 Found 343 hits with Last Name = 'iijima' and Initial = 'd'
Found 343 hits with Last Name = 'iijima' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM18012
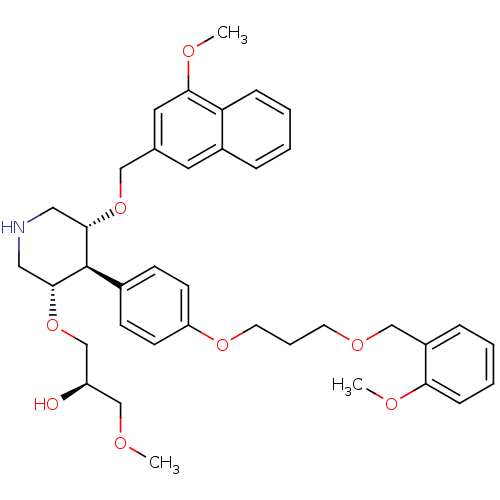
(trans,trans-4-arylpiperidine-based compound, 1)Show SMILES COC[C@@H](O)CO[C@@H]1CNC[C@H](OCc2cc(OC)c3ccccc3c2)[C@H]1c1ccc(OCCCOCc2ccccc2OC)cc1 |r| Show InChI InChI=1S/C38H47NO8/c1-41-25-31(40)26-47-37-22-39-21-36(46-23-27-19-29-9-4-6-11-33(29)35(20-27)43-3)38(37)28-13-15-32(16-14-28)45-18-8-17-44-24-30-10-5-7-12-34(30)42-2/h4-7,9-16,19-20,31,36-40H,8,17-18,21-26H2,1-3H3/t31-,36+,37-,38-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18012

(trans,trans-4-arylpiperidine-based compound, 1)Show SMILES COC[C@@H](O)CO[C@@H]1CNC[C@H](OCc2cc(OC)c3ccccc3c2)[C@H]1c1ccc(OCCCOCc2ccccc2OC)cc1 |r| Show InChI InChI=1S/C38H47NO8/c1-41-25-31(40)26-47-37-22-39-21-36(46-23-27-19-29-9-4-6-11-33(29)35(20-27)43-3)38(37)28-13-15-32(16-14-28)45-18-8-17-44-24-30-10-5-7-12-34(30)42-2/h4-7,9-16,19-20,31,36-40H,8,17-18,21-26H2,1-3H3/t31-,36+,37-,38-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00280
BindingDB Entry DOI: 10.7270/Q28K7F4S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM212579
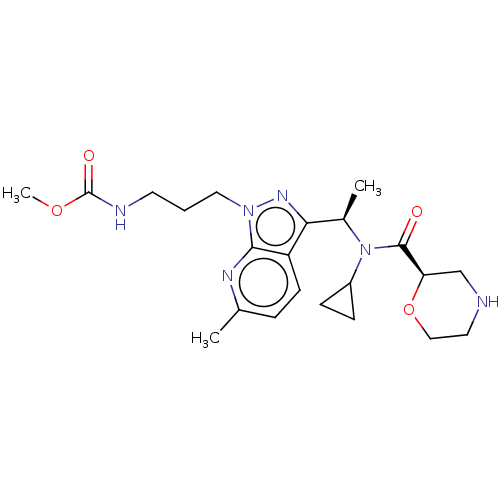
(US10155731, Example 270 | US9278944, 270)Show SMILES COC(=O)NCCCn1nc([C@@H](C)N(C2CC2)C(=O)[C@H]2CNCCO2)c2ccc(C)nc12 |r| Show InChI InChI=1S/C22H32N6O4/c1-14-5-8-17-19(26-27(20(17)25-14)11-4-9-24-22(30)31-3)15(2)28(16-6-7-16)21(29)18-13-23-10-12-32-18/h5,8,15-16,18,23H,4,6-7,9-13H2,1-3H3,(H,24,30)/t15-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50598963
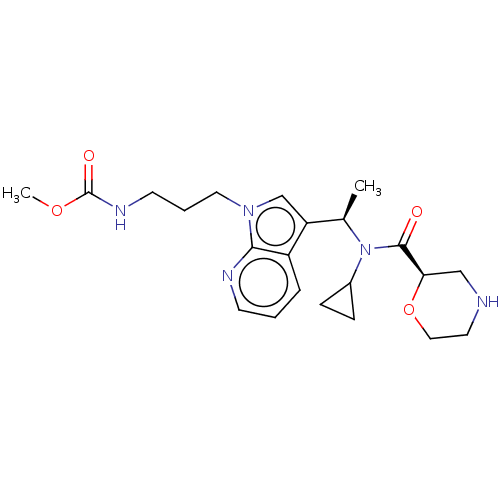
(CHEMBL5174310)Show SMILES COC(=O)NCCCn1cc([C@@H](C)N(C2CC2)C(=O)[C@H]2CNCCO2)c2cccnc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM212579

