 Found 280 hits with Last Name = 'jayaraman' and Initial = 'l'
Found 280 hits with Last Name = 'jayaraman' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50258790
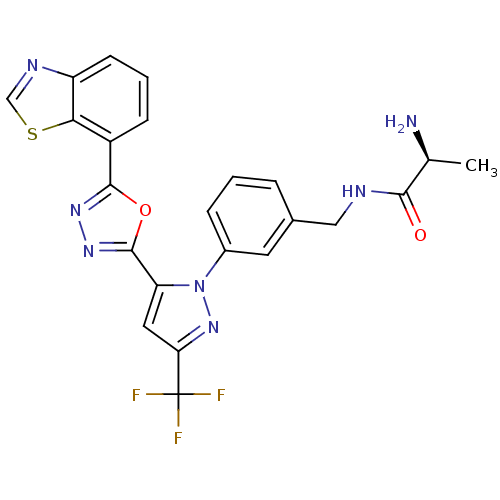
((S)-2-amino-N-(3-(5-(5-(benzo[d]thiazol-7-yl)-1,3,...)Show SMILES C[C@H](N)C(=O)NCc1cccc(c1)-n1nc(cc1-c1nnc(o1)-c1cccc2ncsc12)C(F)(F)F |r| Show InChI InChI=1S/C23H18F3N7O2S/c1-12(27)20(34)28-10-13-4-2-5-14(8-13)33-17(9-18(32-33)23(24,25)26)22-31-30-21(35-22)15-6-3-7-16-19(15)36-11-29-16/h2-9,11-12H,10,27H2,1H3,(H,28,34)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301077
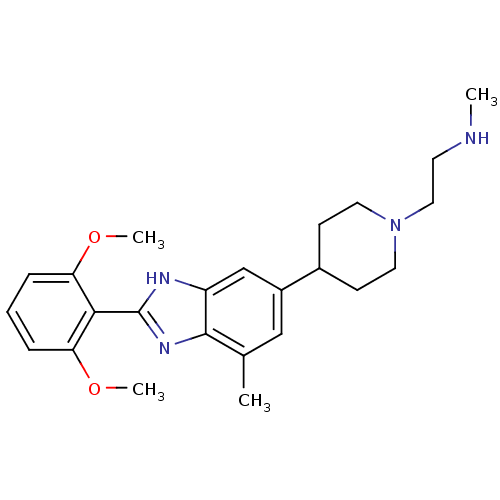
(2-(4-(2-(2,6-dimethoxyphenyl)-7-methyl-1H-benzo[d]...)Show SMILES CNCCN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(OC)cccc1OC Show InChI InChI=1S/C24H32N4O2/c1-16-14-18(17-8-11-28(12-9-17)13-10-25-2)15-19-23(16)27-24(26-19)22-20(29-3)6-5-7-21(22)30-4/h5-7,14-15,17,25H,8-13H2,1-4H3,(H,26,27) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301079

(2-(4-(2-(2-fluoro-6-methoxyphenyl)-7-methyl-1H-ben...)Show SMILES CNCCN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(F)cccc1OC Show InChI InChI=1S/C23H29FN4O/c1-15-13-17(16-7-10-28(11-8-16)12-9-25-2)14-19-22(15)27-23(26-19)21-18(24)5-4-6-20(21)29-3/h4-6,13-14,16,25H,7-12H2,1-3H3,(H,26,27) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301078

(2-(4-(2-(4-fluoro-2-methoxyphenyl)-7-methyl-1H-ben...)Show SMILES CNCCN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1ccc(F)cc1OC Show InChI InChI=1S/C23H29FN4O/c1-15-12-17(16-6-9-28(10-7-16)11-8-25-2)13-20-22(15)27-23(26-20)19-5-4-18(24)14-21(19)29-3/h4-5,12-14,16,25H,6-11H2,1-3H3,(H,26,27) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301076
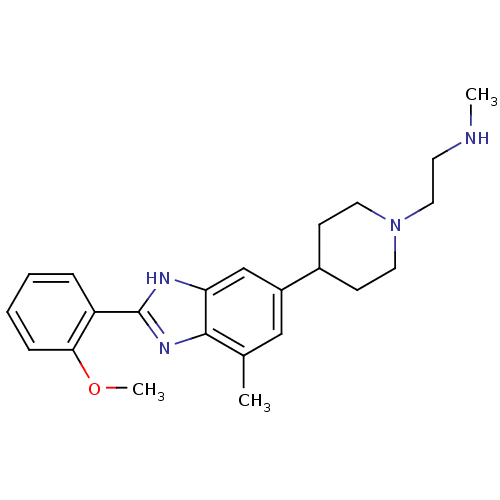
(2-(4-(2-(2-methoxyphenyl)-4-methyl-1H-benzo[d]imid...)Show SMILES CNCCN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1ccccc1OC Show InChI InChI=1S/C23H30N4O/c1-16-14-18(17-8-11-27(12-9-17)13-10-24-2)15-20-22(16)26-23(25-20)19-6-4-5-7-21(19)28-3/h4-7,14-15,17,24H,8-13H2,1-3H3,(H,25,26) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301075

