 Found 85 hits with Last Name = 'inada' and Initial = 'y'
Found 85 hits with Last Name = 'inada' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor B
(RAT) | BDBM50240609
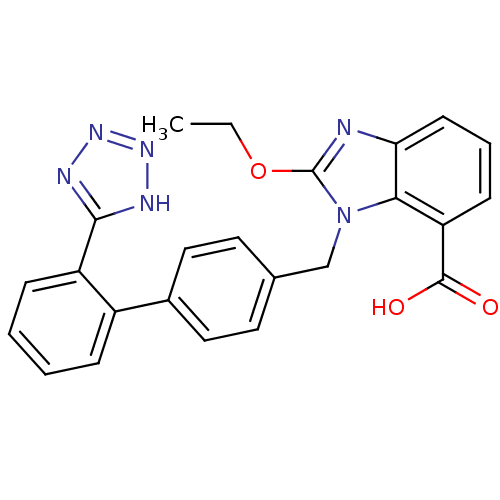
(2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCOc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O3/c1-2-33-24-25-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-26-28-29-27-22/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50240609

(2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCOc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O3/c1-2-33-24-25-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-26-28-29-27-22/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50240609

(2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCOc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O3/c1-2-33-24-25-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-26-28-29-27-22/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM82077
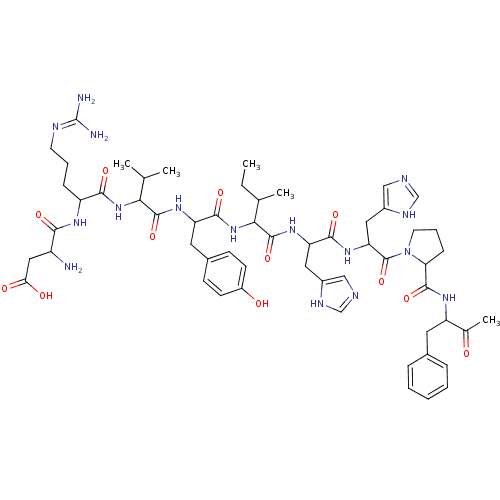
(Angiotensin II | CAS_11128-99-7 | NSC_439662)Show SMILES CCC(C)C(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)C(N)CC(O)=O)C(C)C)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(Cc1cnc[nH]1)C(=O)N1CCCC1C(=O)NC(Cc1ccccc1)C(C)=O |(1.13,.52,;1.58,1.99,;.53,3.12,;-.97,2.77,;.98,4.59,;2.48,4.94,;3.54,3.81,;3.08,2.34,;5.04,4.16,;5.49,5.63,;4.44,6.76,;2.93,6.41,;1.88,7.54,;2.33,9.01,;1.28,10.14,;3.84,9.36,;4.89,8.23,;6.09,3.03,;7.59,3.38,;8.04,4.85,;8.64,2.25,;10.14,2.6,;11.19,1.47,;10.74,-0,;12.69,1.82,;13.74,.69,;13.29,-.78,;14.34,-1.91,;13.89,-3.38,;12.39,-3.73,;11.94,-5.2,;11.34,-2.6,;13.14,3.29,;14.64,3.64,;15.69,2.51,;15.09,5.11,;14.04,6.24,;16.59,5.46,;17.04,6.93,;15.99,8.06,;18.54,7.28,;8.19,.78,;9.24,-.35,;6.69,.43,;-.07,5.72,;.38,7.19,;-1.57,5.37,;-2.62,6.5,;-2.17,7.97,;-3.22,9.1,;-2.92,10.61,;-4.27,11.35,;-5.4,10.3,;-4.75,8.91,;-4.12,6.15,;-4.57,4.68,;-5.17,7.28,;-6.67,6.93,;-6.64,5.39,;-7.96,4.6,;-9.38,5.2,;-10.39,4.04,;-9.6,2.72,;-8.1,3.06,;-7.72,8.06,;-7.27,9.53,;-9.22,7.71,;-10.38,8.72,;-11.7,7.93,;-11.36,6.43,;-9.82,6.29,;-10.42,4.87,;-10.87,3.4,;-11.95,4.69,;-12.55,3.27,;-14.08,3.08,;-14.68,1.66,;-13.76,.43,;-14.36,-.98,;-15.89,-1.17,;-16.81,.06,;-16.21,1.48,;-11.63,2.04,;-10.1,2.23,;-12.23,.62,)| Show InChI InChI=1S/C57H80N16O12/c1-6-32(4)48(72-52(81)42(23-35-16-18-38(75)19-17-35)68-54(83)47(31(2)3)71-50(79)40(14-10-20-63-57(59)60)66-49(78)39(58)26-46(76)77)55(84)69-43(24-36-27-61-29-64-36)51(80)70-44(25-37-28-62-30-65-37)56(85)73-21-11-15-45(73)53(82)67-41(33(5)74)22-34-12-8-7-9-13-34/h7-9,12-13,16-19,27-32,39-45,47-48,75H,6,10-11,14-15,20-26,58H2,1-5H3,(H,61,64)(H,62,65)(H,66,78)(H,67,82)(H,68,83)(H,69,84)(H,70,80)(H,71,79)(H,72,81)(H,76,77)(H4,59,60,63) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM82077