(US10155731, Example 270 | US9278944, 270)Show SMILES COC(=O)NCCCn1nc([C@@H](C)N(C2CC2)C(=O)[C@H]2CNCCO2)c2ccc(C)nc12 |r| Show InChI InChI=1S/C22H32N6O4/c1-14-5-8-17-19(26-27(20(17)25-14)11-4-9-24-22(30)31-3)15(2)28(16-6-7-16)21(29)18-13-23-10-12-32-18/h5,8,15-16,18,23H,4,6-7,9-13H2,1-3H3,(H,24,30)/t15-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00280
BindingDB Entry DOI: 10.7270/Q28K7F4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00280
BindingDB Entry DOI: 10.7270/Q28K7F4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50598963

(CHEMBL5174310)Show SMILES COC(=O)NCCCn1cc([C@@H](C)N(C2CC2)C(=O)[C@H]2CNCCO2)c2cccnc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00280
BindingDB Entry DOI: 10.7270/Q28K7F4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50598961
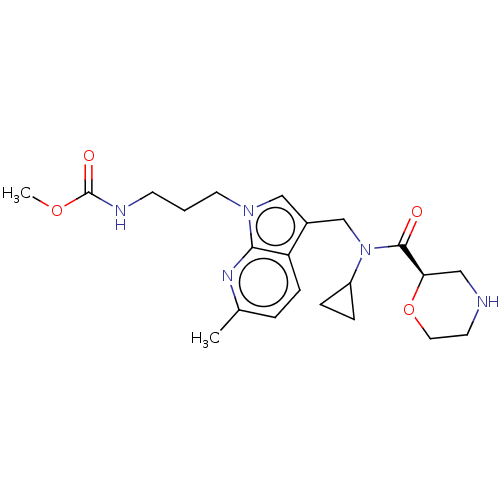
(CHEMBL5200104)Show SMILES COC(=O)NCCCn1cc(CN(C2CC2)C(=O)[C@H]2CNCCO2)c2ccc(C)nc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50598954

(CHEMBL5187830)Show SMILES COC(=O)NCCCn1cc(CN(C2CC2)C(=O)[C@H]2CNCCO2)c2ccccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00280
BindingDB Entry DOI: 10.7270/Q28K7F4S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50598954

(CHEMBL5187830)Show SMILES COC(=O)NCCCn1cc(CN(C2CC2)C(=O)[C@H]2CNCCO2)c2ccccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50601200
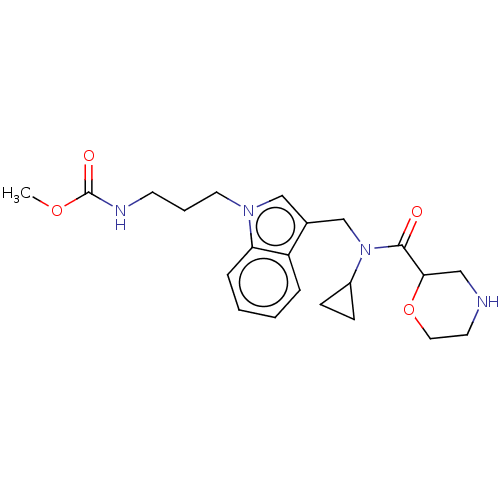
(CHEMBL5203297)Show SMILES COC(=O)NCCCn1cc(CN(C2CC2)C(=O)C2CNCCO2)c2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00280
BindingDB Entry DOI: 10.7270/Q28K7F4S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM212577
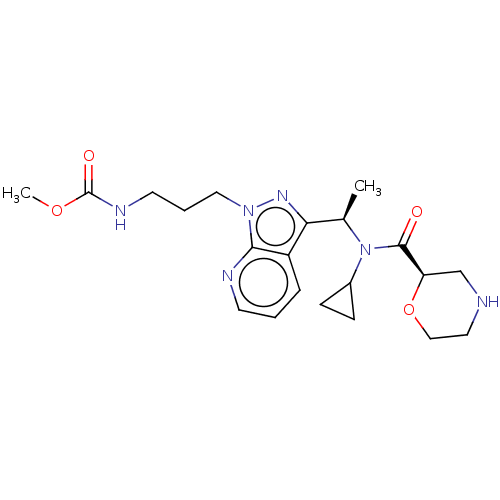
(US10155731, Example 268 | US9278944, 268)Show SMILES COC(=O)NCCCn1nc([C@@H](C)N(C2CC2)C(=O)[C@H]2CNCCO2)c2cccnc12 |r| Show InChI InChI=1S/C21H30N6O4/c1-14(27(15-6-7-15)20(28)17-13-22-10-12-31-17)18-16-5-3-8-23-19(16)26(25-18)11-4-9-24-21(29)30-2/h3,5,8,14-15,17,22H,4,6-7,9-13H2,1-2H3,(H,24,29)/t14-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50598965
