 Found 10484 hits with Last Name = 'ide' and Initial = 's'
Found 10484 hits with Last Name = 'ide' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]nociceptin from human recombinant NOP receptor expressed in HEK293 cells by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00256
BindingDB Entry DOI: 10.7270/Q2VX0MDH |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 1
(Homo sapiens (Human)) | BDBM50029188

(CHEMBL2165920)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C45H72N14O10/c1-24(2)21-31(57-40(65)30(23-35(47)61)56-42(67)33-15-10-20-59(33)44(69)36(48)25(3)4)43(68)58-19-9-14-32(58)41(66)54-28(16-17-34(46)60)39(64)53-27(13-8-18-52-45(50)51)38(63)55-29(37(49)62)22-26-11-6-5-7-12-26/h5-7,11-12,24-25,27-33,36H,8-10,13-23,48H2,1-4H3,(H2,46,60)(H2,47,61)(H2,49,62)(H,53,64)(H,54,66)(H,55,63)(H,56,67)(H,57,65)(H4,50,51,52)/t27-,28-,29-,30-,31-,32-,33-,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-1DMeNPFF from recombinant human NPFF1 receptor expressed in CHO cells by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00256
BindingDB Entry DOI: 10.7270/Q2VX0MDH |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 2
(Homo sapiens (Human)) | BDBM50037557
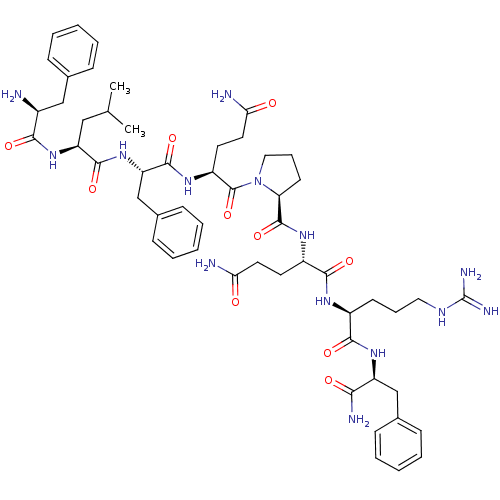
(2-{[1-(2-{2-[2-(2-Amino-3-phenyl-propionylamino)-4...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H76N14O10/c1-32(2)28-41(66-47(72)36(55)29-33-14-6-3-7-15-33)50(75)67-42(31-35-18-10-5-11-19-35)51(76)64-39(23-25-45(57)70)53(78)68-27-13-21-43(68)52(77)63-38(22-24-44(56)69)49(74)62-37(20-12-26-61-54(59)60)48(73)65-40(46(58)71)30-34-16-8-4-9-17-34/h3-11,14-19,32,36-43H,12-13,20-31,55H2,1-2H3,(H2,56,69)(H2,57,70)(H2,58,71)(H,62,74)(H,63,77)(H,64,76)(H,65,73)(H,66,72)(H,67,75)(H4,59,60,61)/t36-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-1DMeNPFF from recombinant human NPFF2 receptor expressed in CHO cells by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00256
BindingDB Entry DOI: 10.7270/Q2VX0MDH |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123058