(Angiotensin II | CAS_11128-99-7 | NSC_439662)Show SMILES CCC(C)C(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)C(N)CC(O)=O)C(C)C)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(Cc1cnc[nH]1)C(=O)N1CCCC1C(=O)NC(Cc1ccccc1)C(C)=O |(1.13,.52,;1.58,1.99,;.53,3.12,;-.97,2.77,;.98,4.59,;2.48,4.94,;3.54,3.81,;3.08,2.34,;5.04,4.16,;5.49,5.63,;4.44,6.76,;2.93,6.41,;1.88,7.54,;2.33,9.01,;1.28,10.14,;3.84,9.36,;4.89,8.23,;6.09,3.03,;7.59,3.38,;8.04,4.85,;8.64,2.25,;10.14,2.6,;11.19,1.47,;10.74,-0,;12.69,1.82,;13.74,.69,;13.29,-.78,;14.34,-1.91,;13.89,-3.38,;12.39,-3.73,;11.94,-5.2,;11.34,-2.6,;13.14,3.29,;14.64,3.64,;15.69,2.51,;15.09,5.11,;14.04,6.24,;16.59,5.46,;17.04,6.93,;15.99,8.06,;18.54,7.28,;8.19,.78,;9.24,-.35,;6.69,.43,;-.07,5.72,;.38,7.19,;-1.57,5.37,;-2.62,6.5,;-2.17,7.97,;-3.22,9.1,;-2.92,10.61,;-4.27,11.35,;-5.4,10.3,;-4.75,8.91,;-4.12,6.15,;-4.57,4.68,;-5.17,7.28,;-6.67,6.93,;-6.64,5.39,;-7.96,4.6,;-9.38,5.2,;-10.39,4.04,;-9.6,2.72,;-8.1,3.06,;-7.72,8.06,;-7.27,9.53,;-9.22,7.71,;-10.38,8.72,;-11.7,7.93,;-11.36,6.43,;-9.82,6.29,;-10.42,4.87,;-10.87,3.4,;-11.95,4.69,;-12.55,3.27,;-14.08,3.08,;-14.68,1.66,;-13.76,.43,;-14.36,-.98,;-15.89,-1.17,;-16.81,.06,;-16.21,1.48,;-11.63,2.04,;-10.1,2.23,;-12.23,.62,)| Show InChI InChI=1S/C57H80N16O12/c1-6-32(4)48(72-52(81)42(23-35-16-18-38(75)19-17-35)68-54(83)47(31(2)3)71-50(79)40(14-10-20-63-57(59)60)66-49(78)39(58)26-46(76)77)55(84)69-43(24-36-27-61-29-64-36)51(80)70-44(25-37-28-62-30-65-37)56(85)73-21-11-15-45(73)53(82)67-41(33(5)74)22-34-12-8-7-9-13-34/h7-9,12-13,16-19,27-32,39-45,47-48,75H,6,10-11,14-15,20-26,58H2,1-5H3,(H,61,64)(H,62,65)(H,66,78)(H,67,82)(H,68,83)(H,69,84)(H,70,80)(H,71,79)(H,72,81)(H,76,77)(H4,59,60,63) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82077

(Angiotensin II | CAS_11128-99-7 | NSC_439662)Show SMILES CCC(C)C(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)C(N)CC(O)=O)C(C)C)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(Cc1cnc[nH]1)C(=O)N1CCCC1C(=O)NC(Cc1ccccc1)C(C)=O |(1.13,.52,;1.58,1.99,;.53,3.12,;-.97,2.77,;.98,4.59,;2.48,4.94,;3.54,3.81,;3.08,2.34,;5.04,4.16,;5.49,5.63,;4.44,6.76,;2.93,6.41,;1.88,7.54,;2.33,9.01,;1.28,10.14,;3.84,9.36,;4.89,8.23,;6.09,3.03,;7.59,3.38,;8.04,4.85,;8.64,2.25,;10.14,2.6,;11.19,1.47,;10.74,-0,;12.69,1.82,;13.74,.69,;13.29,-.78,;14.34,-1.91,;13.89,-3.38,;12.39,-3.73,;11.94,-5.2,;11.34,-2.6,;13.14,3.29,;14.64,3.64,;15.69,2.51,;15.09,5.11,;14.04,6.24,;16.59,5.46,;17.04,6.93,;15.99,8.06,;18.54,7.28,;8.19,.78,;9.24,-.35,;6.69,.43,;-.07,5.72,;.38,7.19,;-1.57,5.37,;-2.62,6.5,;-2.17,7.97,;-3.22,9.1,;-2.92,10.61,;-4.27,11.35,;-5.4,10.3,;-4.75,8.91,;-4.12,6.15,;-4.57,4.68,;-5.17,7.28,;-6.67,6.93,;-6.64,5.39,;-7.96,4.6,;-9.38,5.2,;-10.39,4.04,;-9.6,2.72,;-8.1,3.06,;-7.72,8.06,;-7.27,9.53,;-9.22,7.71,;-10.38,8.72,;-11.7,7.93,;-11.36,6.43,;-9.82,6.29,;-10.42,4.87,;-10.87,3.4,;-11.95,4.69,;-12.55,3.27,;-14.08,3.08,;-14.68,1.66,;-13.76,.43,;-14.36,-.98,;-15.89,-1.17,;-16.81,.06,;-16.21,1.48,;-11.63,2.04,;-10.1,2.23,;-12.23,.62,)| Show InChI InChI=1S/C57H80N16O12/c1-6-32(4)48(72-52(81)42(23-35-16-18-38(75)19-17-35)68-54(83)47(31(2)3)71-50(79)40(14-10-20-63-57(59)60)66-49(78)39(58)26-46(76)77)55(84)69-43(24-36-27-61-29-64-36)51(80)70-44(25-37-28-62-30-65-37)56(85)73-21-11-15-45(73)53(82)67-41(33(5)74)22-34-12-8-7-9-13-34/h7-9,12-13,16-19,27-32,39-45,47-48,75H,6,10-11,14-15,20-26,58H2,1-5H3,(H,61,64)(H,62,65)(H,66,78)(H,67,82)(H,68,83)(H,69,84)(H,70,80)(H,71,79)(H,72,81)(H,76,77)(H4,59,60,63) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM86060