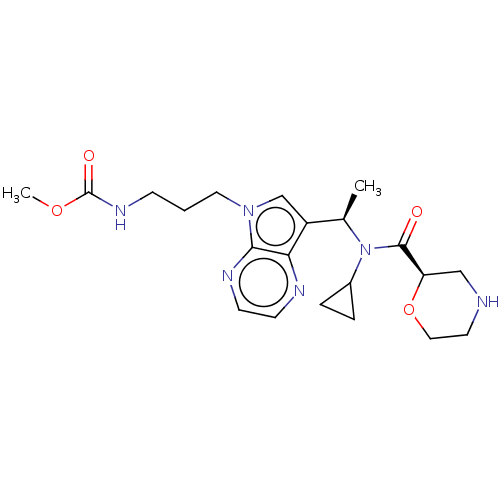
(CHEMBL5188936)Show SMILES COC(=O)NCCCn1cc([C@@H](C)N(C2CC2)C(=O)[C@H]2CNCCO2)c2nccnc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50598954

(CHEMBL5187830)Show SMILES COC(=O)NCCCn1cc(CN(C2CC2)C(=O)[C@H]2CNCCO2)c2ccccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00280
BindingDB Entry DOI: 10.7270/Q28K7F4S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50598954

(CHEMBL5187830)Show SMILES COC(=O)NCCCn1cc(CN(C2CC2)C(=O)[C@H]2CNCCO2)c2ccccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00280
BindingDB Entry DOI: 10.7270/Q28K7F4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50598961

(CHEMBL5200104)Show SMILES COC(=O)NCCCn1cc(CN(C2CC2)C(=O)[C@H]2CNCCO2)c2ccc(C)nc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50598960
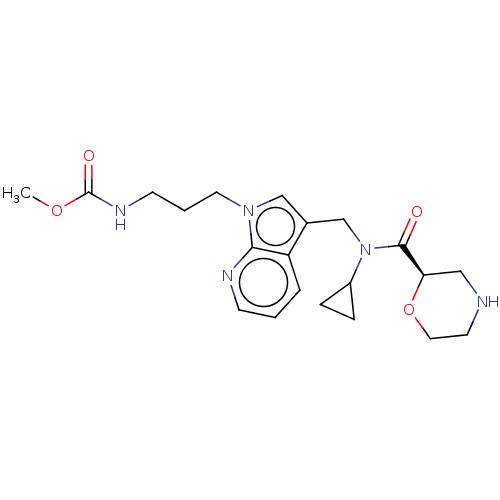
(CHEMBL5176170)Show SMILES COC(=O)NCCCn1cc(CN(C2CC2)C(=O)[C@H]2CNCCO2)c2cccnc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM212577

(US10155731, Example 268 | US9278944, 268)Show SMILES COC(=O)NCCCn1nc([C@@H](C)N(C2CC2)C(=O)[C@H]2CNCCO2)c2cccnc12 |r| Show InChI InChI=1S/C21H30N6O4/c1-14(27(15-6-7-15)20(28)17-13-22-10-12-31-17)18-16-5-3-8-23-19(16)26(25-18)11-4-9-24-21(29)30-2/h3,5,8,14-15,17,22H,4,6-7,9-13H2,1-2H3,(H,24,29)/t14-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM212580
(US10155731, Example 271 | US9278944, 271)Show SMILES COC(=O)NCCCn1nc([C@H](C)N(C2CC2)C(=O)[C@H]2CNCCO2)c2ccc(C)nc12 |r| Show InChI InChI=1S/C22H32N6O4/c1-14-5-8-17-19(26-27(20(17)25-14)11-4-9-24-22(30)31-3)15(2)28(16-6-7-16)21(29)18-13-23-10-12-32-18/h5,8,15-16,18,23H,4,6-7,9-13H2,1-3H3,(H,24,30)/t15-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273135
((R)-N-benzyl-1-{5-[difluoro(phenyl)methyl]-7-oxo-6...)Show SMILES FC(F)(c1ccccc1)c1nc2sc(nc2c(=O)[nH]1)N1CCC[C@@H]1C(=O)NCc1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50601198