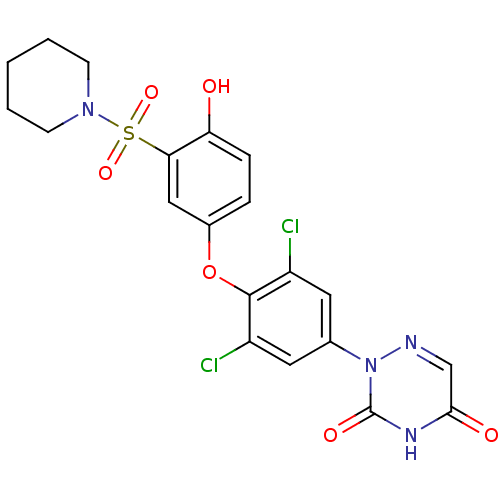
(2-(3,5-dichloro-4-(4-hydroxy-3-(piperidin-1-ylsulf...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)N1CCCCC1 Show InChI InChI=1S/C20H18Cl2N4O6S/c21-14-8-12(26-20(29)24-18(28)11-23-26)9-15(22)19(14)32-13-4-5-16(27)17(10-13)33(30,31)25-6-2-1-3-7-25/h4-5,8-11,27H,1-3,6-7H2,(H,24,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602306
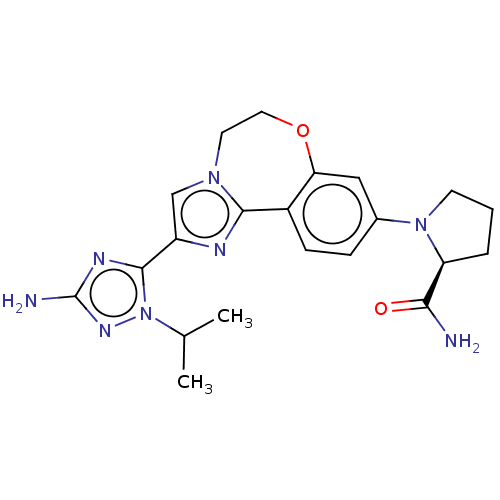
(CHEMBL5208487)Show SMILES CC(C)n1nc(N)nc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149477
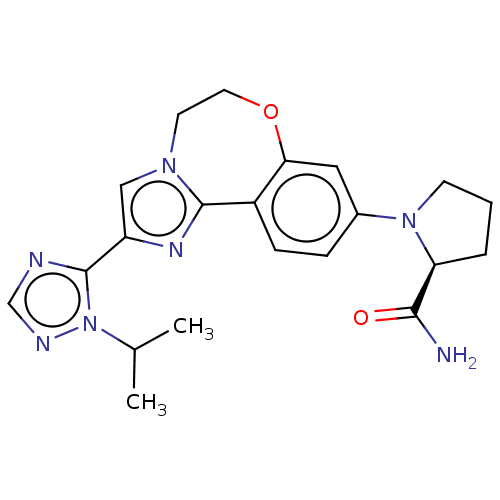
(CHEMBL3770993 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C21H25N7O2/c1-13(2)28-21(23-12-24-28)16-11-26-8-9-30-18-10-14(5-6-15(18)20(26)25-16)27-7-3-4-17(27)19(22)29/h5-6,10-13,17H,3-4,7-9H2,1-2H3,(H2,22,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388901

(CHEMBL2063237)Show SMILES CC(C)C(=O)N[C@@H]1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl |r| Show InChI InChI=1S/C23H23Cl2N3O2/c1-14(2)23(29)26-18-7-5-13-30-22-20(18)27-28(19-8-4-3-6-17(19)25)21(22)15-9-11-16(24)12-10-15/h3-4,6,8-12,14,18H,5,7,13H2,1-2H3,(H,26,29)/t18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123046
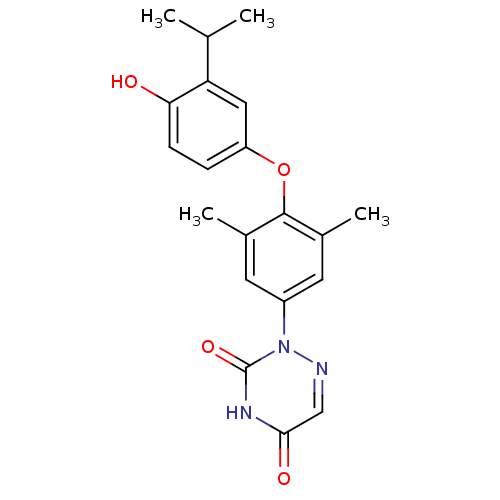
(2-[4-(4-Hydroxy-3-isopropyl-phenoxy)-3,5-dimethyl-...)Show SMILES CC(C)c1cc(Oc2c(C)cc(cc2C)-n2ncc(=O)[nH]c2=O)ccc1O Show InChI InChI=1S/C20H21N3O4/c1-11(2)16-9-15(5-6-17(16)24)27-19-12(3)7-14(8-13(19)4)23-20(26)22-18(25)10-21-23/h5-11,24H,1-4H3,(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM295665
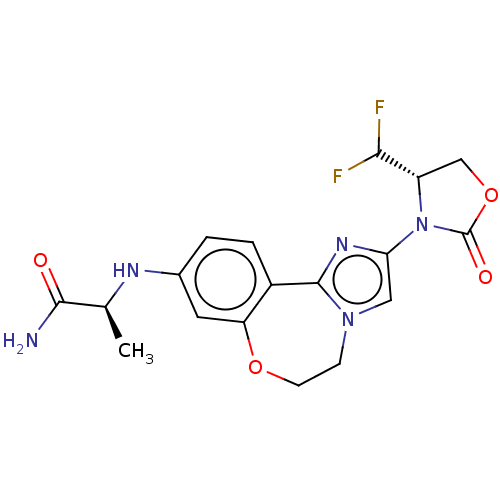
((S)-2-((2-((S)-4-(difluoromethyl)- 2-oxooxazolidin...)Show SMILES C[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F)C(N)=O |r| Show InChI InChI=1S/C18H19F2N5O4/c1-9(16(21)26)22-10-2-3-11-13(6-10)28-5-4-24-7-14(23-17(11)24)25-12(15(19)20)8-29-18(25)27/h2-3,6-7,9,12,15,22H,4-5,8H2,1H3,(H2,21,26)/t9-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123044
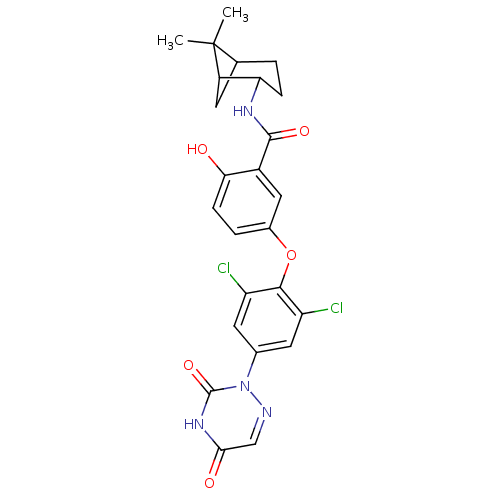
