

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18784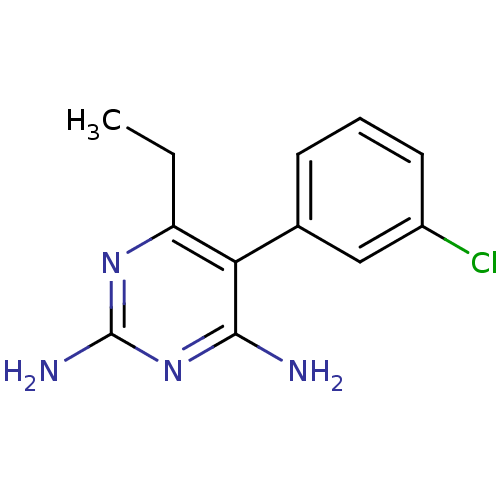 (5-(3-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology at Thailand Curated by ChEMBL | Assay Description Inhibition of human DHFR | ACS Med Chem Lett 9: 1235-1240 (2018) Article DOI: 10.1021/acsmedchemlett.8b00389 BindingDB Entry DOI: 10.7270/Q23J3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50507459 (CHEMBL4462726) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology at Thailand Curated by ChEMBL | Assay Description Inhibition of human DHFR assessed as reduction in NADPH consumption by spectrophotometric method | ACS Med Chem Lett 9: 1235-1240 (2018) Article DOI: 10.1021/acsmedchemlett.8b00389 BindingDB Entry DOI: 10.7270/Q23J3H80 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50507457 (CHEMBL4454693) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology at Thailand Curated by ChEMBL | Assay Description Inhibition of human DHFR assessed as reduction in NADPH consumption by spectrophotometric method | ACS Med Chem Lett 9: 1235-1240 (2018) Article DOI: 10.1021/acsmedchemlett.8b00389 BindingDB Entry DOI: 10.7270/Q23J3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50507456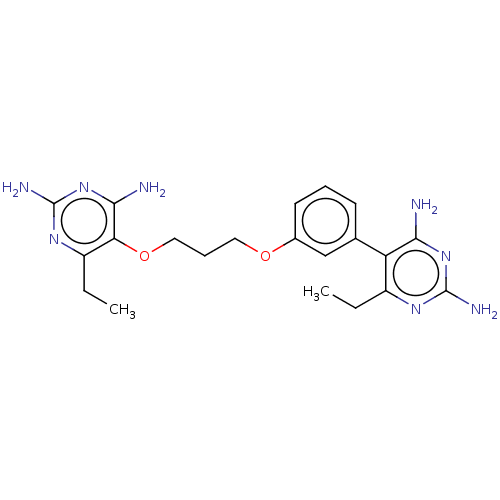 (CHEMBL4442735) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology at Thailand Curated by ChEMBL | Assay Description Inhibition of human DHFR assessed as