(CAS_123794 | CGP 42112 | NSC_123794)Show SMILES CCC(C)C(NC(=O)C1CCCN1C(=O)C(Cc1cnc[nH]1)NC(=O)C(CCCCNC(=O)C(CCCN=C(N)N)NC(=O)OCc1ccccc1)NC(=O)C(Cc1ccc(O)cc1)NC(=O)c1cccnc1)C(O)=O |(27.48,-27.05,;26.03,-27.58,;24.85,-26.6,;25.11,-25.08,;23.4,-27.13,;22.22,-26.15,;20.78,-26.68,;20.51,-28.19,;19.59,-25.69,;19.69,-24.16,;18.26,-23.58,;17.28,-24.77,;18.1,-26.07,;17.53,-27.5,;18.48,-28.71,;16,-27.72,;15.43,-29.15,;16.39,-30.36,;15.97,-31.84,;17.25,-32.7,;18.46,-31.75,;17.92,-30.3,;15.05,-26.51,;13.53,-26.73,;12.96,-28.16,;12.57,-25.52,;11.05,-25.74,;10.1,-24.53,;8.57,-24.75,;7.62,-23.54,;6.1,-23.76,;5.14,-22.55,;5.72,-21.12,;3.62,-22.77,;3.05,-24.2,;4,-25.41,;3.43,-26.84,;4.38,-28.05,;5.91,-27.83,;6.86,-29.04,;6.48,-26.4,;2.67,-21.56,;1.14,-21.78,;.57,-23.21,;.19,-20.57,;-1.33,-20.79,;-2.29,-19.58,;-3.81,-19.8,;-4.76,-18.59,;-4.19,-17.16,;-2.67,-16.94,;-1.71,-18.15,;13.15,-24.09,;14.67,-23.87,;15.62,-25.08,;15.24,-22.44,;14.29,-21.23,;12.77,-21.45,;12.19,-22.88,;10.67,-23.1,;9.72,-21.89,;8.19,-22.11,;10.29,-20.46,;11.81,-20.24,;16.77,-22.22,;17.34,-20.79,;16.39,-19.58,;18.86,-20.57,;19.81,-21.78,;21.34,-21.56,;21.91,-20.13,;20.96,-18.92,;19.43,-19.14,;23.14,-28.65,;24.33,-29.63,;21.7,-29.18,)| Show InChI InChI=1S/C52H69N13O11/c1-3-32(2)43(50(73)74)64-48(71)42-17-11-25-65(42)49(72)41(27-36-29-56-31-59-36)62-46(69)39(60-47(70)40(26-33-18-20-37(66)21-19-33)61-44(67)35-14-9-22-55-28-35)15-7-8-23-57-45(68)38(16-10-24-58-51(53)54)63-52(75)76-30-34-12-5-4-6-13-34/h4-6,9,12-14,18-22,28-29,31-32,38-43,66H,3,7-8,10-11,15-17,23-27,30H2,1-2H3,(H,56,59)(H,57,68)(H,60,70)(H,61,67)(H,62,69)(H,63,75)(H,64,71)(H,73,74)(H4,53,54,58) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50006909
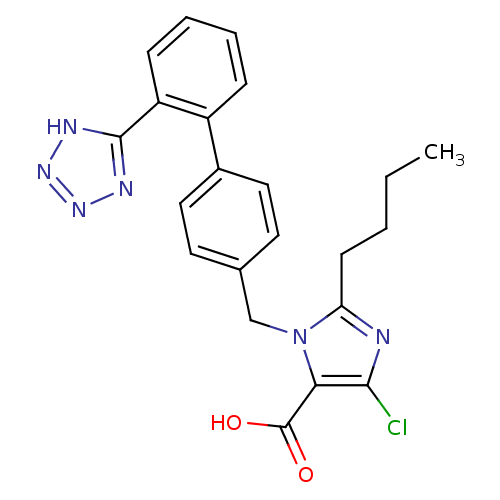
(2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50006909

(2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50287290
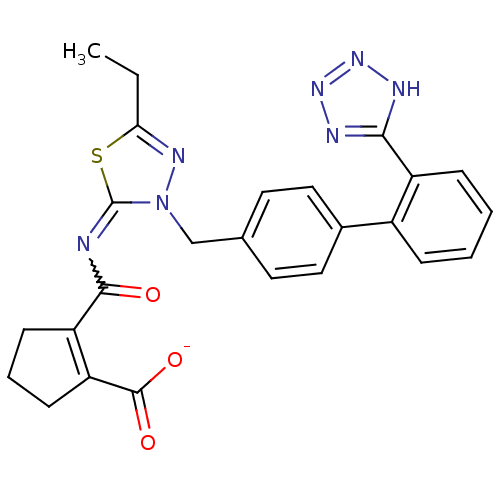
(CHEMBL34866 | KRH-594 | Potassium; 2-[5-ethyl-3-[2...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)C2=C(CCC2)C([O-])=O)s1 |w:24.27,t:30| Show InChI InChI=1S/C25H23N7O3S/c1-2-21-29-32(25(36-21)26-23(33)19-8-5-9-20(19)24(34)35)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-27-30-31-28-22/h3-4,6-7,10-13H,2,5,8-9,14H2,1H3,(H,34,35)(H,27,28,30,31)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50006909

(2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50287290

(CHEMBL34866 | KRH-594 | Potassium; 2-[5-ethyl-3-[2...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)C2=C(CCC2)C([O-])=O)s1 |w:24.27,t:30| Show InChI InChI=1S/C25H23N7O3S/c1-2-21-29-32(25(36-21)26-23(33)19-8-5-9-20(19)24(34)35)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-27-30-31-28-22/h3-4,6-7,10-13H,2,5,8-9,14H2,1H3,(H,34,35)(H,27,28,30,31)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50287290

(CHEMBL34866 | KRH-594 | Potassium; 2-[5-ethyl-3-[2...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)C2=C(CCC2)C([O-])=O)s1 |w:24.27,t:30| Show InChI InChI=1S/C25H23N7O3S/c1-2-21-29-32(25(36-21)26-23(33)19-8-5-9-20(19)24(34)35)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-27-30-31-28-22/h3-4,6-7,10-13H,2,5,8-9,14H2,1H3,(H,34,35)(H,27,28,30,31)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50287290

(CHEMBL34866 | KRH-594 | Potassium; 2-[5-ethyl-3-[2...)Show SMILES CCc1nn(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(=NC(=O)C2=C(CCC2)C([O-])=O)s1 |w:24.27,t:30| Show InChI InChI=1S/C25H23N7O3S/c1-2-21-29-32(25(36-21)26-23(33)19-8-5-9-20(19)24(34)35)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-27-30-31-28-22/h3-4,6-7,10-13H,2,5,8-9,14H2,1H3,(H,34,35)(H,27,28,30,31)/p-1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50006909