(5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...)Show SMILES CC1(C)C2CC1C(CC2)NC(=O)c1cc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)ccc1O |THB:9:6:1:4| Show InChI InChI=1S/C25H24Cl2N4O5/c1-25(2)12-3-5-19(16(25)7-12)29-23(34)15-10-14(4-6-20(15)32)36-22-17(26)8-13(9-18(22)27)31-24(35)30-21(33)11-28-31/h4,6,8-12,16,19,32H,3,5,7H2,1-2H3,(H,29,34)(H,30,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388885
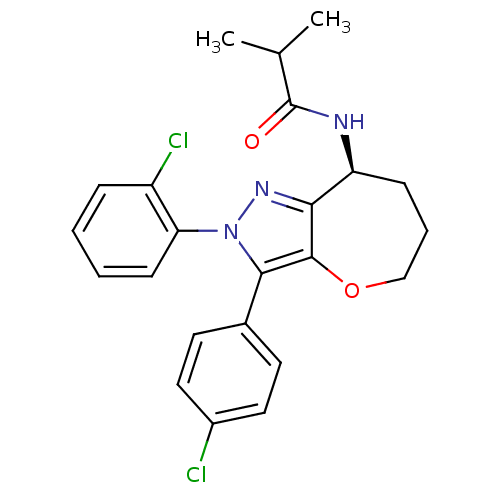
(CHEMBL2063238)Show SMILES CC(C)C(=O)N[C@H]1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl |r| Show InChI InChI=1S/C23H23Cl2N3O2/c1-14(2)23(29)26-18-7-5-13-30-22-20(18)27-28(19-8-4-3-6-17(19)25)21(22)15-9-11-16(24)12-10-15/h3-4,6,8-12,14,18H,5,7,13H2,1-2H3,(H,26,29)/t18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123044

(5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...)Show SMILES CC1(C)C2CC1C(CC2)NC(=O)c1cc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)ccc1O |THB:9:6:1:4| Show InChI InChI=1S/C25H24Cl2N4O5/c1-25(2)12-3-5-19(16(25)7-12)29-23(34)15-10-14(4-6-20(15)32)36-22-17(26)8-13(9-18(22)27)31-24(35)30-21(33)11-28-31/h4,6,8-12,16,19,32H,3,5,7H2,1-2H3,(H,29,34)(H,30,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602320
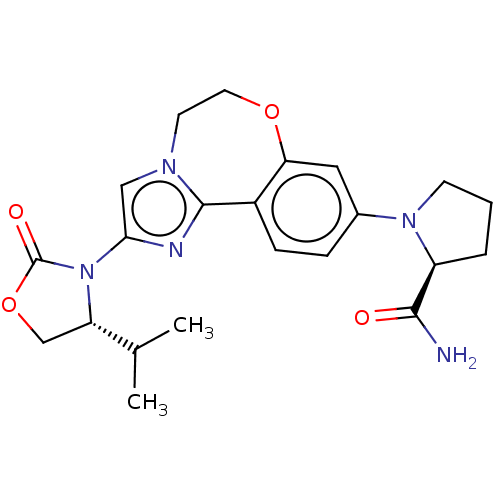
(CHEMBL5199631)Show SMILES CC(C)[C@@H]1COC(=O)N1c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602324
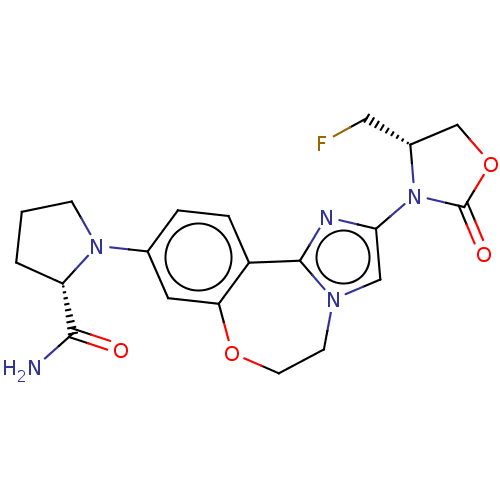
(CHEMBL5198796)Show SMILES NC(=O)[C@@H]1CCCN1c1ccc2-c3nc(cn3CCOc2c1)N1[C@H](CF)COC1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50274047

(CHEMBL3613796)Show SMILES OB(O)CNS(=O)(=O)c1ccc(cc1C(F)(F)F)-c1nnn[nH]1 Show InChI InChI=1S/C9H9BF3N5O4S/c11-9(12,13)6-3-5(8-15-17-18-16-8)1-2-7(6)23(21,22)14-4-10(19)20/h1-3,14,19-20H,4H2,(H,15,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCL School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli AmpC using CENTA as substrate by spectrometry based Lineweaver-Burk plot analysis |
Bioorg Med Chem 26: 2921-2927 (2018)
