

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426652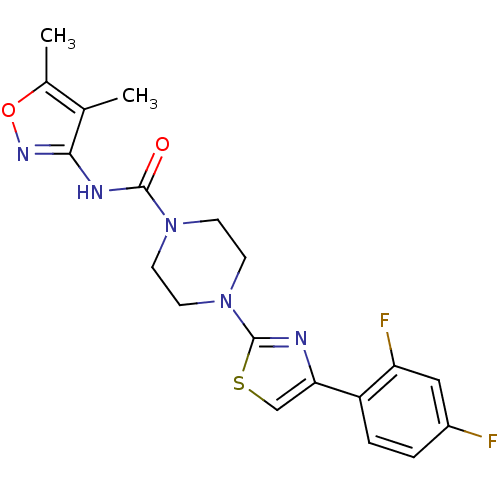 (CHEMBL2326178) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426652 (CHEMBL2326178) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426651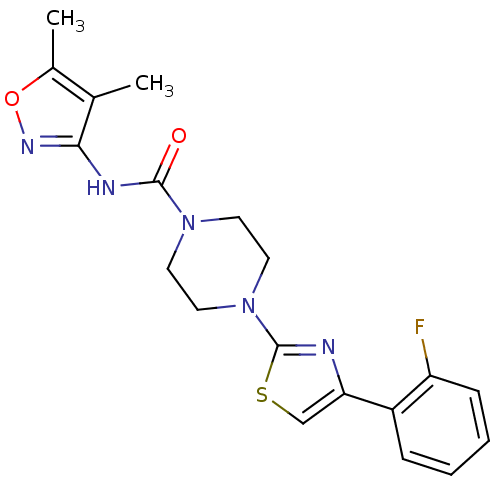 (CHEMBL2326194) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426650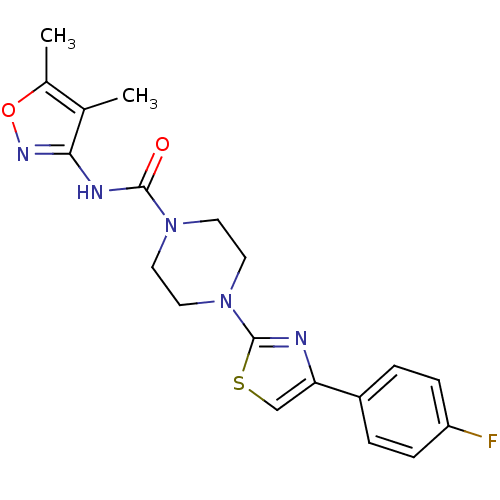 (CHEMBL2326177) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426651 (CHEMBL2326194) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426648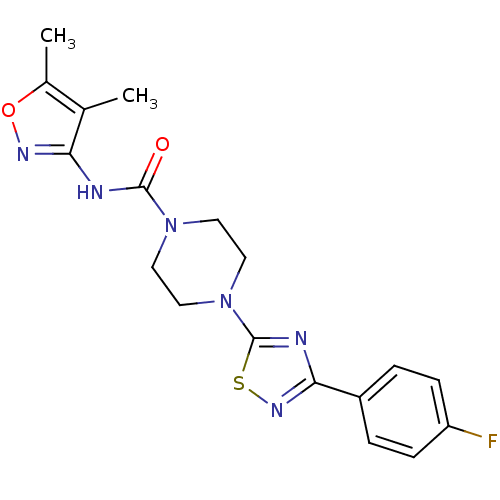 (CHEMBL2326197) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426650 (CHEMBL2326177) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426649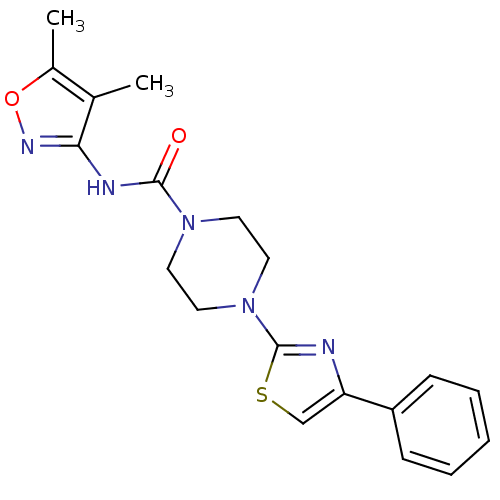 (CHEMBL2326192) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426649 (CHEMBL2326192) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426647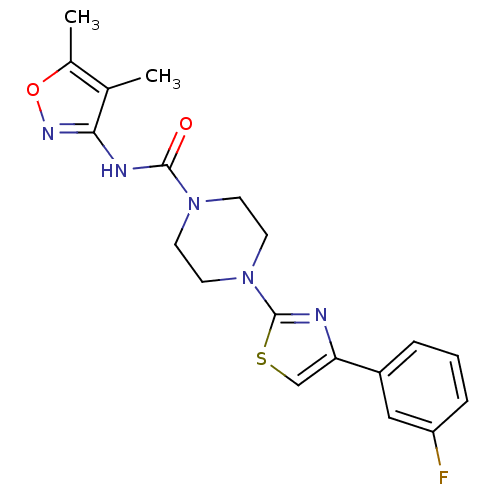 (CHEMBL2326196) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426645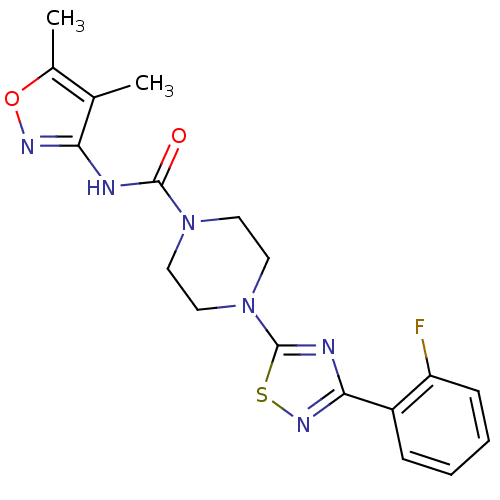 (CHEMBL2326193) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426648 (CHEMBL2326197) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426646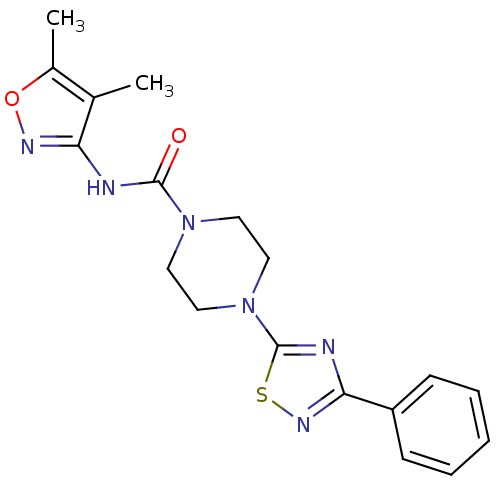 (CHEMBL2326189) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426647 (CHEMBL2326196) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426646 (CHEMBL2326189) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426644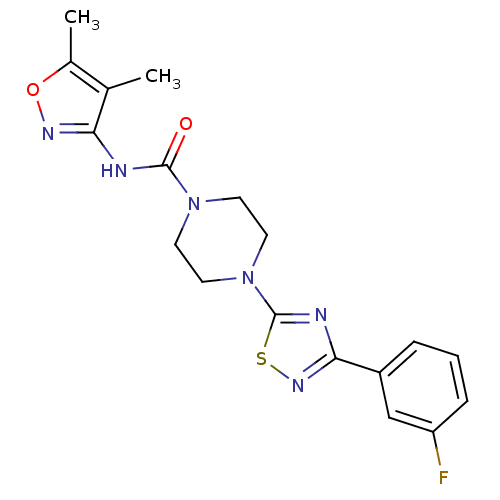 (CHEMBL2326195) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426645 (CHEMBL2326193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50244993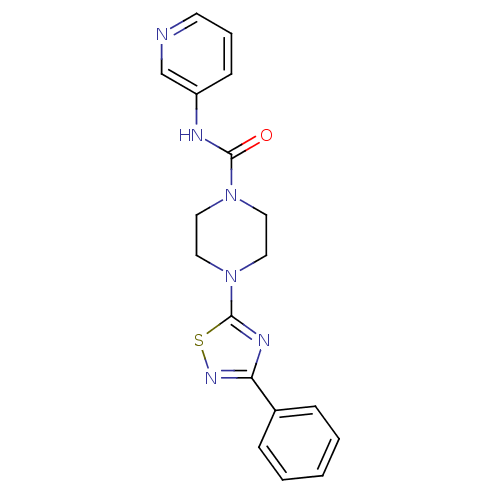 (4-(3-phenyl-1,2,4-thiadiazol-5-yl)-N-(pyridin-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50161957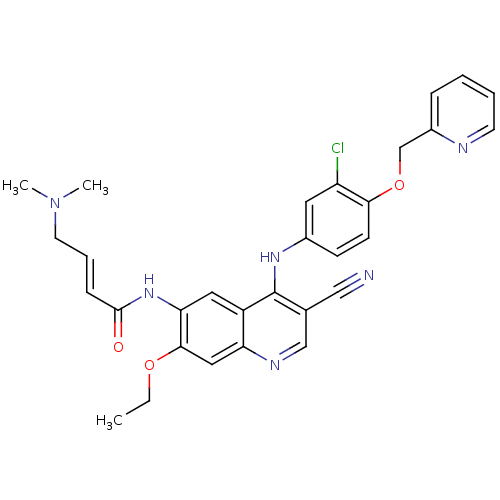 (4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human wild type EGFR expressed in Sf9 cells using [gamma32P]-ATP after 10 mins by scintillation counting | ACS Med Chem Lett 4: 201-5 (2013) Article DOI: 10.1021/ml300327z BindingDB Entry DOI: 10.7270/Q2T1550C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426643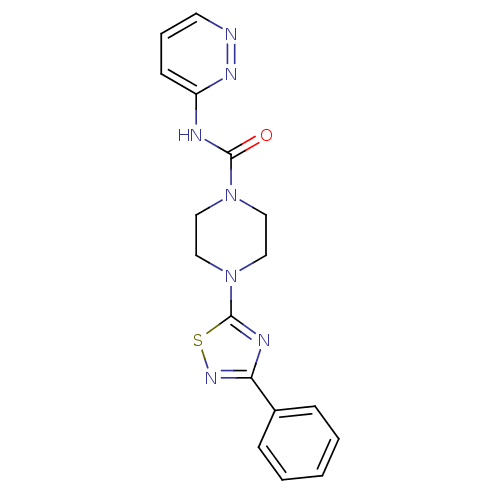 (CHEMBL2326190) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--CoA ligase 1 (Homo sapiens) | BDBM50549459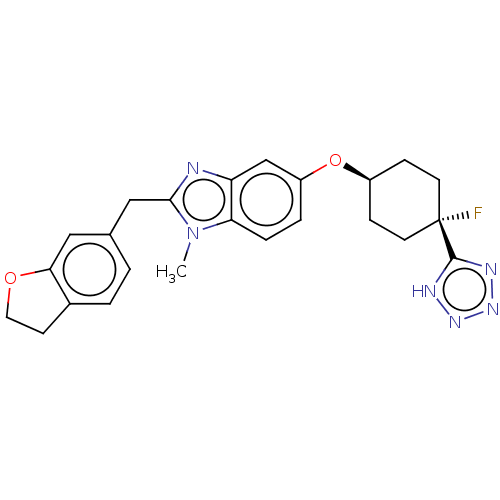 (CHEMBL4764916) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACSL1 assessed as reduction in acetyl-coA prodution incubated for 90 mins in presence of Coenzyme A, ATP and MgCl2 by... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127722 BindingDB Entry DOI: 10.7270/Q2VT1WQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426644 (CHEMBL2326195) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426643 (CHEMBL2326190) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426641 (CHEMBL2326181) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50244993 (4-(3-phenyl-1,2,4-thiadiazol-5-yl)-N-(pyridin-3-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50358430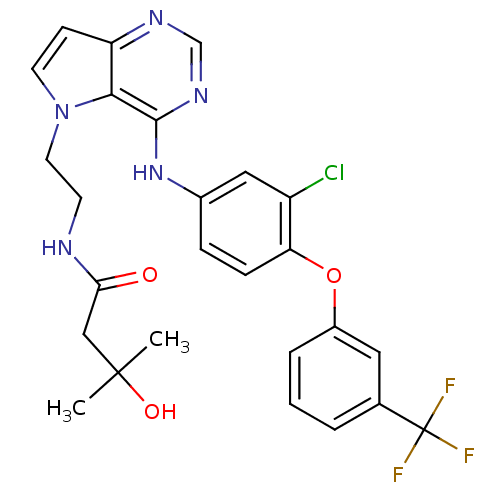 (CHEMBL1614725) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human wild type EGFR expressed in Sf9 cells using [gamma32P]-ATP after 10 mins by scintillation counting | ACS Med Chem Lett 4: 201-5 (2013) Article DOI: 10.1021/ml300327z BindingDB Entry DOI: 10.7270/Q2T1550C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26739 (3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of FAAH (unknown origin) | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50426426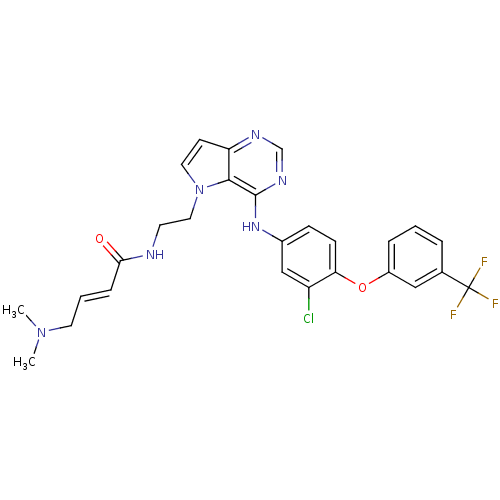 (CHEMBL2322329) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human wild type EGFR expressed in Sf9 cells using [gamma32P]-ATP after 10 mins by scintillation counting | ACS Med Chem Lett 4: 201-5 (2013) Article DOI: 10.1021/ml300327z BindingDB Entry DOI: 10.7270/Q2T1550C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426642 (CHEMBL459152) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426642 (CHEMBL459152) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426641 (CHEMBL2326181) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50426425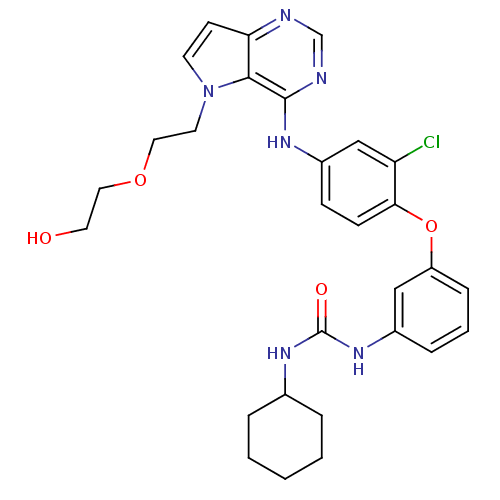 (CHEMBL2322330) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human wild type EGFR expressed in Sf9 cells using [gamma32P]-ATP after 10 mins by scintillation counting | ACS Med Chem Lett 4: 201-5 (2013) Article DOI: 10.1021/ml300327z BindingDB Entry DOI: 10.7270/Q2T1550C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50244718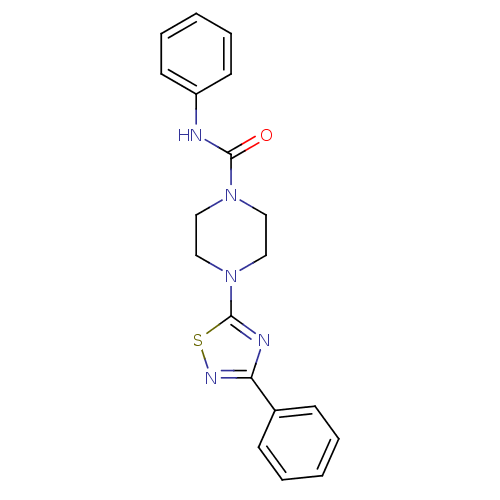 (CHEMBL460273 | JNJ-1661010 | N-phenyl-4-(3-phenyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26736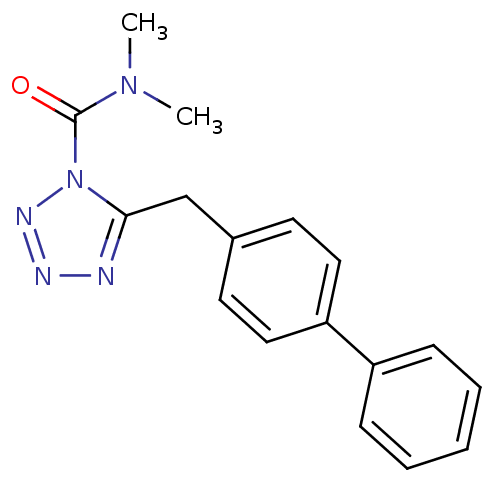 (CHEMBL509860 | LY2183240 | N,N-dimethyl-5-[(4-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of FAAH (unknown origin) | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--CoA ligase 1 (Homo sapiens) | BDBM50549460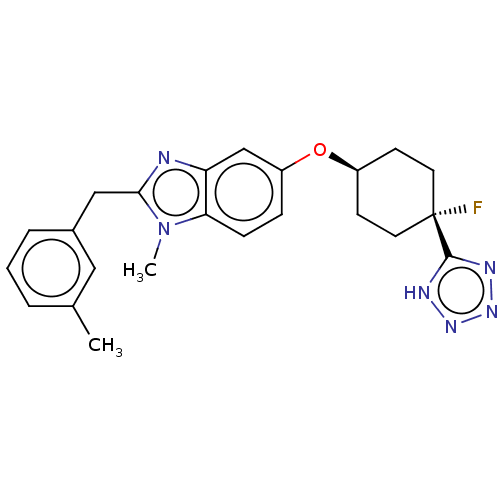 (CHEMBL4750346) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACSL1 assessed as reduction in acetyl-coA prodution incubated for 90 mins in presence of Coenzyme A, ATP and MgCl2 by... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127722 BindingDB Entry DOI: 10.7270/Q2VT1WQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--CoA ligase 1 (Homo sapiens) | BDBM50549472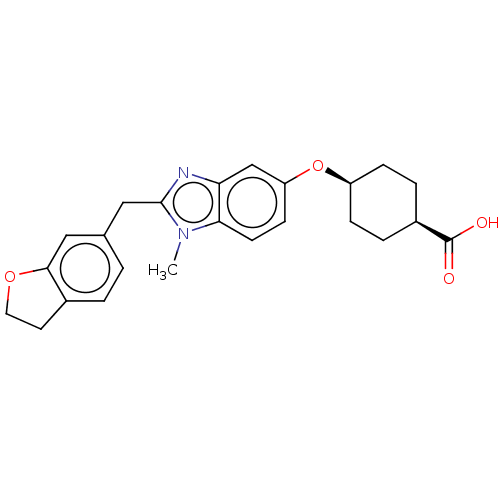 (CHEMBL4746996) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACSL1 assessed as reduction in acetyl-coA prodution incubated for 90 mins in presence of Coenzyme A, ATP and MgCl2 by... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127722 BindingDB Entry DOI: 10.7270/Q2VT1WQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--CoA ligase 1 (Mus musculus) | BDBM50549460 (CHEMBL4750346) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse ACSL1 assessed as reduction in acetyl-coA prodution incubated for 90 mins in presence of Coenzyme A, ATP and MgCl2 by... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127722 BindingDB Entry DOI: 10.7270/Q2VT1WQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--CoA ligase 1 (Mus musculus) | BDBM50549459 (CHEMBL4764916) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse ACSL1 assessed as reduction in acetyl-coA prodution incubated for 90 mins in presence of Coenzyme A, ATP and MgCl2 by... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127722 BindingDB Entry DOI: 10.7270/Q2VT1WQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26740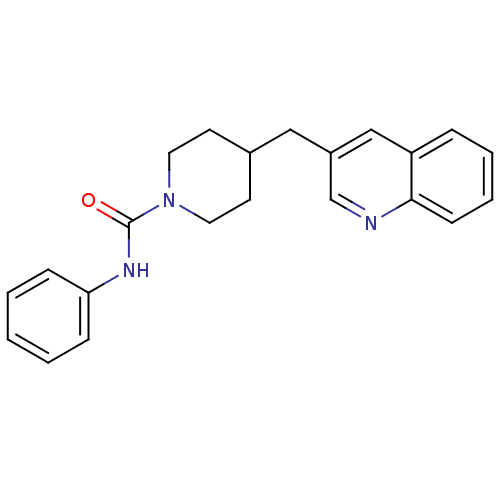 (N-phenyl-4-(quinolin-3-ylmethyl)piperidine-1-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of FAAH (unknown origin) | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--CoA ligase 1 (Mus musculus) | BDBM50549462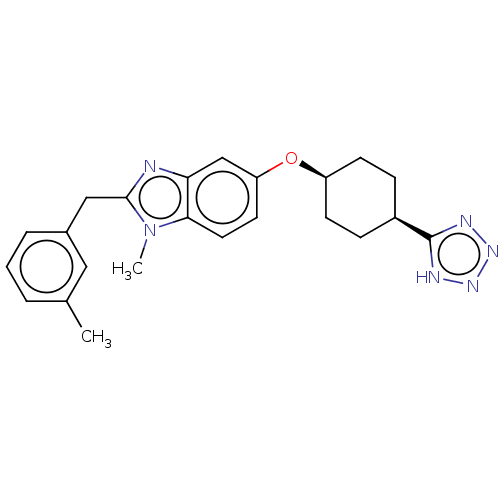 (CHEMBL4749130) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse ACSL1 assessed as reduction in acetyl-coA prodution incubated for 90 mins in presence of Coenzyme A, ATP and MgCl2 by... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127722 BindingDB Entry DOI: 10.7270/Q2VT1WQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--CoA ligase 1 (Homo sapiens) | BDBM50549468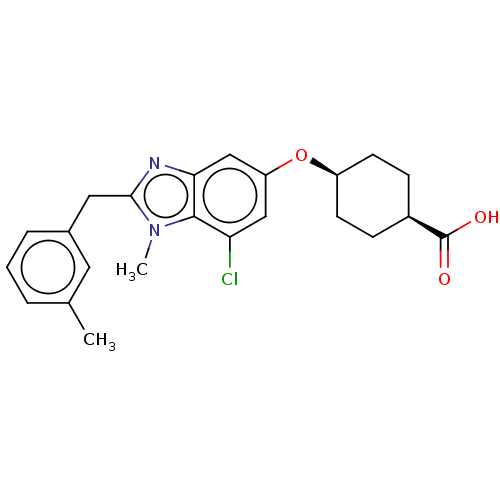 (CHEMBL4787746) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACSL1 assessed as reduction in acetyl-coA prodution incubated for 90 mins in presence of Coenzyme A, ATP and MgCl2 by... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127722 BindingDB Entry DOI: 10.7270/Q2VT1WQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--CoA ligase 1 (Mus musculus) | BDBM50549456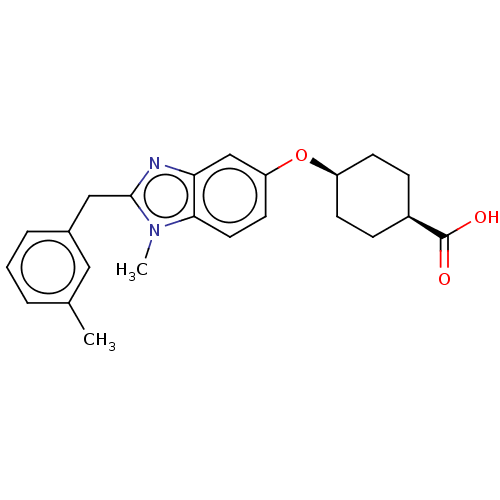 (CHEMBL4778736) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse ACSL1 assessed as reduction in acetyl-coA prodution incubated for 90 mins in presence of Coenzyme A, ATP and MgCl2 by... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127722 BindingDB Entry DOI: 10.7270/Q2VT1WQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--CoA ligase 1 (Homo sapiens) | BDBM50549462 (CHEMBL4749130) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACSL1 assessed as reduction in acetyl-coA prodution incubated for 90 mins in presence of Coenzyme A, ATP and MgCl2 by... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127722 BindingDB Entry DOI: 10.7270/Q2VT1WQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--CoA ligase 1 (Mus musculus) | BDBM50549472 (CHEMBL4746996) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse ACSL1 assessed as reduction in acetyl-coA prodution incubated for 90 mins in presence of Coenzyme A, ATP and MgCl2 by... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127722 BindingDB Entry DOI: 10.7270/Q2VT1WQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--CoA ligase 1 (Homo sapiens) | BDBM50549483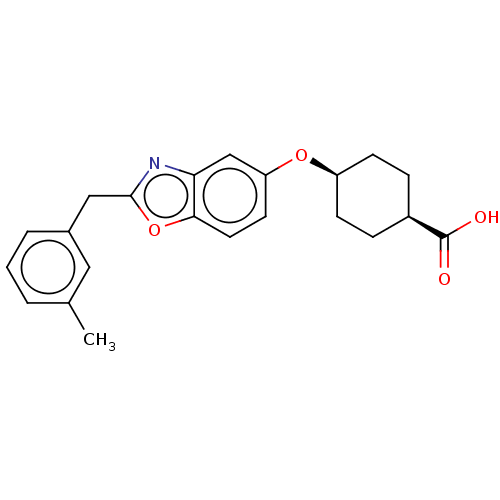 (CHEMBL4781788) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACSL1 assessed as reduction in acetyl-coA prodution incubated for 90 mins in presence of Coenzyme A, ATP and MgCl2 by... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127722 BindingDB Entry DOI: 10.7270/Q2VT1WQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--CoA ligase 1 (Homo sapiens) | BDBM50549456 (CHEMBL4778736) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACSL1 assessed as reduction in acetyl-coA prodution incubated for 90 mins in presence of Coenzyme A, ATP and MgCl2 by... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127722 BindingDB Entry DOI: 10.7270/Q2VT1WQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426639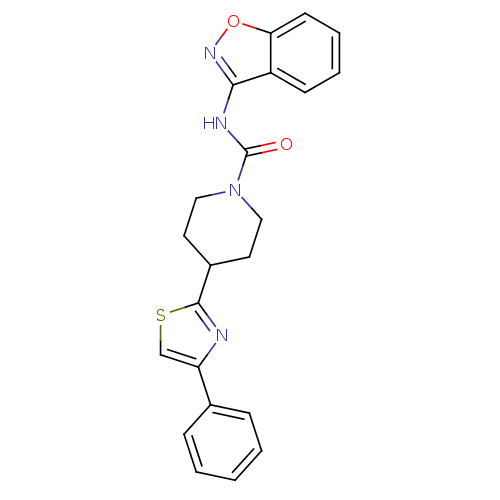 (CHEMBL2326184) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--CoA ligase 1 (Homo sapiens) | BDBM50549465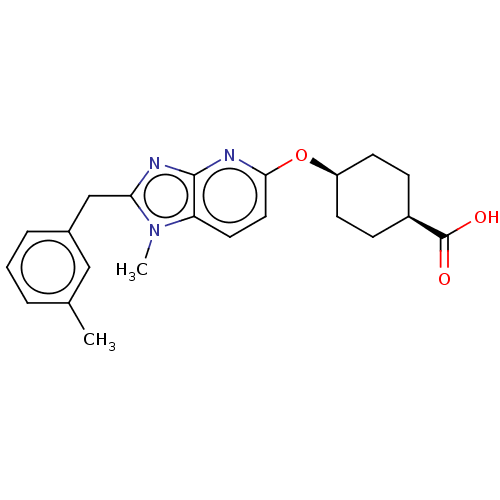 (CHEMBL4797544) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACSL1 assessed as reduction in acetyl-coA prodution incubated for 90 mins in presence of Coenzyme A, ATP and MgCl2 by... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127722 BindingDB Entry DOI: 10.7270/Q2VT1WQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--CoA ligase 1 (Homo sapiens) | BDBM50549482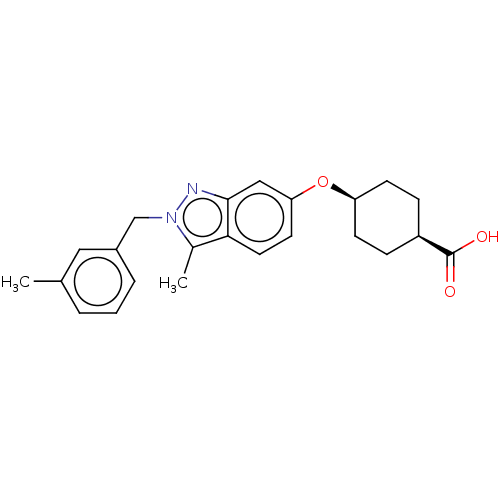 (CHEMBL4755109) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACSL1 assessed as reduction in acetyl-coA prodution incubated for 90 mins in presence of Coenzyme A, ATP and MgCl2 by... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127722 BindingDB Entry DOI: 10.7270/Q2VT1WQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426640 (CHEMBL2326179) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 151 total ) | Next | Last >> |