(2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50240609

(2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCOc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O3/c1-2-33-24-25-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-26-28-29-27-22/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shinshu University
Curated by PDSP Ki Database
| |
Fundam Clin Pharmacol 16: 317-23 (2002)
Article DOI: 10.1046/j.1472-8206.2002.00076.x
BindingDB Entry DOI: 10.7270/Q2697259 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50005097
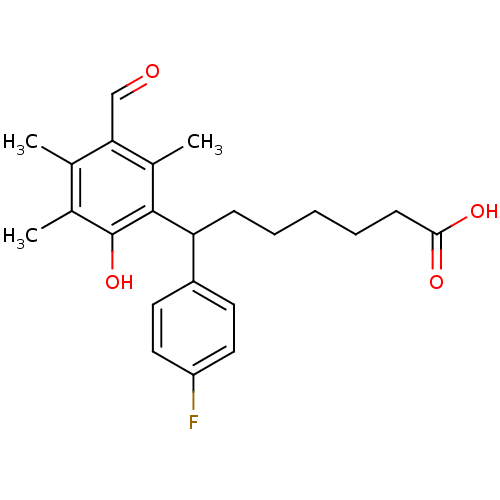
(7-(4-Fluoro-phenyl)-7-(3-formyl-6-hydroxy-2,4,5-tr...)Show SMILES Cc1c(C)c(C=O)c(C)c(C(CCCCCC(O)=O)c2ccc(F)cc2)c1O Show InChI InChI=1S/C23H27FO4/c1-14-15(2)23(28)22(16(3)20(14)13-25)19(7-5-4-6-8-21(26)27)17-9-11-18(24)12-10-17/h9-13,19,28H,4-8H2,1-3H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to inhibit specific binding of [3H]-U-46,619 to Thromboxane A2/ Prostaglandin H2 receptor in guinea pig platel... |
J Med Chem 35: 2202-9 (1992)
BindingDB Entry DOI: 10.7270/Q2JQ0ZZZ |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50005108
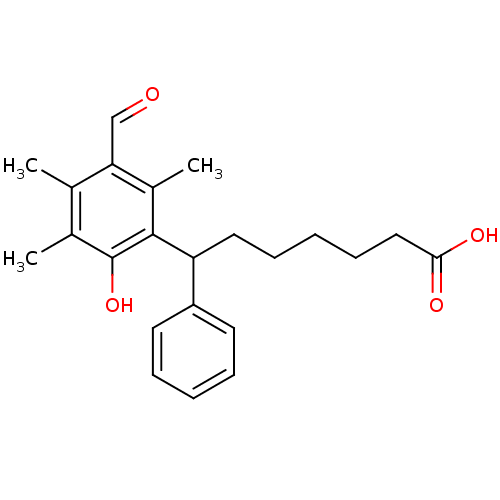
(7-(3-Formyl-6-hydroxy-2,4,5-trimethyl-phenyl)-7-ph...)Show SMILES Cc1c(C)c(C=O)c(C)c(C(CCCCCC(O)=O)c2ccccc2)c1O Show InChI InChI=1S/C23H28O4/c1-15-16(2)23(27)22(17(3)20(15)14-24)19(18-10-6-4-7-11-18)12-8-5-9-13-21(25)26/h4,6-7,10-11,14,19,27H,5,8-9,12-13H2,1-3H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to inhibit specific binding of [3H]-U-46,619 to Thromboxane A2/ Prostaglandin H2 receptor in guinea pig platel... |
J Med Chem 35: 2202-9 (1992)
BindingDB Entry DOI: 10.7270/Q2JQ0ZZZ |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50005102

(7-(4-Fluoro-phenyl)-7-(4-formyl-2-hydroxy-3,5,6-tr...)Show SMILES Cc1c(C)c(C(CCCCCC(O)=O)c2ccc(F)cc2)c(O)c(C)c1C=O Show InChI InChI=1S/C23H27FO4/c1-14-15(2)22(23(28)16(3)20(14)13-25)19(7-5-4-6-8-21(26)27)17-9-11-18(24)12-10-17/h9-13,19,28H,4-8H2,1-3H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to inhibit specific binding of [3H]-U-46,619 to Thromboxane A2/ Prostaglandin H2 receptor in guinea pig platel... |
J Med Chem 35: 2202-9 (1992)
BindingDB Entry DOI: 10.7270/Q2JQ0ZZZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044403
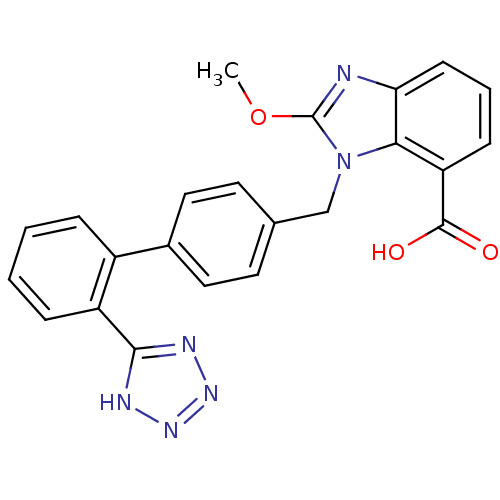
(2-Methoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylme...)Show SMILES COc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H18N6O3/c1-32-23-24-19-8-4-7-18(22(30)31)20(19)29(23)13-14-9-11-15(12-10-14)16-5-2-3-6-17(16)21-25-27-28-26-21/h2-12H,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50282377
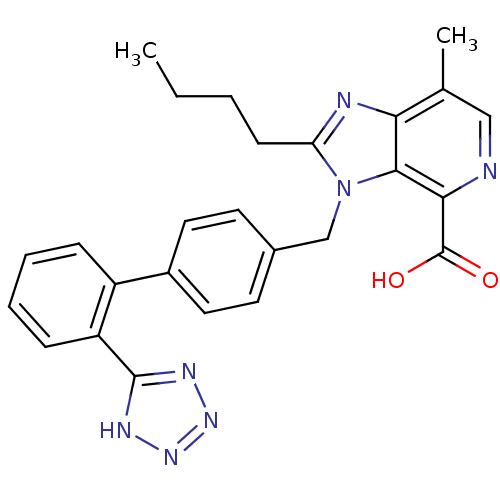
(2-Butyl-7-methyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2c(C)cnc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H25N7O2/c1-3-4-9-21-28-22-16(2)14-27-23(26(34)35)24(22)33(21)15-17-10-12-18(13-11-17)19-7-5-6-8-20(19)25-29-31-32-30-25/h5-8,10-14H,3-4,9,15H2,1-2H3,(H,34,35)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [125I]-AII binding to Angiotensin II receptor from membrane fractions of bovine adrenal cortex |
Bioorg Med Chem Lett 4: 35-40 (1994)
Article DOI: 10.1016/S0960-894X(01)81118-5
BindingDB Entry DOI: 10.7270/Q21Z44CP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044379