(2-(4-(2-(2-methoxypyridin-3-yl)-3H-imidazo[4,5-b]p...)Show SMILES CNCCN1CCC(CC1)c1cnc2nc([nH]c2c1)-c1cccnc1OC Show InChI InChI=1S/C20H26N6O/c1-21-8-11-26-9-5-14(6-10-26)15-12-17-19(23-13-15)25-18(24-17)16-4-3-7-22-20(16)27-2/h3-4,7,12-14,21H,5-6,8-11H2,1-2H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301069

(2-(4-(2-(2-methoxypyridin-3-yl)-4-methyl-1H-benzo[...)Show SMILES CNCCN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1cccnc1OC Show InChI InChI=1S/C22H29N5O/c1-15-13-17(16-6-10-27(11-7-16)12-9-23-2)14-19-20(15)26-21(25-19)18-5-4-8-24-22(18)28-3/h4-5,8,13-14,16,23H,6-7,9-12H2,1-3H3,(H,25,26) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM144614
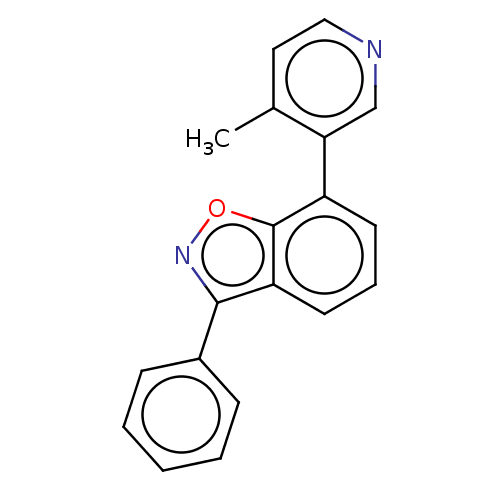
(US8969586, 1 | US9598436, 1)Show InChI InChI=1S/C19H14N2O/c1-13-10-11-20-12-17(13)15-8-5-9-16-18(21-22-19(15)16)14-6-3-2-4-7-14/h2-12H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301073

(2-(4-(2-(2-methoxypyridin-3-yl)-1H-benzo[d]imidazo...)Show SMILES CNCCN1CCC(CC1)c1ccc2nc([nH]c2c1)-c1cccnc1OC Show InChI InChI=1S/C21H27N5O/c1-22-10-13-26-11-7-15(8-12-26)16-5-6-18-19(14-16)25-20(24-18)17-4-3-9-23-21(17)27-2/h3-6,9,14-15,22H,7-8,10-13H2,1-2H3,(H,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614709

(CHEMBL5273615)Show SMILES Cc1ccc(NC(=O)Nc2cc(cc3C(CCCOc23)c2ccccc2)-c2ccccc2-c2nc(=O)[nH]o2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Macaca fascicularis) | BDBM50405216
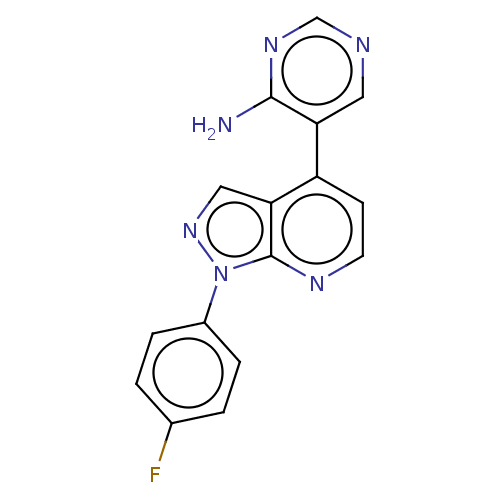
(CHEMBL5290029)Show InChI InChI=1S/C8H13N5O2/c1-3-13(4-2)6-5(12-15)7(14)11-8(9)10-6/h3-4H2,1-2H3,(H3,9,10,11,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against cell free dihydrofolate reductase (DHFR) from rat |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Macaca fascicularis) | BDBM50405216