Article DOI: 10.1016/j.bmc.2018.04.055
BindingDB Entry DOI: 10.7270/Q25T3P0V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388902
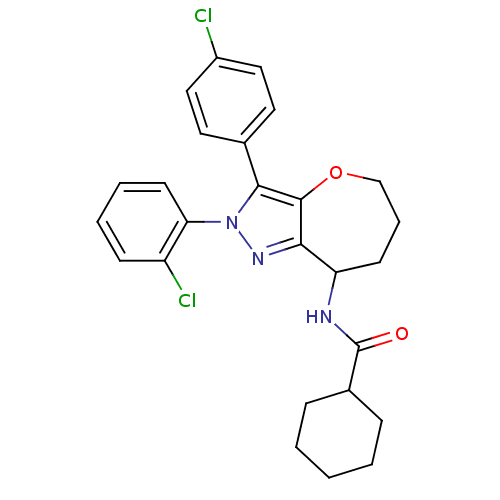
(CHEMBL2063239)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)C3CCCCC3)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C26H27Cl2N3O2/c27-19-14-12-17(13-15-19)24-25-23(30-31(24)22-11-5-4-9-20(22)28)21(10-6-16-33-25)29-26(32)18-7-2-1-3-8-18/h4-5,9,11-15,18,21H,1-3,6-8,10,16H2,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM295669
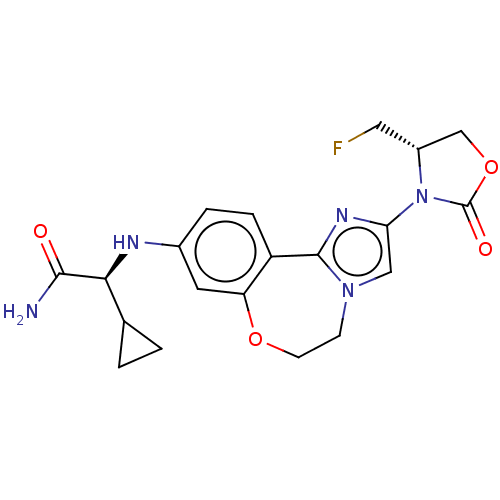
((S)-2-cyclopropyl-2-((2-((S)-4- (fluoromethyl)-2-o...)Show SMILES NC(=O)[C@@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@H](CF)COC1=O)C1CC1 |r| Show InChI InChI=1S/C20H22FN5O4/c21-8-13-10-30-20(28)26(13)16-9-25-5-6-29-15-7-12(3-4-14(15)19(25)24-16)23-17(18(22)27)11-1-2-11/h3-4,7,9,11,13,17,23H,1-2,5-6,8,10H2,(H2,22,27)/t13-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602323
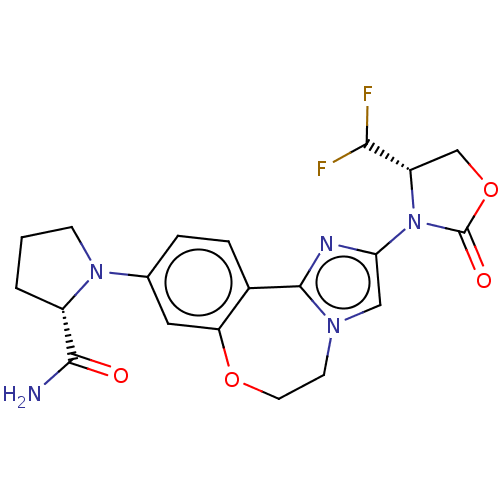
(CHEMBL5209168)Show SMILES NC(=O)[C@@H]1CCCN1c1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM475607
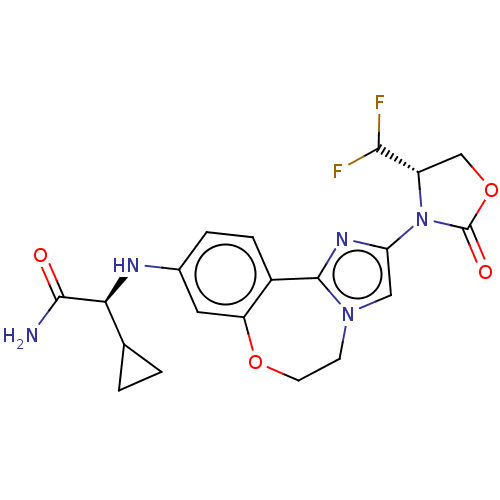
(US10851091, Compound 103)Show SMILES NC(=O)[C@@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F)C1CC1 |r| Show InChI InChI=1S/C20H21F2N5O4/c21-17(22)13-9-31-20(29)27(13)15-8-26-5-6-30-14-7-11(3-4-12(14)19(26)25-15)24-16(18(23)28)10-1-2-10/h3-4,7-8,10,13,16-17,24H,1-2,5-6,9H2,(H2,23,28)/t13-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 1
(Homo sapiens (Human)) | BDBM50037557

(2-{[1-(2-{2-[2-(2-Amino-3-phenyl-propionylamino)-4...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H76N14O10/c1-32(2)28-41(66-47(72)36(55)29-33-14-6-3-7-15-33)50(75)67-42(31-35-18-10-5-11-19-35)51(76)64-39(23-25-45(57)70)53(78)68-27-13-21-43(68)52(77)63-38(22-24-44(56)69)49(74)62-37(20-12-26-61-54(59)60)48(73)65-40(46(58)71)30-34-16-8-4-9-17-34/h3-11,14-19,32,36-43H,12-13,20-31,55H2,1-2H3,(H2,56,69)(H2,57,70)(H2,58,71)(H,62,74)(H,63,77)(H,64,76)(H,65,73)(H,66,72)(H,67,75)(H4,59,60,61)/t36-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-1DMeNPFF from recombinant human NPFF1 receptor expressed in CHO cells by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00256