reduction in NADPH consumption by spectrophotometric method | ACS Med Chem Lett 9: 1235-1240 (2018) Article DOI: 10.1021/acsmedchemlett.8b00389 BindingDB Entry DOI: 10.7270/Q23J3H80 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology at Thailand Curated by ChEMBL | Assay Description Inhibition of human DHFR | ACS Med Chem Lett 9: 1235-1240 (2018) Article DOI: 10.1021/acsmedchemlett.8b00389 BindingDB Entry DOI: 10.7270/Q23J3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50507458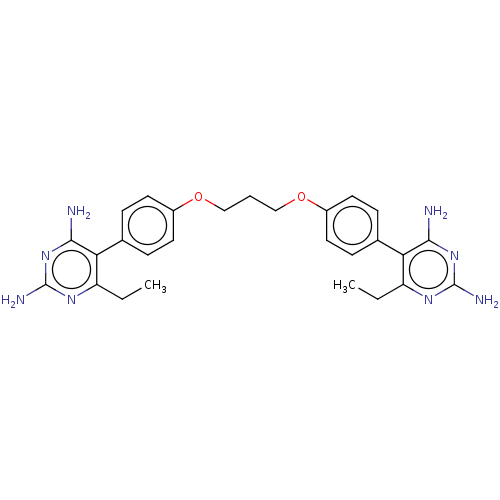 (CHEMBL4460601) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology at Thailand Curated by ChEMBL | Assay Description Inhibition of human DHFR assessed as reduction in NADPH consumption by spectrophotometric method | ACS Med Chem Lett 9: 1235-1240 (2018) Article DOI: 10.1021/acsmedchemlett.8b00389 BindingDB Entry DOI: 10.7270/Q23J3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50452169 (CHEMBL3819601) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology at Thailand Curated by ChEMBL | Assay Description Inhibition of human DHFR assessed as reduction in NADPH consumption by spectrophotometric method | ACS Med Chem Lett 9: 1235-1240 (2018) Article DOI: 10.1021/acsmedchemlett.8b00389 BindingDB Entry DOI: 10.7270/Q23J3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239647 (CHEMBL4092595) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239671 (CHEMBL4063186) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239672 (CHEMBL4093534) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus type-I specific thymidine kinase | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239667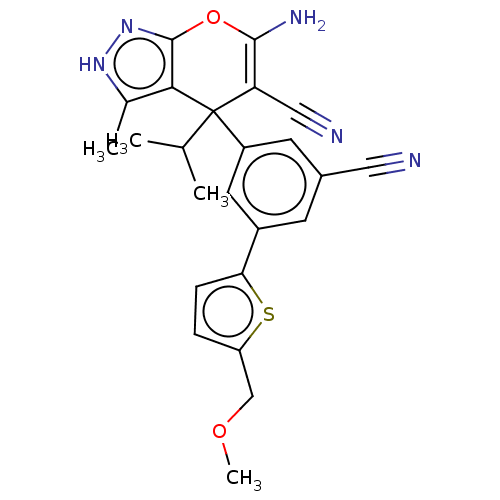 (CHEMBL4066725) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239656 (CHEMBL4103145) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239641 (CHEMBL4085568) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239663 (CHEMBL4061729) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239657 (CHEMBL4074964) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239654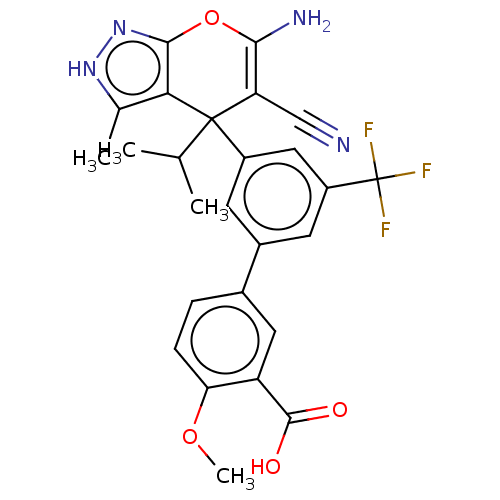 (CHEMBL4104071) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239676 (CHEMBL4078219) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239661 (CHEMBL4102497) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239640 (CHEMBL4064002) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239664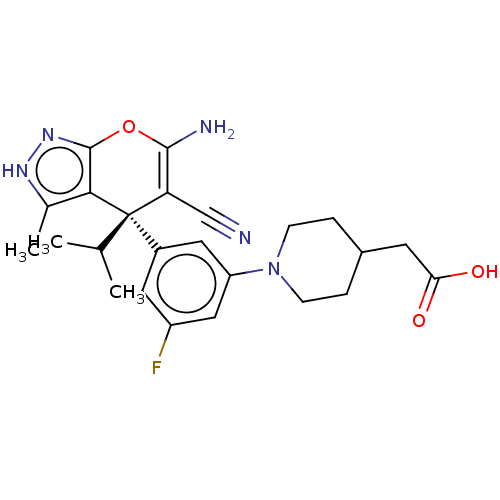 (CHEMBL4088892) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239653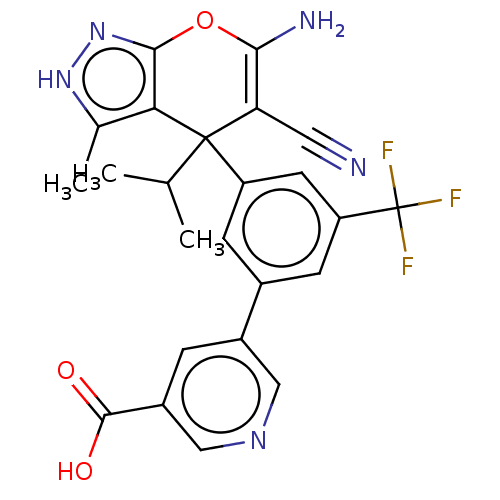 (CHEMBL4087703) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heart | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239662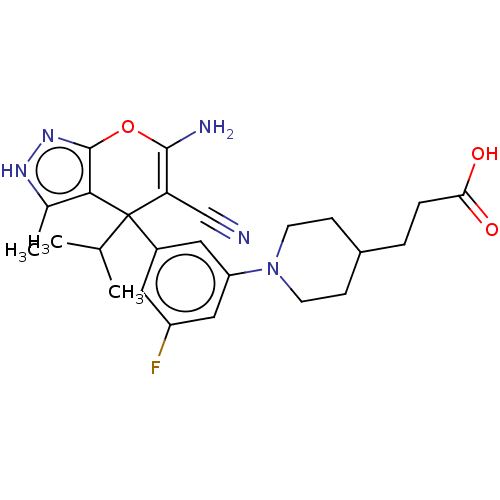 (CHEMBL4101602) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239679 (CHEMBL4093312) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus type-I specific thymidine kinase | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239658 (CHEMBL4100679) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239659 (CHEMBL4086113) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239660 (CHEMBL4064601) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239648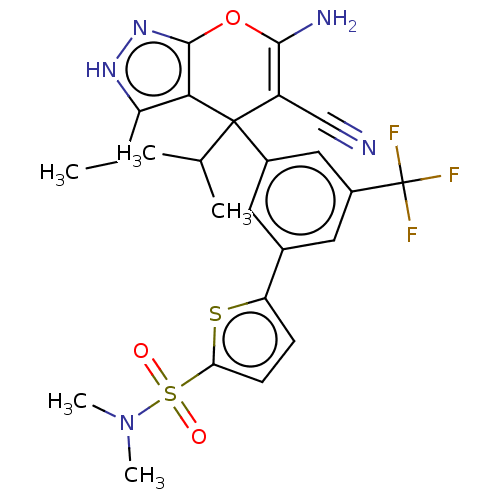 (CHEMBL4071810) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239675 (CHEMBL4095396) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus type-I specific thymidine kinase | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239638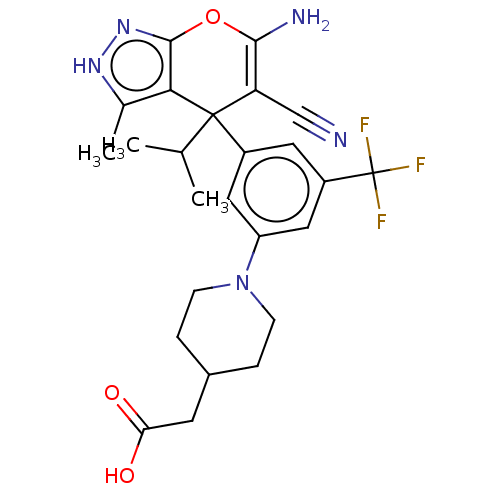 (CHEMBL4073903) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 by displacement of [3H]QNB in rat myocardium | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239673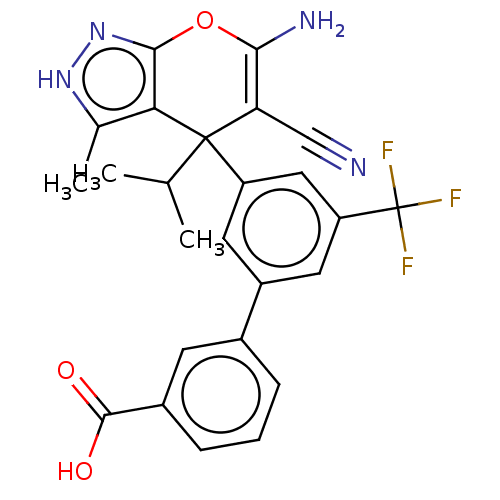 (CHEMBL4066357) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus type-I specific thymidine kinase | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239668 (CHEMBL4072596) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239652 (CHEMBL4102165) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239643 (CHEMBL4100331) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239674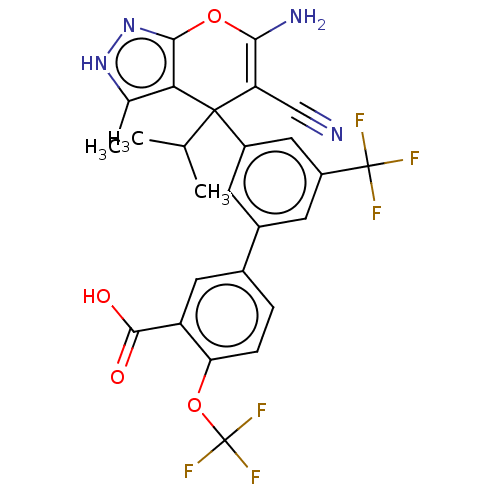 (CHEMBL4079818) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 268 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus type-I specific thymidine kinase | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239655 (CHEMBL4085213) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239666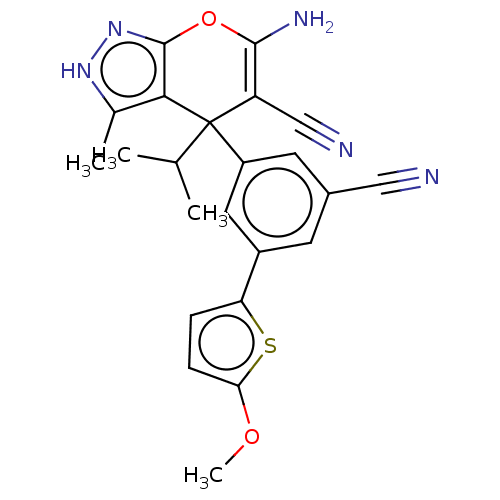 (CHEMBL4094249) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 323 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239650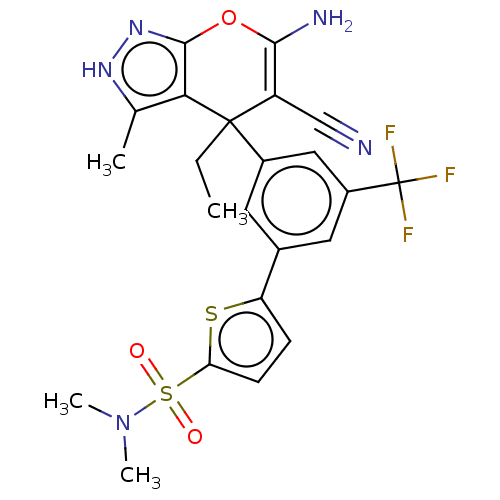 (CHEMBL4081230) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239677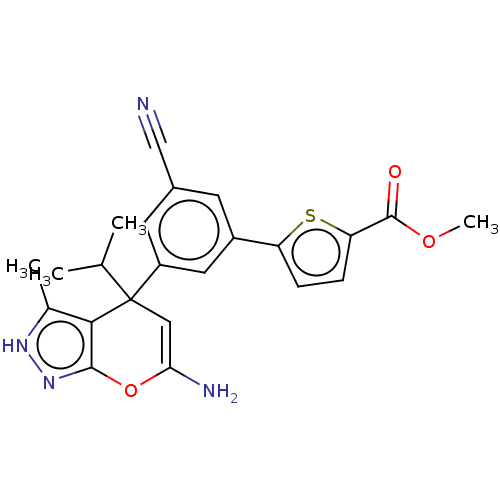 (CHEMBL4073208) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus type-I specific thymidine kinase | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239678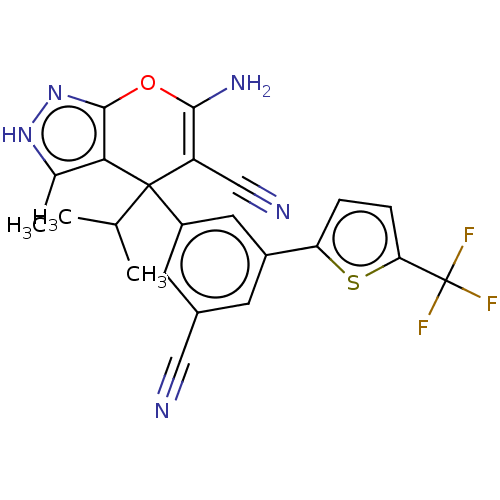 (CHEMBL4077713) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 388 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus type-I specific thymidine kinase | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239669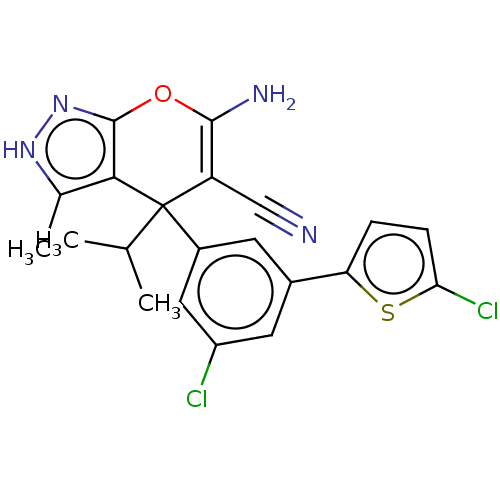 (CHEMBL4067746) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 485 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239637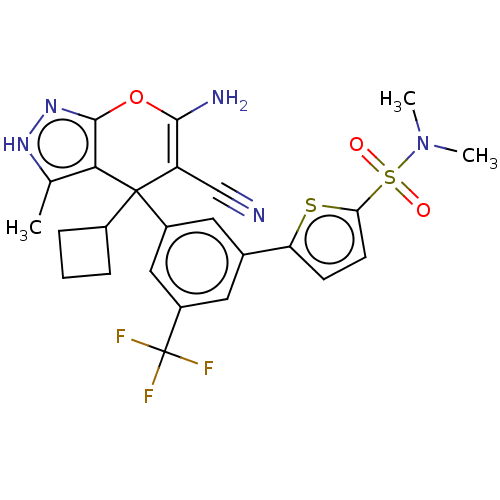 (CHEMBL4089096) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 498 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239649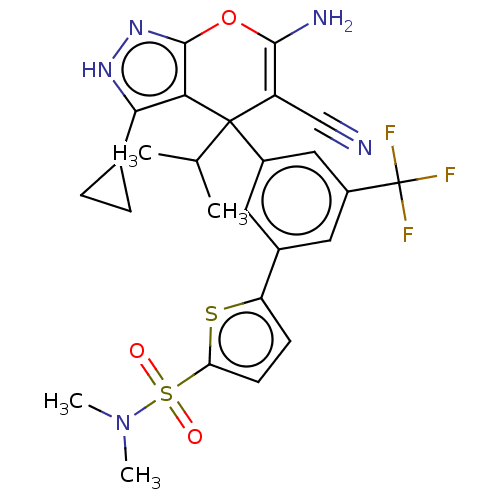 (CHEMBL4065935) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 576 