(CHEMBL5186584)Show SMILES COCCCCn1cc(CN(C2CC2)C(=O)C2CNCCO2)c2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00280
BindingDB Entry DOI: 10.7270/Q28K7F4S |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273091
((R)-N-benzyl-1-[6-methyl-7-oxo-5-(tetrahydro-2H-py...)Show SMILES Cn1c(nc2sc(nc2c1=O)N1CCC[C@@H]1C(=O)NCc1ccccc1)C1CCOCC1 |r| Show InChI InChI=1S/C23H27N5O3S/c1-27-19(16-9-12-31-13-10-16)26-21-18(22(27)30)25-23(32-21)28-11-5-8-17(28)20(29)24-14-15-6-3-2-4-7-15/h2-4,6-7,16-17H,5,8-14H2,1H3,(H,24,29)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273115
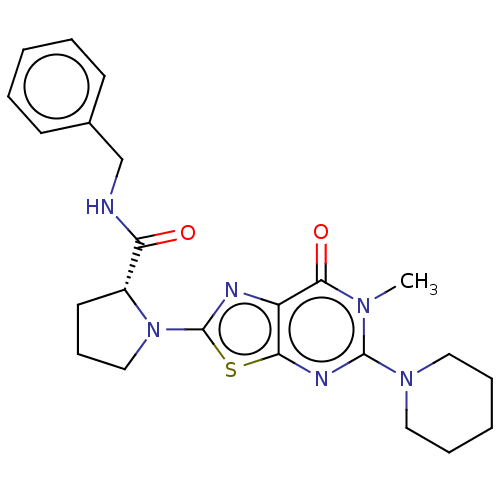
((R)-N-benzyl-1-[6-methyl-7-oxo-5-(piperidin-1-yl)-...)Show SMILES Cn1c(nc2sc(nc2c1=O)N1CCC[C@@H]1C(=O)NCc1ccccc1)N1CCCCC1 |r| Show InChI InChI=1S/C23H28N6O2S/c1-27-21(31)18-20(26-22(27)28-12-6-3-7-13-28)32-23(25-18)29-14-8-11-17(29)19(30)24-15-16-9-4-2-5-10-16/h2,4-5,9-10,17H,3,6-8,11-15H2,1H3,(H,24,30)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273041
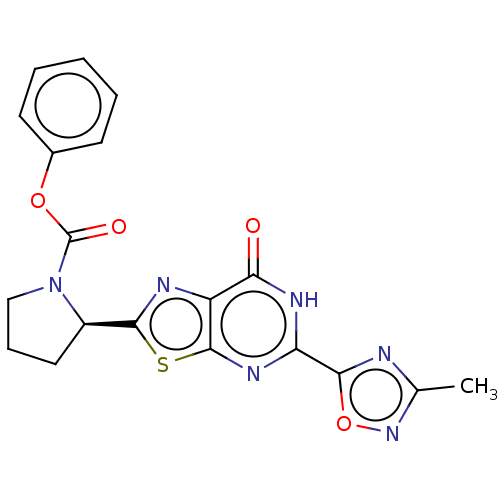
((R)-2-[5-(3-methyl-[1,2,4]oxadiazol-5-yl)-7-oxo-6,...)Show SMILES Cc1noc(n1)-c1nc2sc(nc2c(=O)[nH]1)[C@H]1CCCN1C(=O)Oc1ccccc1 |r| Show InChI InChI=1S/C19H16N6O4S/c1-10-20-16(29-24-10)14-22-15(26)13-18(23-14)30-17(21-13)12-8-5-9-25(12)19(27)28-11-6-3-2-4-7-11/h2-4,6-7,12H,5,8-9H2,1H3,(H,22,23,26)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273079
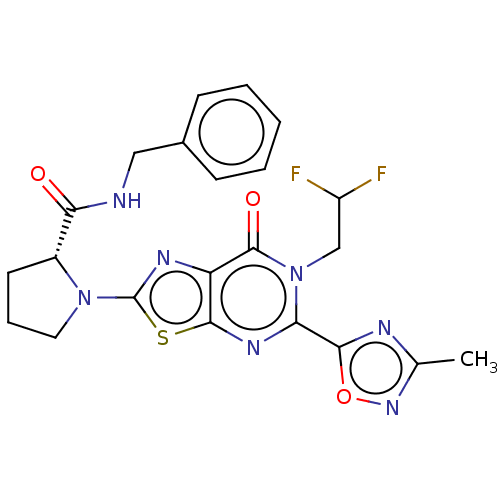
((R)-N-benzyl-1-[6-(2,2-difluoroethyl)-5-(3-methyl-...)Show SMILES Cc1noc(n1)-c1nc2sc(nc2c(=O)n1CC(F)F)N1CCC[C@@H]1C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C22H21F2N7O3S/c1-12-26-19(34-29-12)17-28-20-16(21(33)31(17)11-15(23)24)27-22(35-20)30-9-5-8-14(30)18(32)25-10-13-6-3-2-4-7-13/h2-4,6-7,14-15H,5,8-11H2,1H3,(H,25,32)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273157