BindingDB Entry DOI: 10.7270/Q2VX0MDH |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50045513
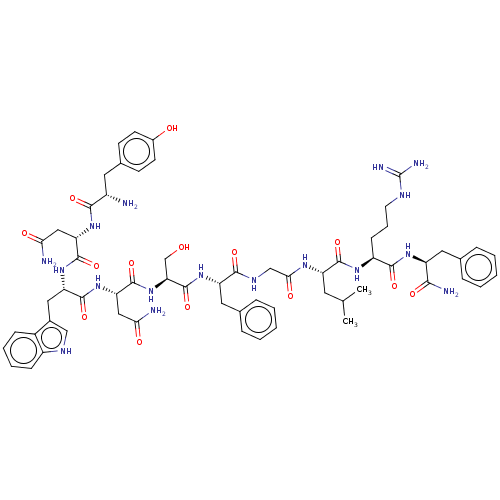
(Kisspeptin-10)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-Kp-10 from human Kiss1 receptor by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00256
BindingDB Entry DOI: 10.7270/Q2VX0MDH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602305
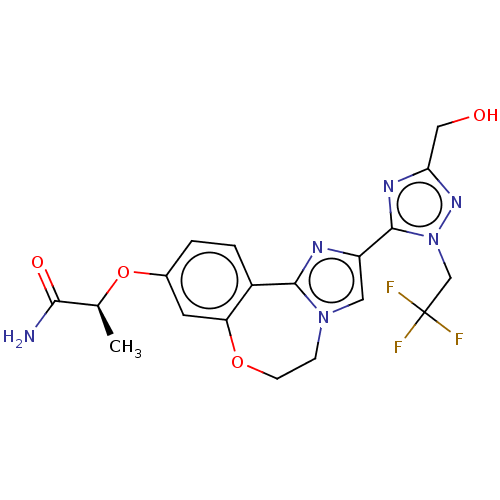
(CHEMBL5209048)Show SMILES C[C@H](Oc1ccc2-c3nc(cn3CCOc2c1)-c1nc(CO)nn1CC(F)(F)F)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123045
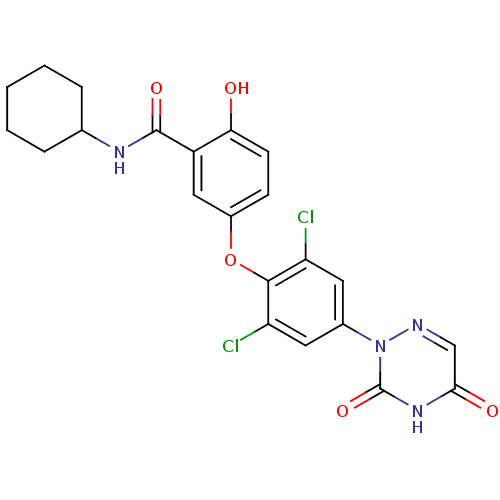
(CHEMBL413699 | N-Cyclohexyl-5-[2,6-dichloro-4-(3,5...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1C(=O)NC1CCCCC1 Show InChI InChI=1S/C22H20Cl2N4O5/c23-16-8-13(28-22(32)27-19(30)11-25-28)9-17(24)20(16)33-14-6-7-18(29)15(10-14)21(31)26-12-4-2-1-3-5-12/h6-12,29H,1-5H2,(H,26,31)(H,27,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388887
(CHEMBL2063240)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)C3CCOCC3)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C25H25Cl2N3O3/c26-18-9-7-16(8-10-18)23-24-22(29-30(23)21-6-2-1-4-19(21)27)20(5-3-13-33-24)28-25(31)17-11-14-32-15-12-17/h1-2,4,6-10,17,20H,3,5,11-15H2,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388896

(CHEMBL2063249)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)c3cnon3)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C22H17Cl2N5O3/c23-14-9-7-13(8-10-14)20-21-19(27-29(20)18-6-2-1-4-15(18)24)16(5-3-11-31-21)26-22(30)17-12-25-32-28-17/h1-2,4,6-10,12,16H,3,5,11H2,(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388893