(CHEMBL5290029)Show InChI InChI=1S/C8H13N5O2/c1-3-13(4-2)6-5(12-15)7(14)11-8(9)10-6/h3-4H2,1-2H3,(H3,9,10,11,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Mycobacterium lufu |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156232
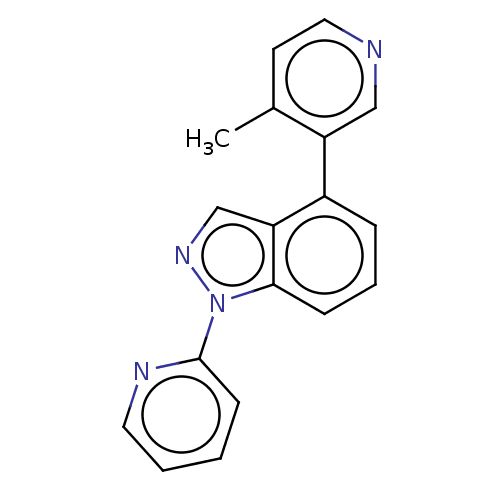
(CHEMBL3782020)Show InChI InChI=1S/C18H14N4/c1-13-8-10-19-11-15(13)14-5-4-6-17-16(14)12-21-22(17)18-7-2-3-9-20-18/h2-12H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Macaca fascicularis) | BDBM50156282
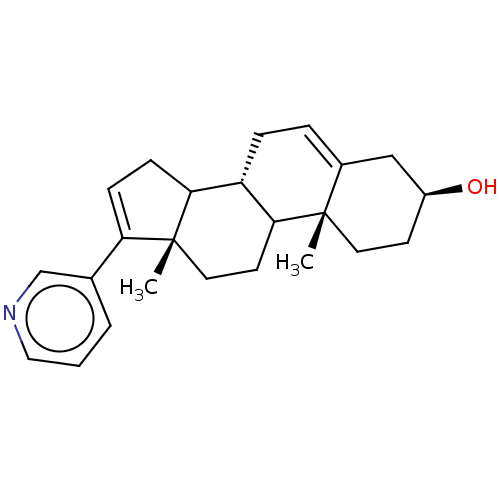
(CHEMBL3780847)Show SMILES [H][C@@]12CC=C3C[C@@H](O)CC[C@]3(C)C1CC[C@@]1(C)C2CC=C1c1cccnc1 |r,c:22,t:3| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21?,22?,23-,24+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 in cynomolgus monkey using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity assay in presence of... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Macaca fascicularis) | BDBM50391846

(CHEMBL2147041 | US9133160, 31)Show InChI InChI=1S/C15H12F3N3/c1-10-5-6-19-7-12(10)11-3-2-4-13-14(11)20-9-21(13)8-15(16,17)18/h2-7,9H,8H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 in cynomolgus monkey adrenal microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity a... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50404835