(2-Propylamino-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-...)Show SMILES CCCNc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H23N7O2/c1-2-14-26-25-27-21-9-5-8-20(24(33)34)22(21)32(25)15-16-10-12-17(13-11-16)18-6-3-4-7-19(18)23-28-30-31-29-23/h3-13H,2,14-15H2,1H3,(H,26,27)(H,33,34)(H,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50285002
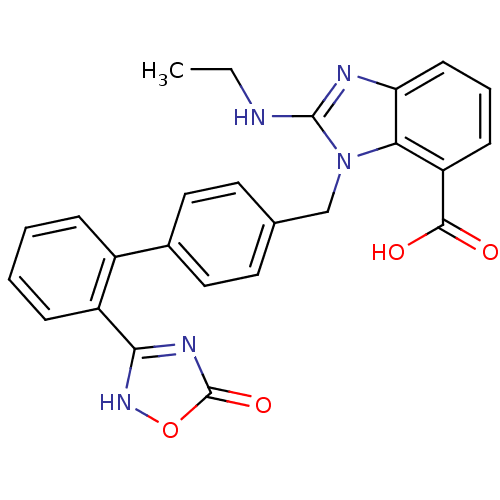
(2-Ethylamino-3-[2'-(5-oxo-4,5-dihydro-[1,2,4]oxadi...)Show SMILES CCNc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nc(=O)o[nH]1 Show InChI InChI=1S/C25H21N5O4/c1-2-26-24-27-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-28-25(33)34-29-22/h3-13H,2,14H2,1H3,(H,26,27)(H,31,32)(H,28,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-AII (0.2 nM) from bovine adrenal cortical membrane angiotensin II (AII) receptor at 10e-7 M |
Bioorg Med Chem Lett 5: 1903-1908 (1995)
Article DOI: 10.1016/0960-894X(95)00319-O
BindingDB Entry DOI: 10.7270/Q26M36SM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044410
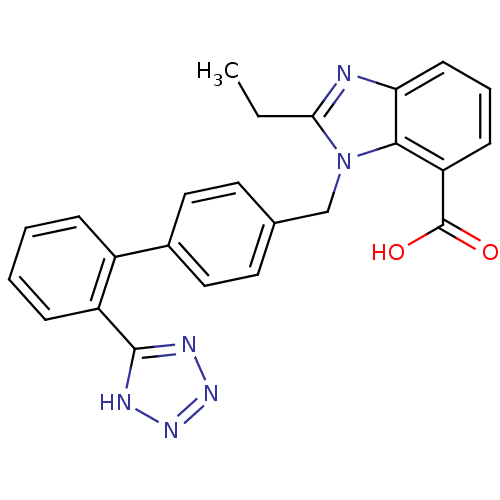
(2-Ethyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O2/c1-2-21-25-20-9-5-8-19(24(31)32)22(20)30(21)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)23-26-28-29-27-23/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50282379
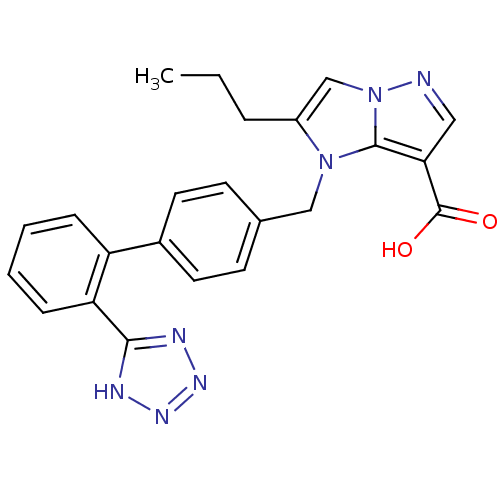
(2-Propyl-1-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCCc1cn2ncc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H21N7O2/c1-2-5-17-14-30-22(20(12-24-30)23(31)32)29(17)13-15-8-10-16(11-9-15)18-6-3-4-7-19(18)21-25-27-28-26-21/h3-4,6-12,14H,2,5,13H2,1H3,(H,31,32)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [125I]-AII binding to Angiotensin II receptor from membrane fractions of bovine adrenal cortex |
Bioorg Med Chem Lett 4: 35-40 (1994)
Article DOI: 10.1016/S0960-894X(01)81118-5
BindingDB Entry DOI: 10.7270/Q21Z44CP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50282373
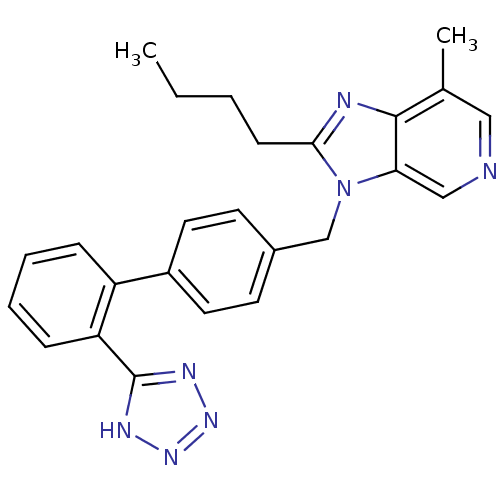
(2-Butyl-7-methyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2c(C)cncc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H25N7/c1-3-4-9-23-27-24-17(2)14-26-15-22(24)32(23)16-18-10-12-19(13-11-18)20-7-5-6-8-21(20)25-28-30-31-29-25/h5-8,10-15H,3-4,9,16H2,1-2H3,(H,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [125I]-AII binding to Angiotensin II receptor from membrane fractions of bovine adrenal cortex |
Bioorg Med Chem Lett 4: 35-40 (1994)
Article DOI: 10.1016/S0960-894X(01)81118-5
BindingDB Entry DOI: 10.7270/Q21Z44CP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044414
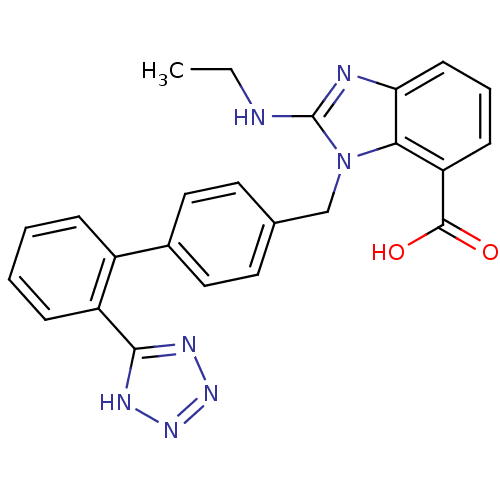
(2-Ethylamino-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-y...)Show SMILES CCNc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H21N7O2/c1-2-25-24-26-20-9-5-8-19(23(32)33)21(20)31(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-27-29-30-28-22/h3-13H,2,14H2,1H3,(H,25,26)(H,32,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044396
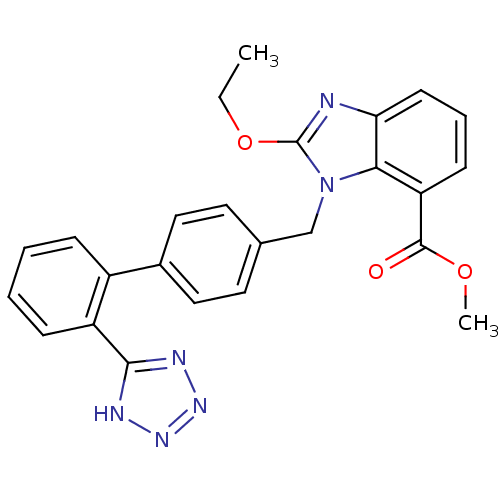
(2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCOc1nc2cccc(C(=O)OC)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H22N6O3/c1-3-34-25-26-21-10-6-9-20(24(32)33-2)22(21)31(25)15-16-11-13-17(14-12-16)18-7-4-5-8-19(18)23-27-29-30-28-23/h4-14H,3,15H2,1-2H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044386