(CHEMBL2063246)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)C3(CC3)C#N)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C24H20Cl2N4O2/c25-16-9-7-15(8-10-16)21-22-20(29-30(21)19-6-2-1-4-17(19)26)18(5-3-13-32-22)28-23(31)24(14-27)11-12-24/h1-2,4,6-10,18H,3,5,11-13H2,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860
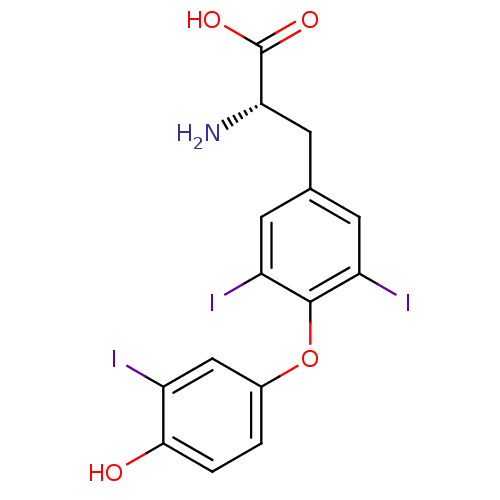
((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388887

(CHEMBL2063240)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)C3CCOCC3)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C25H25Cl2N3O3/c26-18-9-7-16(8-10-18)23-24-22(29-30(23)21-6-2-1-4-19(21)27)20(5-3-13-33-24)28-25(31)17-11-14-32-15-12-17/h1-2,4,6-10,17,20H,3,5,11-15H2,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434806
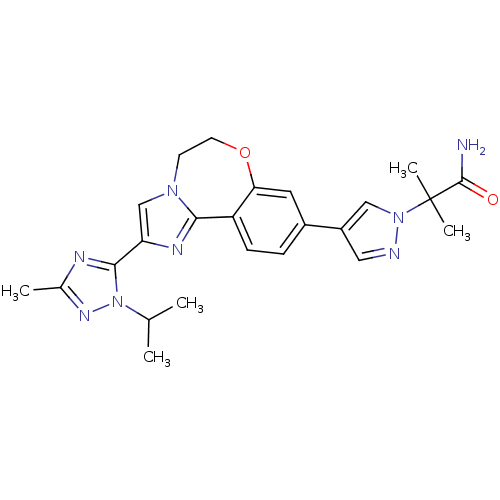
(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602328
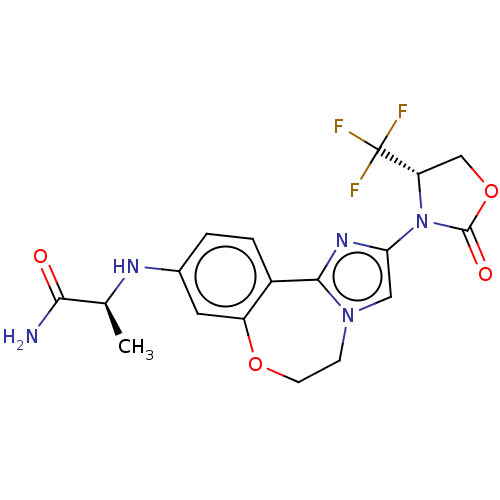
(CHEMBL5205438)Show SMILES C[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602326
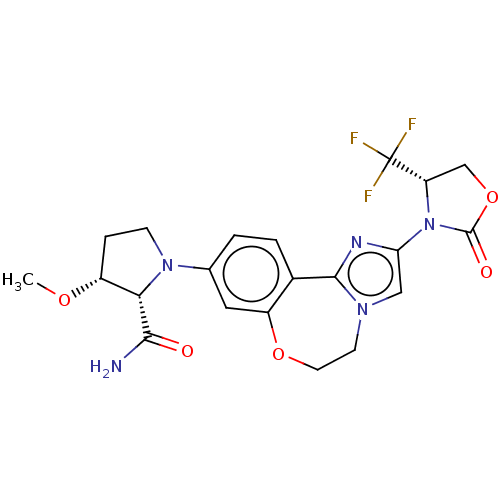
(CHEMBL5181348)Show SMILES CO[C@@H]1CCN([C@@H]1C(N)=O)c1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50301747
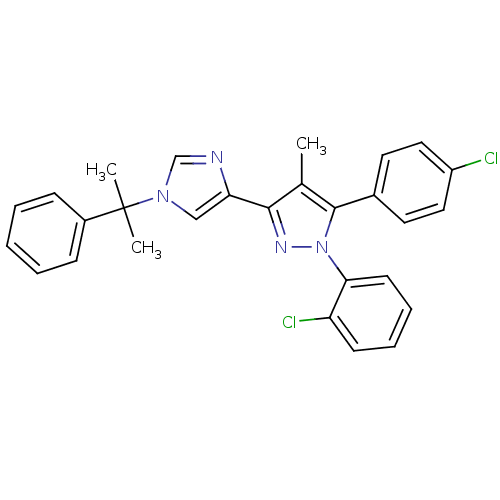
(1-(2-chlorophenyl)-5-(4-chlorophenyl)-4-methyl-3-(...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccccc1Cl)-c1cn(cn1)C(C)(C)c1ccccc1 Show InChI InChI=1S/C28H24Cl2N4/c1-19-26(24-17-33(18-31-24)28(2,3)21-9-5-4-6-10-21)32-34(25-12-8-7-11-23(25)30)27(19)20-13-15-22(29)16-14-20/h4-18H,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor transfected in CHO-K1cells by GTPgamma[35S] binding assay |
Bioorg Med Chem Lett 19: 5351-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.130
BindingDB Entry DOI: 10.7270/Q2KP827S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149553

(CHEMBL3770306)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CC[C@H]1C(N)=O |r| Show InChI InChI=1S/C20H23N7O2/c1-12(2)27-20(22-11-23-27)15-10-25-7-8-29-17-9-13(3-4-14(17)19(25)24-15)26-6-5-16(26)18(21)28/h3-4,9-12,16H,5-8H2,1-2H3,(H2,21,28)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123054
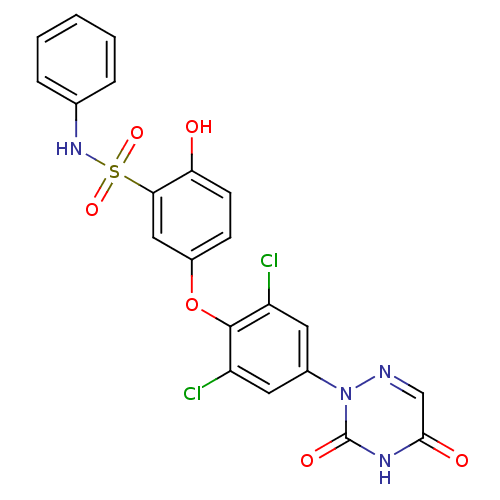