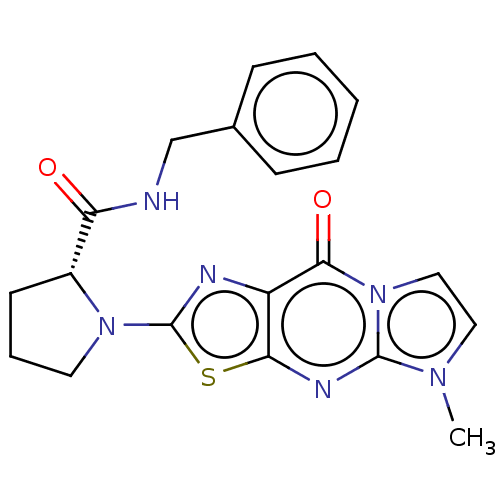
((R)-N-benzyl-1-(5-methyl-9-oxo-5,9-dihydroimidazo[...)Show SMILES Cn1ccn2c1nc1sc(nc1c2=O)N1CCC[C@@H]1C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C20H20N6O2S/c1-24-10-11-26-18(28)15-17(23-19(24)26)29-20(22-15)25-9-5-8-14(25)16(27)21-12-13-6-3-2-4-7-13/h2-4,6-7,10-11,14H,5,8-9,12H2,1H3,(H,21,27)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273070
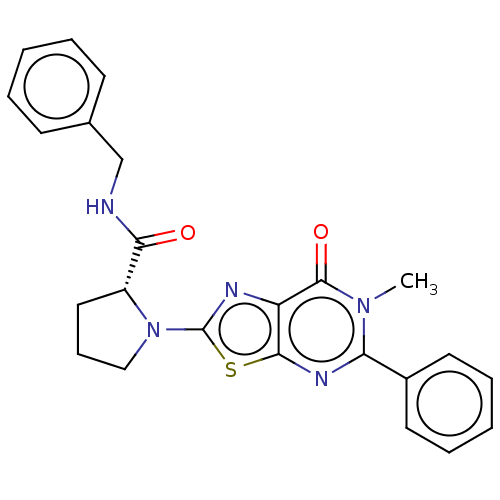
((R)-N-benzyl-1-(6-methyl-7-oxo-5-phenyl-6,7-dihydr...)Show SMILES Cn1c(nc2sc(nc2c1=O)N1CCC[C@@H]1C(=O)NCc1ccccc1)-c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2S/c1-28-20(17-11-6-3-7-12-17)27-22-19(23(28)31)26-24(32-22)29-14-8-13-18(29)21(30)25-15-16-9-4-2-5-10-16/h2-7,9-12,18H,8,13-15H2,1H3,(H,25,30)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273096
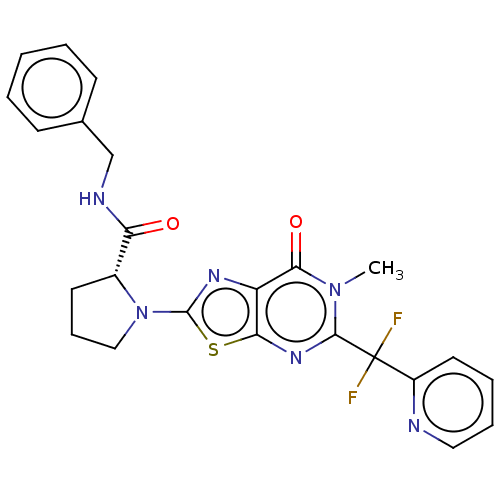
((R)-N-benzyl-1-{5-[difluoro(pyridin-2-yl)methyl]-6...)Show SMILES Cn1c(nc2sc(nc2c1=O)N1CCC[C@@H]1C(=O)NCc1ccccc1)C(F)(F)c1ccccn1 |r| Show InChI InChI=1S/C24H22F2N6O2S/c1-31-21(34)18-20(30-22(31)24(25,26)17-11-5-6-12-27-17)35-23(29-18)32-13-7-10-16(32)19(33)28-14-15-8-3-2-4-9-15/h2-6,8-9,11-12,16H,7,10,13-14H2,1H3,(H,28,33)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
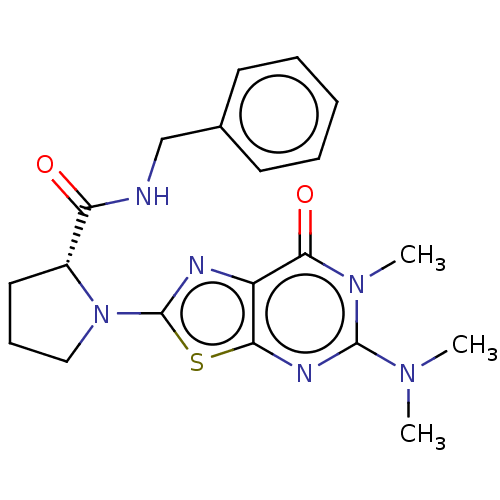
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273114
((R)-N-benzyl-1-[5-(N',N'-dimethylamino)-6-methyl-7...)Show SMILES CN(C)c1nc2sc(nc2c(=O)n1C)N1CCC[C@@H]1C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C20H24N6O2S/c1-24(2)19-23-17-15(18(28)25(19)3)22-20(29-17)26-11-7-10-14(26)16(27)21-12-13-8-5-4-6-9-13/h4-6,8-9,14H,7,10-12H2,1-3H3,(H,21,27)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50601192
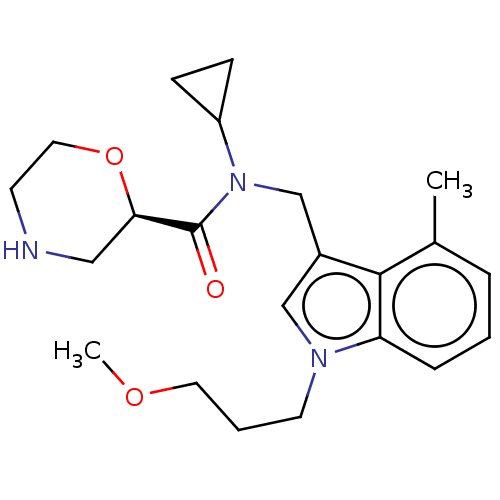
(CHEMBL5176733)Show SMILES COCCCn1cc(CN(C2CC2)C(=O)[C@H]2CNCCO2)c2c(C)cccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00280