(CHEMBL5284599)Show InChI InChI=1S/C11H18O3S/c1-7(6-15)10(12)8-4-2-3-5-9(8)11(13)14/h7-9,15H,2-6H2,1H3,(H,13,14)/t7-,8-,9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301074
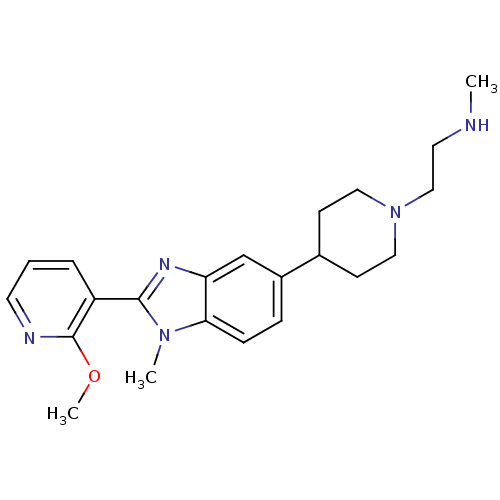
(2-(4-(2-(2-methoxypyridin-3-yl)-1-methyl-1H-benzo[...)Show SMILES CNCCN1CCC(CC1)c1ccc2n(C)c(nc2c1)-c1cccnc1OC Show InChI InChI=1S/C22H29N5O/c1-23-11-14-27-12-8-16(9-13-27)17-6-7-20-19(15-17)25-21(26(20)2)18-5-4-10-24-22(18)28-3/h4-7,10,15-16,23H,8-9,11-14H2,1-3H3 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Macaca fascicularis) | BDBM50156282