(5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)Nc1ccccc1 Show InChI InChI=1S/C21H14Cl2N4O6S/c22-15-8-13(27-21(30)25-19(29)11-24-27)9-16(23)20(15)33-14-6-7-17(28)18(10-14)34(31,32)26-12-4-2-1-3-5-12/h1-11,26,28H,(H,25,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50301739
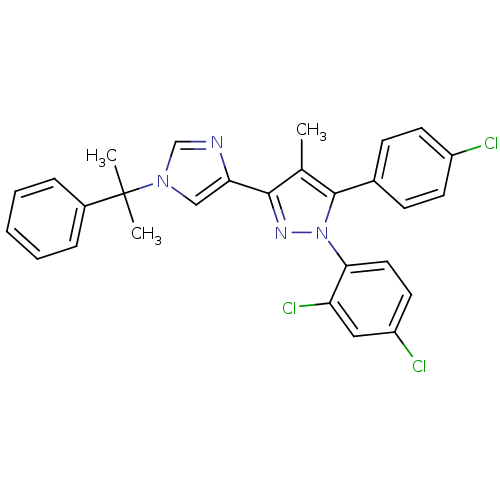
(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)-c1cn(cn1)C(C)(C)c1ccccc1 Show InChI InChI=1S/C28H23Cl3N4/c1-18-26(24-16-34(17-32-24)28(2,3)20-7-5-4-6-8-20)33-35(25-14-13-22(30)15-23(25)31)27(18)19-9-11-21(29)12-10-19/h4-17H,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor transfected in CHO-K1cells by GTPgamma[35S] binding assay |
Bioorg Med Chem Lett 19: 5351-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.130
BindingDB Entry DOI: 10.7270/Q2KP827S |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged EGFR catalytic domain (669 to 1210 residues) expressed in baculovirus expression system by mass... |
ACS Med Chem Lett 7: 100-4 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00428
BindingDB Entry DOI: 10.7270/Q25T3NCC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602331
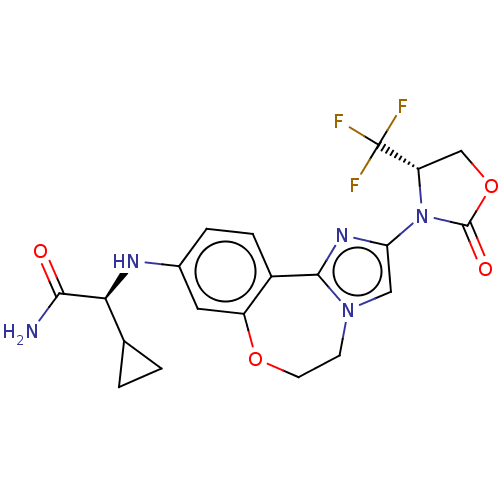
(CHEMBL5182371)Show SMILES NC(=O)[C@@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F)C1CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149482
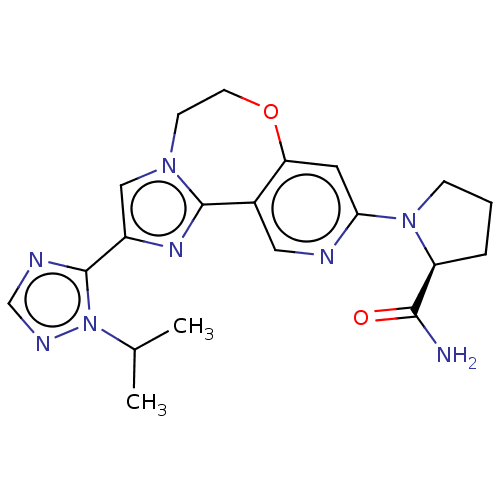
(CHEMBL3770709)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ncc3-c2n1)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C20H24N8O2/c1-12(2)28-20(23-11-24-28)14-10-26-6-7-30-16-8-17(22-9-13(16)19(26)25-14)27-5-3-4-15(27)18(21)29/h8-12,15H,3-7H2,1-2H3,(H2,21,29)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602304
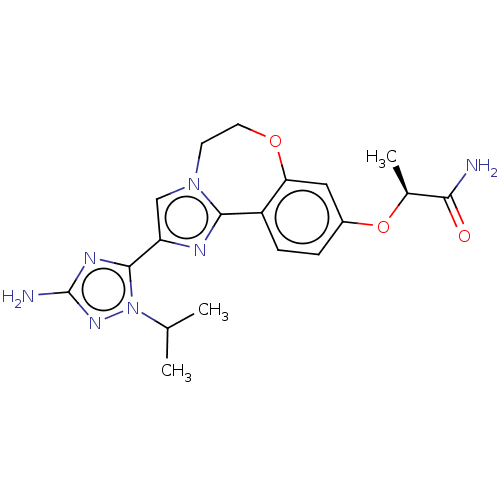
(CHEMBL5182339)Show SMILES CC(C)n1nc(N)nc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ccc3-c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388900
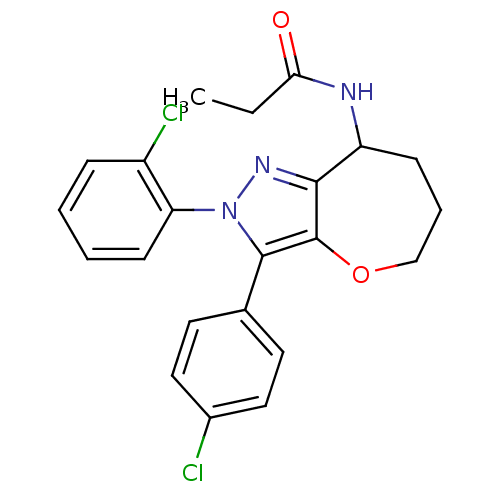