(2-Isopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...)Show SMILES CC(C)c1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H22N6O2/c1-15(2)24-26-21-9-5-8-20(25(32)33)22(21)31(24)14-16-10-12-17(13-11-16)18-6-3-4-7-19(18)23-27-29-30-28-23/h3-13,15H,14H2,1-2H3,(H,32,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044389

(2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-...)Show SMILES OC(=O)c1cccc2nc(C3CC3)n(Cc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c12 Show InChI InChI=1S/C25H20N6O2/c32-25(33)20-6-3-7-21-22(20)31(24(26-21)17-12-13-17)14-15-8-10-16(11-9-15)18-4-1-2-5-19(18)23-27-29-30-28-23/h1-11,17H,12-14H2,(H,32,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044388

(2-Ethanesulfinyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCS(=O)c1nc2cccc(C(=O)OC)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H22N6O3S/c1-3-35(33)25-26-21-10-6-9-20(24(32)34-2)22(21)31(25)15-16-11-13-17(14-12-16)18-7-4-5-8-19(18)23-27-29-30-28-23/h4-14H,3,15H2,1-2H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50240609

(2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCOc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O3/c1-2-33-24-25-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-26-28-29-27-22/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [125I]-AII binding to Angiotensin II receptor from membrane fractions of bovine adrenal cortex |
Bioorg Med Chem Lett 4: 35-40 (1994)
Article DOI: 10.1016/S0960-894X(01)81118-5
BindingDB Entry DOI: 10.7270/Q21Z44CP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50240609

(2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCOc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O3/c1-2-33-24-25-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-26-28-29-27-22/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-AII (0.2 nM) from bovine adrenal cortical membrane angiotensin II (AII) receptor at 10e-7 M |
Bioorg Med Chem Lett 5: 1903-1908 (1995)
Article DOI: 10.1016/0960-894X(95)00319-O
BindingDB Entry DOI: 10.7270/Q26M36SM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50240609

(2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCOc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O3/c1-2-33-24-25-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-26-28-29-27-22/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044413