BindingDB Entry DOI: 10.7270/Q28K7F4S |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273131
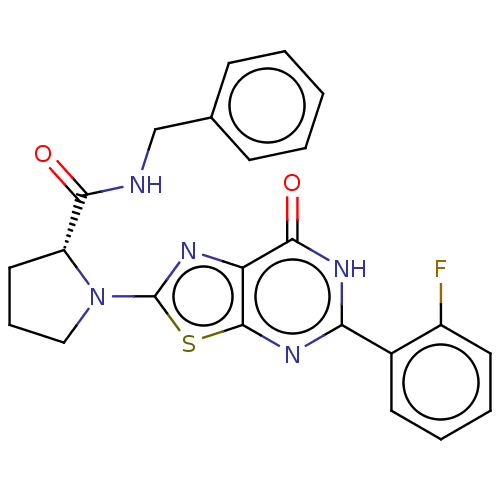
((R)-N-benzyl-1-[5-(2-fluorophenyl)-7-oxo-6,7-dihyd...)Show SMILES Fc1ccccc1-c1nc2sc(nc2c(=O)[nH]1)N1CCC[C@@H]1C(=O)NCc1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273030
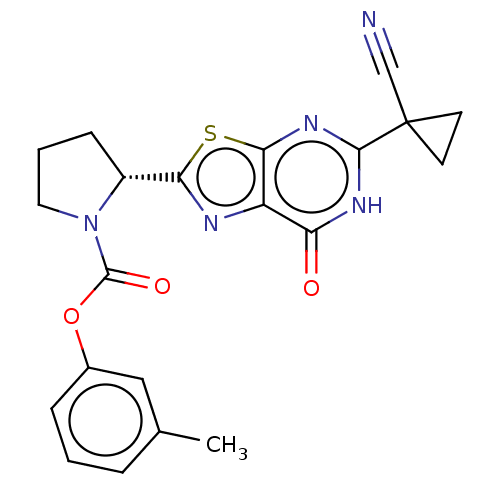
((R)-2-[5-(1-cyanocyclopropyl)-7-oxo-6,7-dihydro[1,...)Show SMILES Cc1cccc(OC(=O)N2CCC[C@@H]2c2nc3c(nc([nH]c3=O)C3(CC3)C#N)s2)c1 |r| Show InChI InChI=1S/C21H19N5O3S/c1-12-4-2-5-13(10-12)29-20(28)26-9-3-6-14(26)17-23-15-16(27)24-19(25-18(15)30-17)21(11-22)7-8-21/h2,4-5,10,14H,3,6-9H2,1H3,(H,24,25,27)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50598965

(CHEMBL5188936)Show SMILES COC(=O)NCCCn1cc([C@@H](C)N(C2CC2)C(=O)[C@H]2CNCCO2)c2nccnc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273139
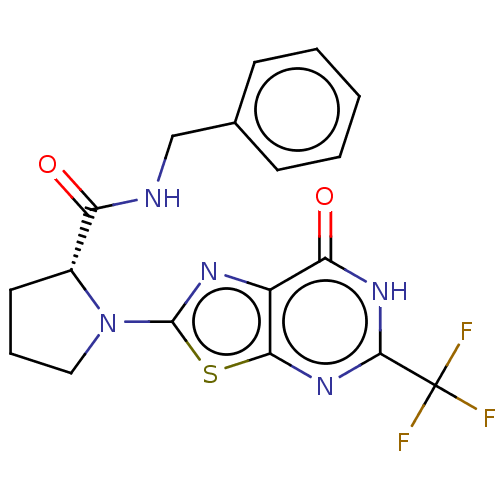
((R)-N-benzyl-1-(7-oxo-5-trifluoromethyl-6,7-dihydr...)Show SMILES FC(F)(F)c1nc2sc(nc2c(=O)[nH]1)N1CCC[C@@H]1C(=O)NCc1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273076
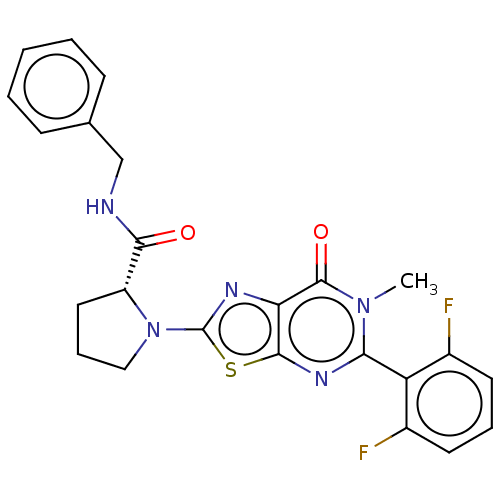
((R)-N-benzyl-1-[5-(2,6-difluorophenyl)-6-methyl-7-...)Show SMILES Cn1c(nc2sc(nc2c1=O)N1CCC[C@@H]1C(=O)NCc1ccccc1)-c1c(F)cccc1F |r,wD:15.18,(6,-.08,;4.66,-.85,;4.66,-2.39,;3.33,-3.16,;1.99,-2.39,;.53,-2.87,;-.37,-1.62,;.53,-.37,;1.99,-.85,;3.33,-.08,;3.33,1.46,;-1.91,-1.62,;-2.82,-2.87,;-4.28,-2.39,;-4.28,-.85,;-2.82,-.37,;-2.42,1.11,;-.93,1.51,;-3.51,2.2,;-5,1.8,;-6.09,2.89,;-5.69,4.38,;-6.78,5.47,;-8.26,5.07,;-8.66,3.58,;-7.57,2.49,;6,-3.16,;6,-4.7,;4.66,-5.47,;7.33,-5.47,;8.66,-4.7,;8.66,-3.16,;7.33,-2.39,;8.1,-1.06,)| Show InChI InChI=1S/C24H21F2N5O2S/c1-30-20(18-15(25)9-5-10-16(18)26)29-22-19(23(30)33)28-24(34-22)31-12-6-11-17(31)21(32)27-13-14-7-3-2-4-8-14/h2-5,7-10,17H,6,11-13H2,1H3,(H,27,32)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273132