(CHEMBL2063235)Show SMILES CCC(=O)NC1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl Show InChI InChI=1S/C22H21Cl2N3O2/c1-2-19(28)25-17-7-5-13-29-22-20(17)26-27(18-8-4-3-6-16(18)24)21(22)14-9-11-15(23)12-10-14/h3-4,6,8-12,17H,2,5,7,13H2,1H3,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434810
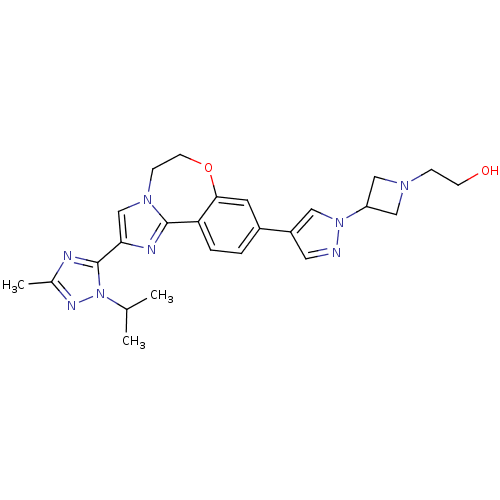
(CHEMBL2386970)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C1CN(CCO)C1 Show InChI InChI=1S/C25H30N8O2/c1-16(2)33-25(27-17(3)29-33)22-15-31-7-9-35-23-10-18(4-5-21(23)24(31)28-22)19-11-26-32(12-19)20-13-30(14-20)6-8-34/h4-5,10-12,15-16,20,34H,6-9,13-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388892
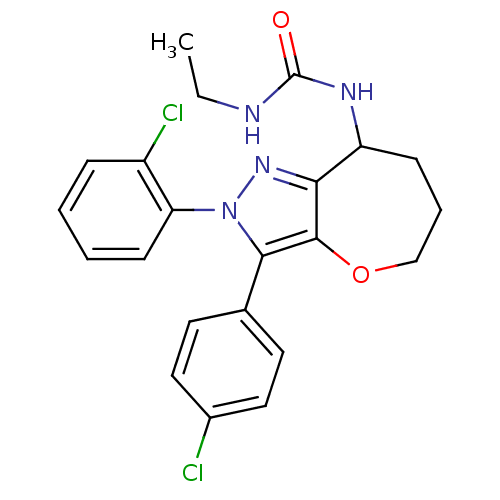
(CHEMBL2063245)Show SMILES CCNC(=O)NC1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl Show InChI InChI=1S/C22H22Cl2N4O2/c1-2-25-22(29)26-17-7-5-13-30-21-19(17)27-28(18-8-4-3-6-16(18)24)20(21)14-9-11-15(23)12-10-14/h3-4,6,8-12,17H,2,5,7,13H2,1H3,(H2,25,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388892

(CHEMBL2063245)Show SMILES CCNC(=O)NC1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl Show InChI InChI=1S/C22H22Cl2N4O2/c1-2-25-22(29)26-17-7-5-13-30-21-19(17)27-28(18-8-4-3-6-16(18)24)20(21)14-9-11-15(23)12-10-14/h3-4,6,8-12,17H,2,5,7,13H2,1H3,(H2,25,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388891
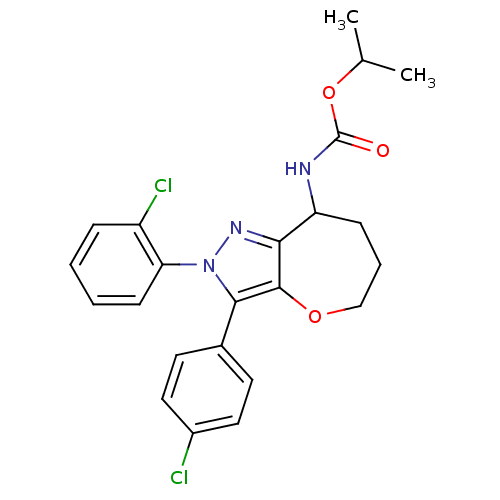
(CHEMBL2063244)Show SMILES CC(C)OC(=O)NC1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl Show InChI InChI=1S/C23H23Cl2N3O3/c1-14(2)31-23(29)26-18-7-5-13-30-22-20(18)27-28(19-8-4-3-6-17(19)25)21(22)15-9-11-16(24)12-10-15/h3-4,6,8-12,14,18H,5,7,13H2,1-2H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388893

(CHEMBL2063246)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)C3(CC3)C#N)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C24H20Cl2N4O2/c25-16-9-7-15(8-10-16)21-22-20(29-30(21)19-6-2-1-4-17(19)26)18(5-3-13-32-22)28-23(31)24(14-27)11-12-24/h1-2,4,6-10,18H,3,5,11-13H2,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data