016/j.bmcl.2017.04.059 BindingDB Entry DOI: 10.7270/Q2H134GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50254123 (CHEMBL4068691) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.458 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Chemiphar Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human recombinant DOR expressed in CHO cell membranes | Bioorg Med Chem Lett 27: 3495-3498 (2017) Article DOI: 10.1016/j.bmcl.2017.05.072 BindingDB Entry DOI: 10.7270/Q25H7JPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121732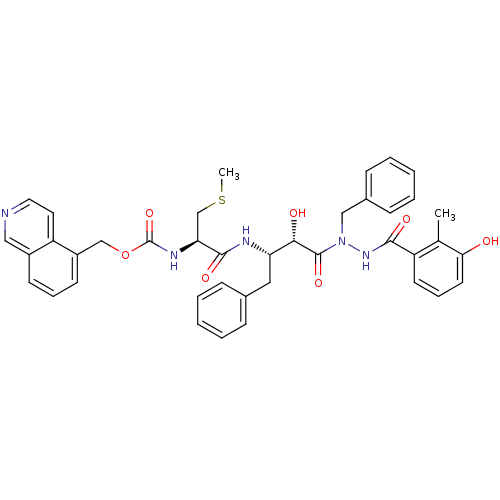 (CHEMBL172850 | {1-[(S)-3-[N-Benzyl-N'-(3-hydroxy-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50254131 (CHEMBL4094845) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.479 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Chemiphar Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from human recombinant KOR expressed in CHO cell membranes | Bioorg Med Chem Lett 27: 3495-3498 (2017) Article DOI: 10.1016/j.bmcl.2017.05.072 BindingDB Entry DOI: 10.7270/Q25H7JPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50547394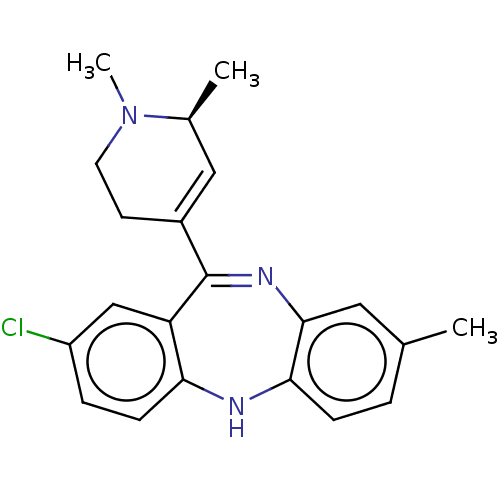 (CHEMBL4756254) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human dopamine D1 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127563 BindingDB Entry DOI: 10.7270/Q26113Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50079652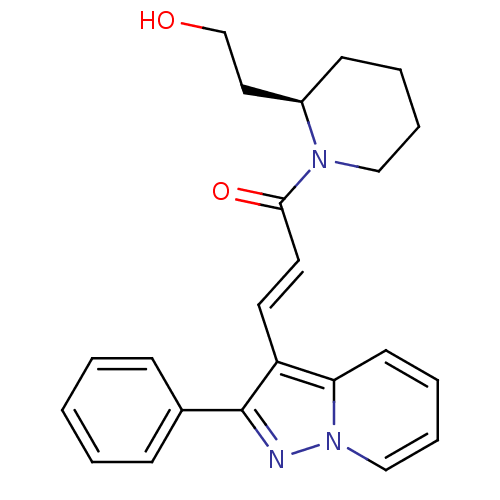 ((E)-1-[(R)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Adenosine A1 receptor binding using [3H]DPCPX in human cortical membranes | J Med Chem 42: 779-83 (1999) Article DOI: 10.1021/jm980671w BindingDB Entry DOI: 10.7270/Q2J105WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50079652 ((E)-1-[(R)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Adenosine A1 receptor binding using [3H]DPCOX in rat cortical membranes | J Med Chem 42: 779-83 (1999) Article DOI: 10.1021/jm980671w BindingDB Entry DOI: 10.7270/Q2J105WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209559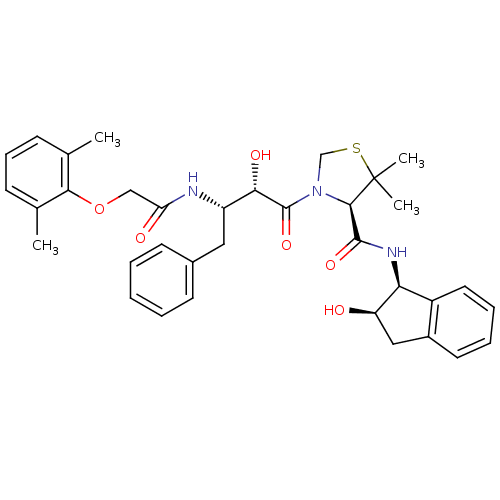 ((4R)-3-[(2S,3S)-3-{[(2,6-dimethylphenoxy)acetyl]am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4119 total ) | Next | Last >> |