(CHEMBL3780847)Show SMILES [H][C@@]12CC=C3C[C@@H](O)CC[C@]3(C)C1CC[C@@]1(C)C2CC=C1c1cccnc1 |r,c:22,t:3| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21?,22?,23-,24+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 in cynomolgus monkey adrenal microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity a... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50404839
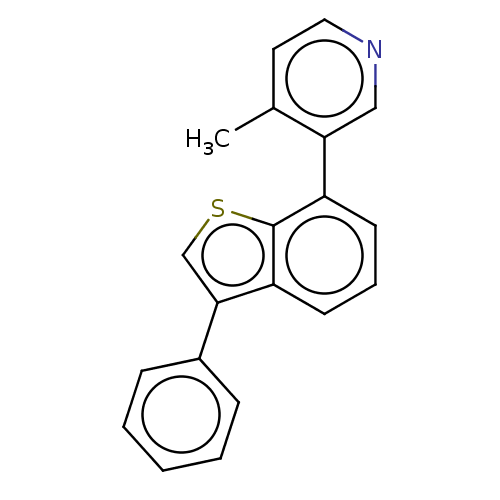
(CHEMBL5286021)Show InChI InChI=1S/C8H12O3S/c9-7(3-4-12)5-1-2-6(5)8(10)11/h5-6,12H,1-4H2,(H,10,11)/t5-,6+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50405184
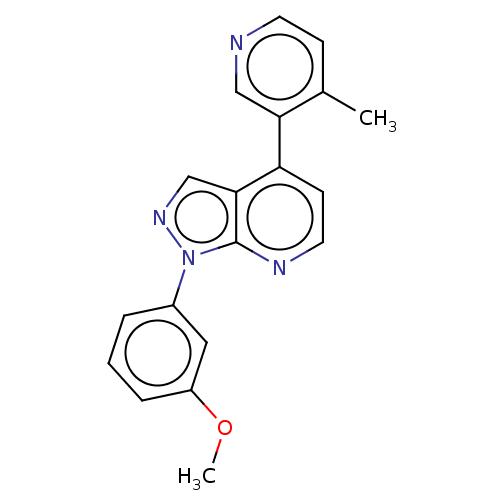
(CHEMBL5290743)Show SMILES CCCCC[C@@H](O)\C=C\[C@H]1C2CCC(O2)[C@@H]1C\C=C/CCCC(O)=O |TLB:16:15:14:12.11| Show InChI InChI=1S/C21H34O4/c1-2-3-6-9-16(22)12-13-18-17(19-14-15-20(18)25-19)10-7-4-5-8-11-21(23)24/h4,7,12-13,16-20,22H,2-3,5-6,8-11,14-15H2,1H3,(H,23,24)/b7-4-,13-12+/t16-,17-,18-,19?,20?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156233
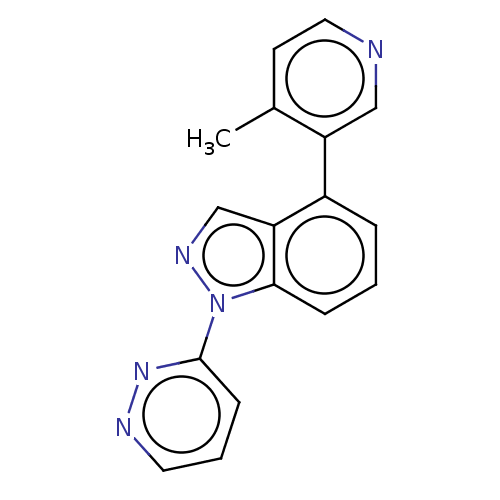
(CHEMBL3780658)Show InChI InChI=1S/C17H13N5/c1-12-7-9-18-10-14(12)13-4-2-5-16-15(13)11-20-22(16)17-6-3-8-19-21-17/h2-11H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614704
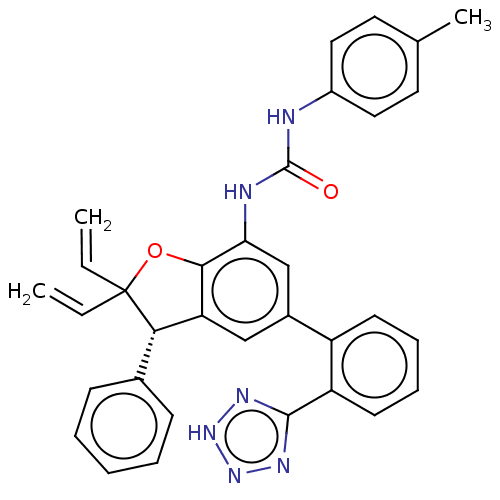
(CHEMBL5279540)Show SMILES Cc1ccc(NC(=O)Nc2cc(cc3[C@H](c4ccccc4)C(Oc23)(C=C)C=C)-c2ccccc2-c2nn[nH]n2)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614710
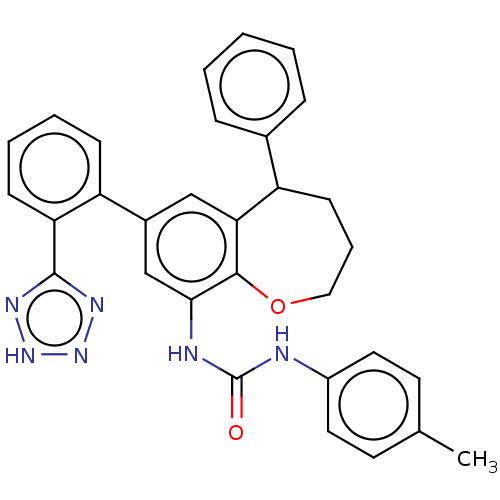
(CHEMBL5267407)Show SMILES Cc1ccc(NC(=O)Nc2cc(cc3C(CCCOc23)c2ccccc2)-c2ccccc2-c2nn[nH]n2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156281
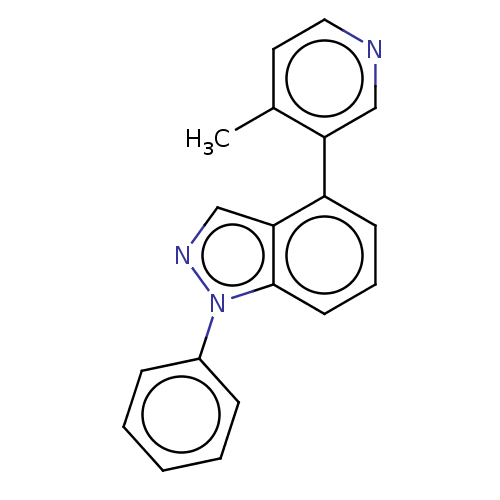
(CHEMBL3780743)Show InChI InChI=1S/C19H15N3/c1-14-10-11-20-12-17(14)16-8-5-9-19-18(16)13-21-22(19)15-6-3-2-4-7-15/h2-13H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156235
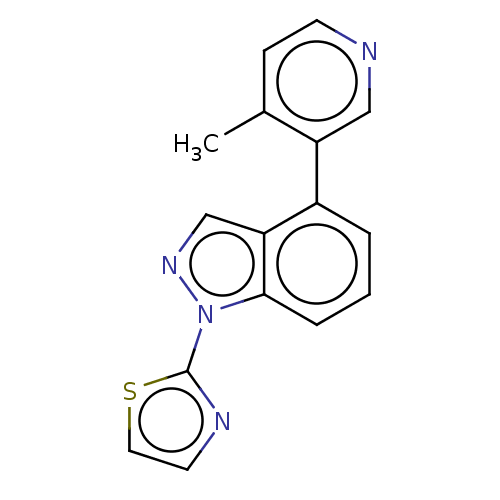
(CHEMBL3780266)Show InChI InChI=1S/C16H12N4S/c1-11-5-6-17-9-13(11)12-3-2-4-15-14(12)10-19-20(15)16-18-7-8-21-16/h2-10H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50405189
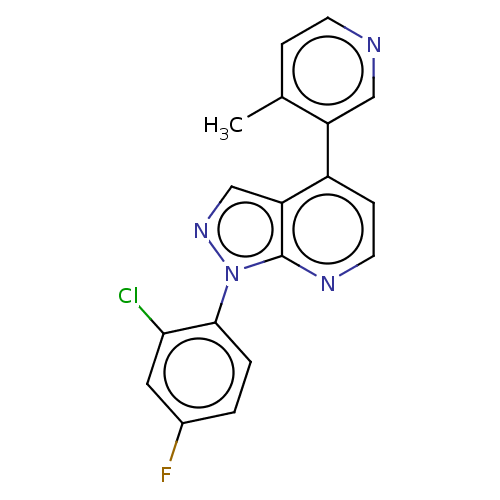
(CHEMBL5284859)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)CC[C@H]4N)ccc5O |r| Show InChI InChI=1S/C17H22N2O3/c1-19-7-6-16-13-9-2-3-11(20)14(13)22-15(16)10(18)4-5-17(16,21)12(19)8-9/h2-3,10,12,15,20-21H,4-8,18H2,1H3/t10-,12-,15+,16+,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity by the displacement of [3H]NMS binding to muscarinic M1 receptor of rat brain |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50405217
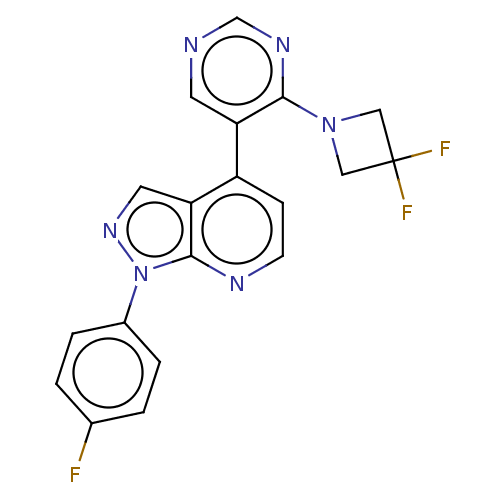
(CHEMBL5265986)Show InChI InChI=1S/C15H19N5O2/c16-15-18-13(12(20-22)14(21)19-15)17-10-6-2-5-9-11-7-3-1-4-8-11/h1,3-4,7-8H,2,5-6,9-10H2,(H4,16,17,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity by the displacement of [3H]NMS binding to muscarinic M1 receptor of rat brain |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Macaca fascicularis) | BDBM50391846