(2-Propylsulfanyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCSc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H22N6O2S/c1-2-14-34-25-26-21-9-5-8-20(24(32)33)22(21)31(25)15-16-10-12-17(13-11-16)18-6-3-4-7-19(18)23-27-29-30-28-23/h3-13H,2,14-15H2,1H3,(H,32,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044377
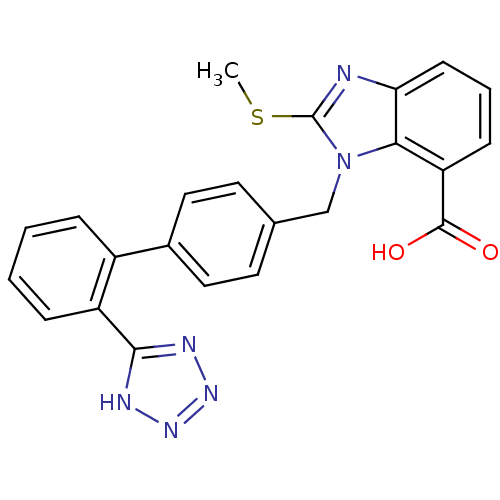
(2-Methylsulfanyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CSc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H18N6O2S/c1-32-23-24-19-8-4-7-18(22(30)31)20(19)29(23)13-14-9-11-15(12-10-14)16-5-2-3-6-17(16)21-25-27-28-26-21/h2-12H,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50406795
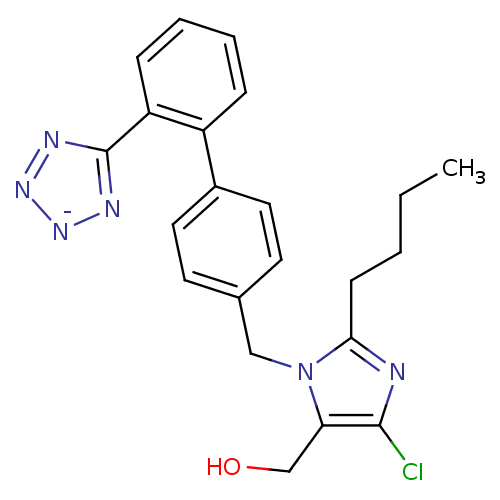
(Cozaar | LOSARTAN POTASSIUM)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C22H22ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3/q-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044406
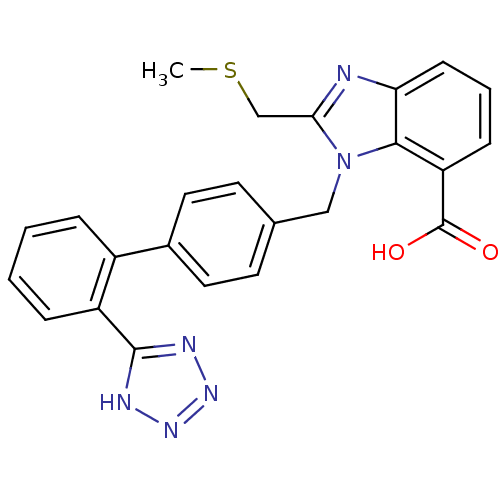
(2-Methylsulfanylmethyl-3-[2'-(1H-tetrazol-5-yl)-bi...)Show SMILES CSCc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O2S/c1-33-14-21-25-20-8-4-7-19(24(31)32)22(20)30(21)13-15-9-11-16(12-10-15)17-5-2-3-6-18(17)23-26-28-29-27-23/h2-12H,13-14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50009714
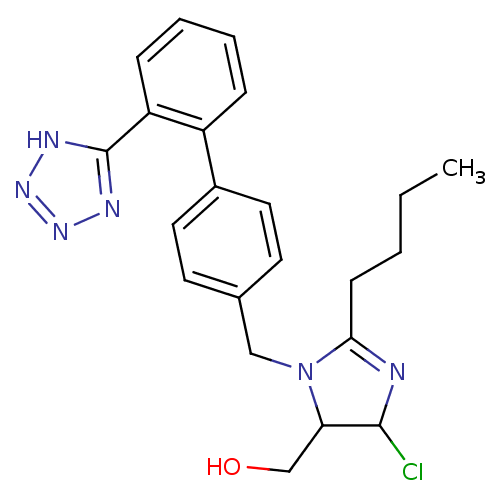
(CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...)Show SMILES CCCCC1=NC(Cl)C(CO)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C22H25ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,19,21,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-AII (0.2 nM) from bovine adrenal cortical membrane angiotensin II (AII) receptor at 10e-7 M |
Bioorg Med Chem Lett 5: 1903-1908 (1995)
Article DOI: 10.1016/0960-894X(95)00319-O
BindingDB Entry DOI: 10.7270/Q26M36SM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044375

(2-Propyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCCc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H22N6O2/c1-2-6-22-26-21-10-5-9-20(25(32)33)23(21)31(22)15-16-11-13-17(14-12-16)18-7-3-4-8-19(18)24-27-29-30-28-24/h3-5,7-14H,2,6,15H2,1H3,(H,32,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044408

(2-Methylamino-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-...)Show SMILES CNc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H19N7O2/c1-24-23-25-19-8-4-7-18(22(31)32)20(19)30(23)13-14-9-11-15(12-10-14)16-5-2-3-6-17(16)21-26-28-29-27-21/h2-12H,13H2,1H3,(H,24,25)(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044382
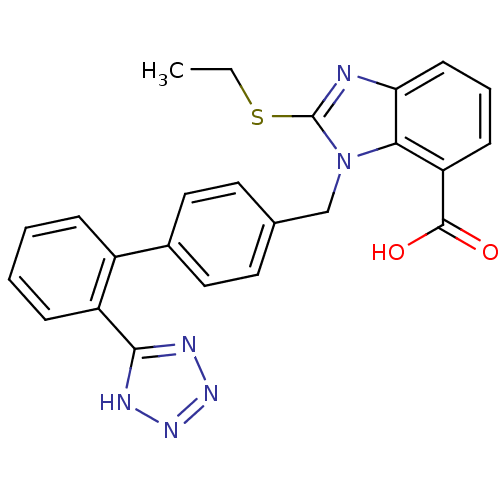
(2-Ethylsulfanyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-...)Show SMILES CCSc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O2S/c1-2-33-24-25-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-26-28-29-27-22/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044391
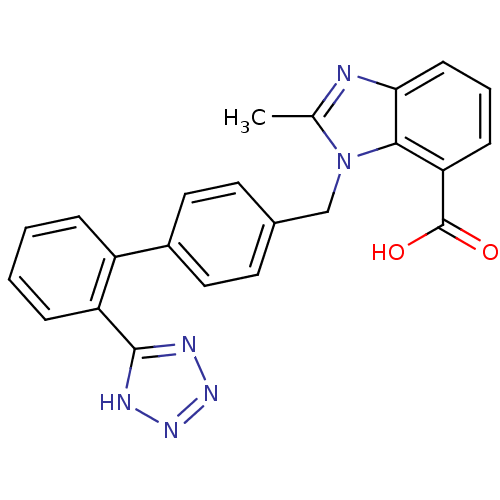
(2-Methyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES Cc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H18N6O2/c1-14-24-20-8-4-7-19(23(30)31)21(20)29(14)13-15-9-11-16(12-10-15)17-5-2-3-6-18(17)22-25-27-28-26-22/h2-12H,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044405
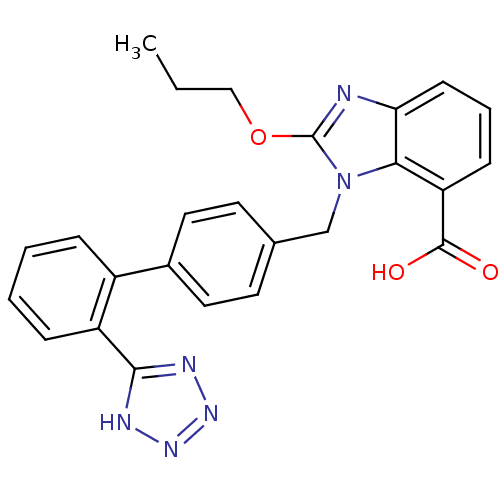
(2-Propoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylme...)Show SMILES CCCOc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H22N6O3/c1-2-14-34-25-26-21-9-5-8-20(24(32)33)22(21)31(25)15-16-10-12-17(13-11-16)18-6-3-4-7-19(18)23-27-29-30-28-23/h3-13H,2,14-15H2,1H3,(H,32,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044407
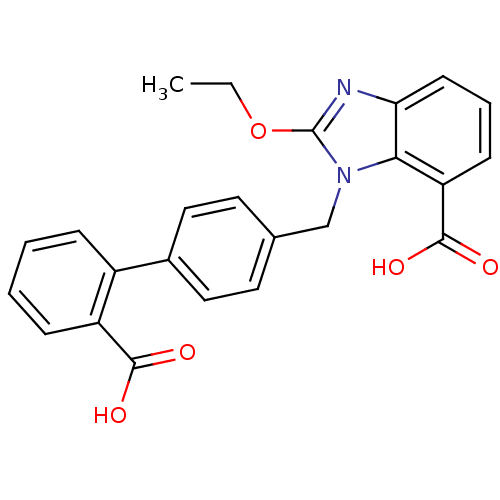
(3-(2'-Carboxy-biphenyl-4-ylmethyl)-2-ethoxy-3H-ben...)Show SMILES CCOc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C24H20N2O5/c1-2-31-24-25-20-9-5-8-19(23(29)30)21(20)26(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22(27)28/h3-13H,2,14H2,1H3,(H,27,28)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50005113
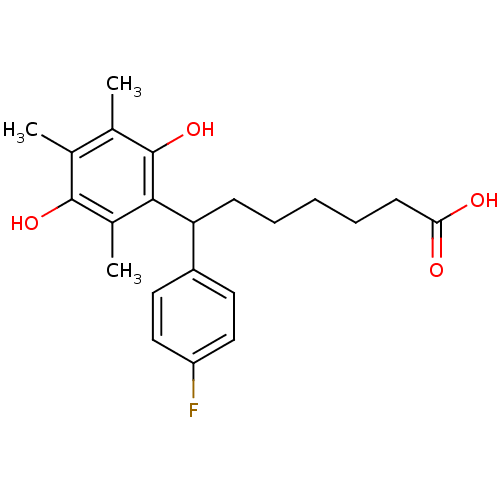
(7-(2,5-Dihydroxy-3,4,6-trimethyl-phenyl)-7-(4-fluo...)Show SMILES Cc1c(C)c(O)c(C(CCCCCC(O)=O)c2ccc(F)cc2)c(C)c1O Show InChI InChI=1S/C22H27FO4/c1-13-14(2)22(27)20(15(3)21(13)26)18(7-5-4-6-8-19(24)25)16-9-11-17(23)12-10-16/h9-12,18,26-27H,4-8H2,1-3H3,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to inhibit specific binding of [3H]-U-46,619 to Thromboxane A2/ Prostaglandin H2 receptor in guinea pig platel... |
J Med Chem 35: 2202-9 (1992)
BindingDB Entry DOI: 10.7270/Q2JQ0ZZZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044387