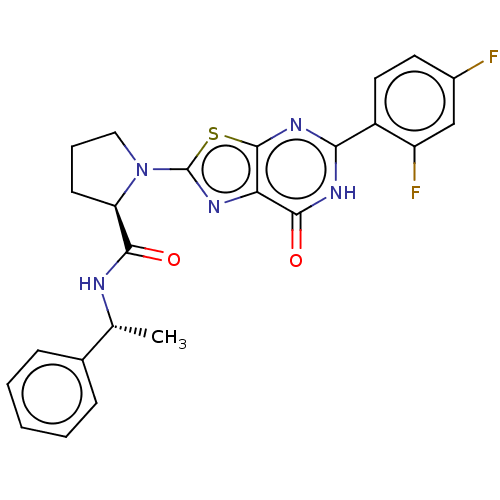
((R)-1-[5-(2,4-difluorophenyl)-7-oxo-6,7-dihydro[1,...)Show SMILES C[C@@H](NC(=O)[C@H]1CCCN1c1nc2c(nc([nH]c2=O)-c2ccc(F)cc2F)s1)c1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273077
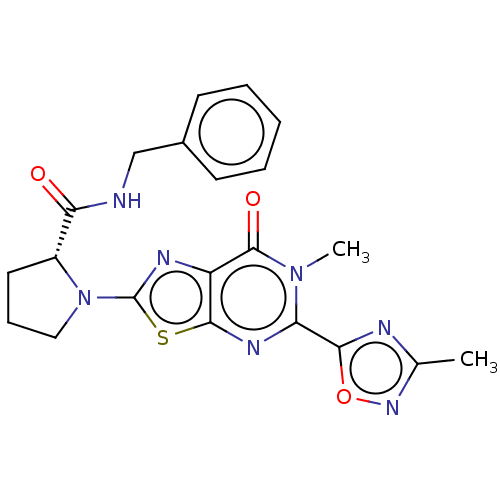
((R)-N-benzyl-1-[6-methyl-5-(3-methyl-1,2,4-oxadiaz...)Show SMILES Cc1noc(n1)-c1nc2sc(nc2c(=O)n1C)N1CCC[C@@H]1C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C21H21N7O3S/c1-12-23-18(31-26-12)16-25-19-15(20(30)27(16)2)24-21(32-19)28-10-6-9-14(28)17(29)22-11-13-7-4-3-5-8-13/h3-5,7-8,14H,6,9-11H2,1-2H3,(H,22,29)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273159
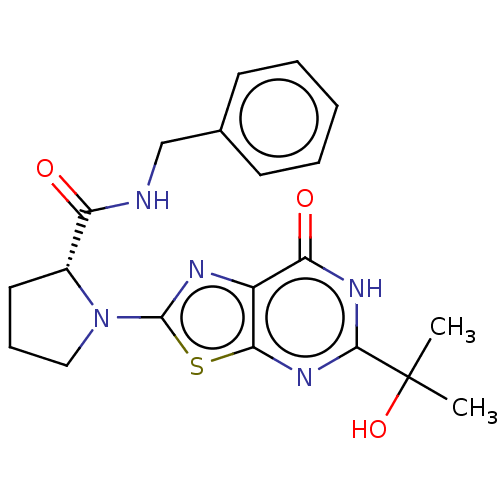
((R)-N-benzyl-1-[5-(2-hydroxypropan-2-yl)-7-oxo-6,7...)Show SMILES CC(C)(O)c1nc2sc(nc2c(=O)[nH]1)N1CCC[C@@H]1C(=O)NCc1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50598962
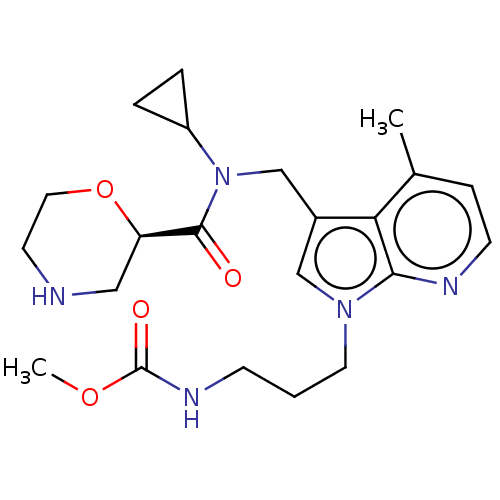
(CHEMBL5190224)Show SMILES COC(=O)NCCCn1cc(CN(C2CC2)C(=O)[C@H]2CNCCO2)c2c(C)ccnc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00834
BindingDB Entry DOI: 10.7270/Q2JS9VH0 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273116
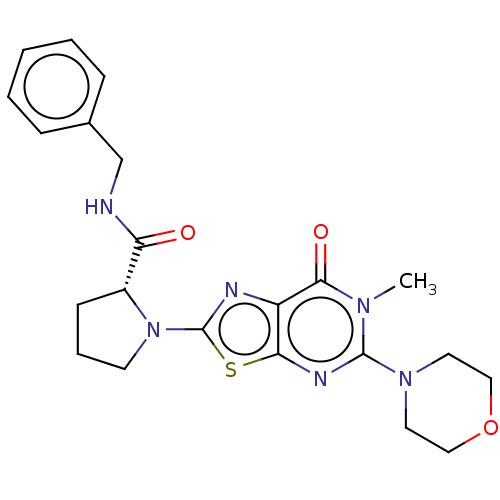
((R)-N-benzyl-1-[6-methyl-5-(morpholin-4-yl)-7-oxo-...)Show SMILES Cn1c(nc2sc(nc2c1=O)N1CCC[C@@H]1C(=O)NCc1ccccc1)N1CCOCC1 |r| Show InChI InChI=1S/C22H26N6O3S/c1-26-20(30)17-19(25-21(26)27-10-12-31-13-11-27)32-22(24-17)28-9-5-8-16(28)18(29)23-14-15-6-3-2-4-7-15/h2-4,6-7,16H,5,8-14H2,1H3,(H,23,29)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273123
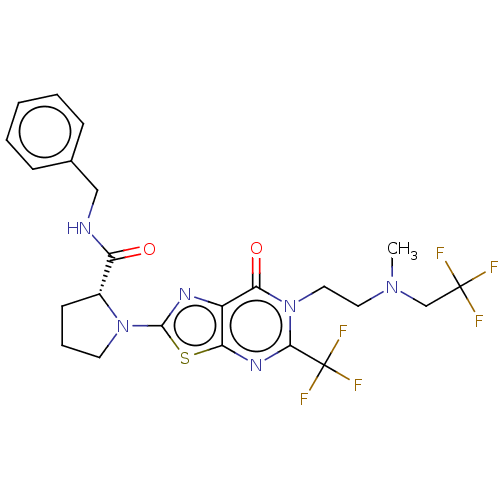
((R)-N-benzyl-1-[6-{2-[N'-methyl-N'-(2,2,2-trifluor...)Show SMILES CN(CCn1c(nc2sc(nc2c1=O)N1CCC[C@@H]1C(=O)NCc1ccccc1)C(F)(F)F)CC(F)(F)F |r| Show InChI InChI=1S/C23H24F6N6O2S/c1-33(13-22(24,25)26)10-11-35-19(37)16-18(32-20(35)23(27,28)29)38-21(31-16)34-9-5-8-15(34)17(36)30-12-14-6-3-2-4-7-14/h2-4,6-7,15H,5,8-13H2,1H3,(H,30,36)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273158