(CHEMBL2147041 | US9133160, 31)Show InChI InChI=1S/C15H12F3N3/c1-10-5-6-19-7-12(10)11-3-2-4-13-14(11)20-9-21(13)8-15(16,17)18/h2-7,9H,8H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 in cynomolgus monkey using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity assay in presence of... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301080
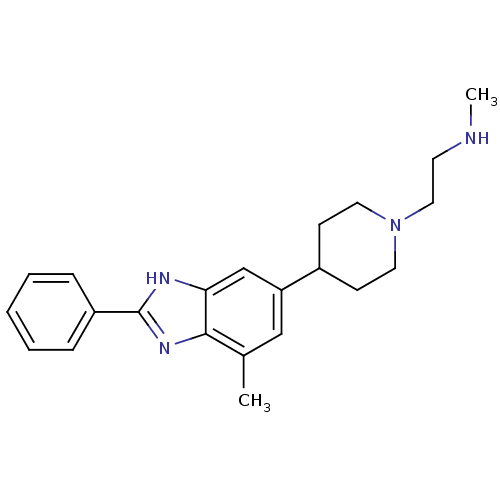
(CHEMBL567298 | N-methyl-2-(4-(7-methyl-2-phenyl-1H...)Show SMILES CNCCN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1ccccc1 Show InChI InChI=1S/C22H28N4/c1-16-14-19(17-8-11-26(12-9-17)13-10-23-2)15-20-21(16)25-22(24-20)18-6-4-3-5-7-18/h3-7,14-15,17,23H,8-13H2,1-2H3,(H,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614709