(2-Methoxymethyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-...)Show SMILES COCc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O3/c1-33-14-21-25-20-8-4-7-19(24(31)32)22(20)30(21)13-15-9-11-16(12-10-15)17-5-2-3-6-18(17)23-26-28-29-27-23/h2-12H,13-14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50284996
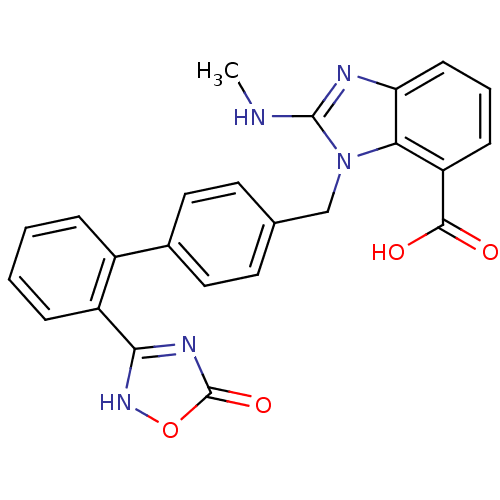
(2-Methylamino-3-[2'-(5-oxo-4,5-dihydro-[1,2,4]oxad...)Show SMILES CNc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nc(=O)o[nH]1 Show InChI InChI=1S/C24H19N5O4/c1-25-23-26-19-8-4-7-18(22(30)31)20(19)29(23)13-14-9-11-15(12-10-14)16-5-2-3-6-17(16)21-27-24(32)33-28-21/h2-12H,13H2,1H3,(H,25,26)(H,30,31)(H,27,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-AII (0.2 nM) from bovine adrenal cortical membrane angiotensin II (AII) receptor at 10e-7 M |
Bioorg Med Chem Lett 5: 1903-1908 (1995)
Article DOI: 10.1016/0960-894X(95)00319-O
BindingDB Entry DOI: 10.7270/Q26M36SM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50044409

(2-Ethylsulfanylmethyl-3-[2'-(1H-tetrazol-5-yl)-bip...)Show SMILES CCSCc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H22N6O2S/c1-2-34-15-22-26-21-9-5-8-20(25(32)33)23(21)31(22)14-16-10-12-17(13-11-16)18-6-3-4-7-19(18)24-27-29-30-28-24/h3-13H,2,14-15H2,1H3,(H,32,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex |
J Med Chem 36: 2182-95 (1993)
BindingDB Entry DOI: 10.7270/Q20Z73X8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50284995

(2-Ethyl-3-[2'-(5-oxo-4,5-dihydro-[1,2,4]oxadiazol-...)Show SMILES CCc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nc(=O)o[nH]1 Show InChI InChI=1S/C25H20N4O4/c1-2-21-26-20-9-5-8-19(24(30)31)22(20)29(21)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)23-27-25(32)33-28-23/h3-13H,2,14H2,1H3,(H,30,31)(H,27,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-AII (0.2 nM) from bovine adrenal cortical membrane angiotensin II (AII) receptor at 10e-7 M |
Bioorg Med Chem Lett 5: 1903-1908 (1995)
Article DOI: 10.1016/0960-894X(95)00319-O
BindingDB Entry DOI: 10.7270/Q26M36SM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data