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239642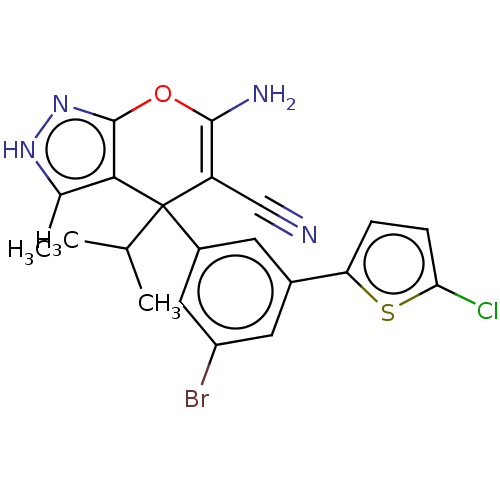 (CHEMBL4088964) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 892 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239651 (CHEMBL4099248) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239665 (CHEMBL4060666) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239644 (CHEMBL4082315) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239645 (CHEMBL4090164) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239646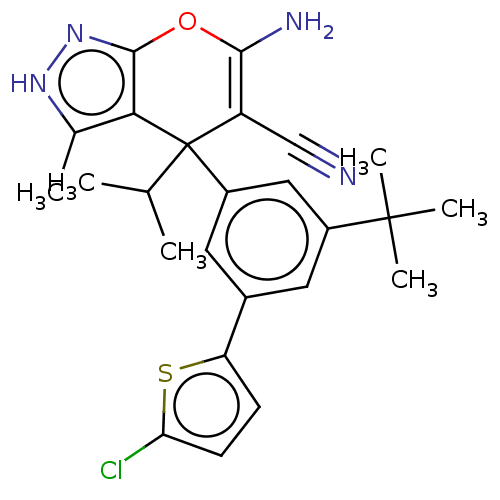 (CHEMBL4084834) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycine hydroxymethyltransferase (Plasmodium falciparum) | BDBM50239670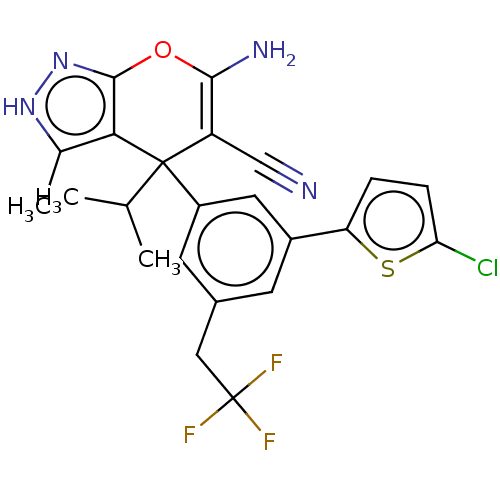 (CHEMBL4062141) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SHMT using L-serine/(6S)-THF as substrate by methylene tetrahydrofolate dehydrogenase coupled enzyme assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50239639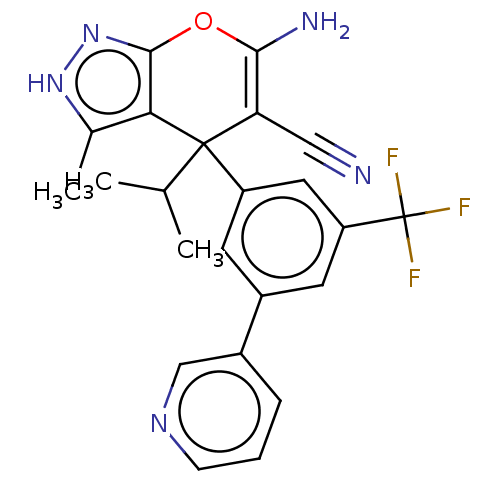 (CHEMBL4080522) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium f�r Organische Chemie, ETH Zurich , Vladimir-Prelog-Weg 3, 8093 Zurich, Switzerland. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells after 30 mins by BTC-AM dye based thallium flux assay | J Med Chem 60: 4840-4860 (2017) Article DOI: 10.1021/acs.jmedchem.7b00008 BindingDB Entry DOI: 10.7270/Q2959KPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||