((R)-N-benzyl-1-(5,5-difluoro-10-oxo-5,7,8,10-tetra...)Show SMILES FC1(F)CCCn2c1nc1sc(nc1c2=O)N1CCC[C@@H]1C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C21H21F2N5O2S/c22-21(23)9-5-11-28-18(30)15-17(26-19(21)28)31-20(25-15)27-10-4-8-14(27)16(29)24-12-13-6-2-1-3-7-13/h1-3,6-7,14H,4-5,8-12H2,(H,24,29)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273028
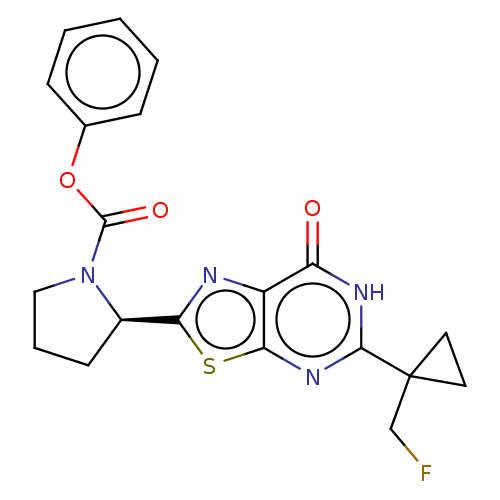
((R)-2-{5-[1-(fluoromethyl)cyclopropyl]-7-oxo-6,7-d...)Show SMILES FCC1(CC1)c1nc2sc(nc2c(=O)[nH]1)[C@H]1CCCN1C(=O)Oc1ccccc1 |r| Show InChI InChI=1S/C20H19FN4O3S/c21-11-20(8-9-20)18-23-15(26)14-17(24-18)29-16(22-14)13-7-4-10-25(13)19(27)28-12-5-2-1-3-6-12/h1-3,5-6,13H,4,7-11H2,(H,23,24,26)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273118
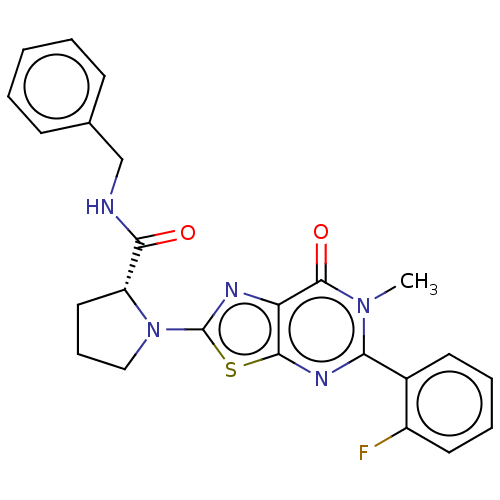
((R)-N-benzyl-1-[5-(2-fluorophenyl)-6-methyl-7-oxo-...)Show SMILES Cn1c(nc2sc(nc2c1=O)N1CCC[C@@H]1C(=O)NCc1ccccc1)-c1ccccc1F |r| Show InChI InChI=1S/C24H22FN5O2S/c1-29-20(16-10-5-6-11-17(16)25)28-22-19(23(29)32)27-24(33-22)30-13-7-12-18(30)21(31)26-14-15-8-3-2-4-9-15/h2-6,8-11,18H,7,12-14H2,1H3,(H,26,31)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM273095
((R)-N-benzyl-1-[5-(1-fluorocyclopropyl)-6-methyl-7...)Show SMILES Cn1c(nc2sc(nc2c1=O)N1CCC[C@@H]1C(=O)NCc1ccccc1)C1(F)CC1 |r| Show InChI InChI=1S/C21H22FN5O2S/c1-26-18(29)15-17(25-19(26)21(22)9-10-21)30-20(24-15)27-11-5-8-14(27)16(28)23-12-13-6-3-2-4-7-13/h2-4,6-7,14H,5,8-12H2,1H3,(H,23,28)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION
US Patent
| Assay Description
The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... |
US Patent US10065972 (2018)
BindingDB Entry DOI: 10.7270/Q23X88P5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data