(CHEMBL5273615)Show SMILES Cc1ccc(NC(=O)Nc2cc(cc3C(CCCOc23)c2ccccc2)-c2ccccc2-c2nc(=O)[nH]o2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
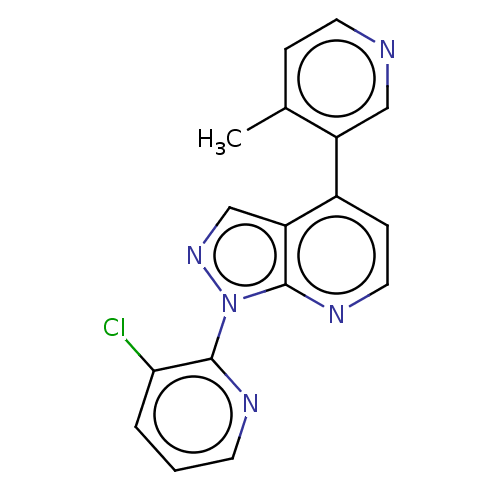
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50405210
(CHEMBL5269382)Show InChI InChI=1S/C17H23N5O2/c18-17-20-15(14(22-24)16(23)21-17)19-12-8-3-1-2-5-9-13-10-6-4-7-11-13/h4,6-7,10-11H,1-3,5,8-9,12H2,(H4,18,19,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity by the displacement of [3H]NMS binding to A9 L cells transfected with muscarinic M1 receptor of rat brain |
Citation and Details
|
More data for this
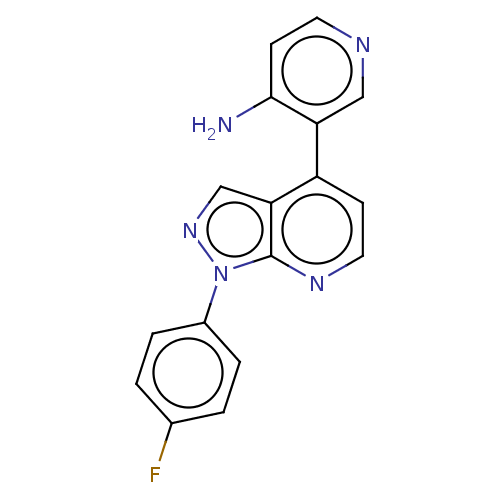
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50405215
(CHEMBL5290106)Show InChI InChI=1S/C11H11N5O2/c12-11-14-9(8(16-18)10(17)15-11)13-6-7-4-2-1-3-5-7/h1-5H,6H2,(H4,12,13,14,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity by the displacement of [3H]NMS binding to muscarinic M2 receptor of rat heart |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50405225
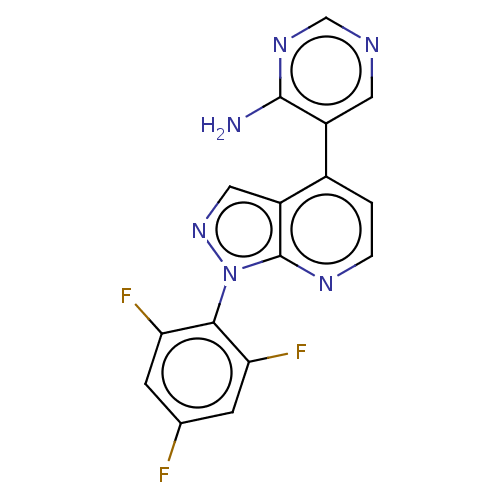
(CHEMBL5286147)Show InChI InChI=1S/C15H18N4O2/c16-15-18-13(12(10-20)14(21)19-15)17-9-5-4-8-11-6-2-1-3-7-11/h1-3,6-7,10H,4-5,8-9H2,(H4,16,17,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity by the displacement of [3H]NMS binding to muscarinic M1 receptor of rat brain |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156231

(CHEMBL3781112)Show InChI InChI=1S/C19H14FN3/c1-13-9-10-21-11-17(13)16-3-2-4-19-18(16)12-22-23(19)15-7-5-14(20)6-8-15/h2-12H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156234
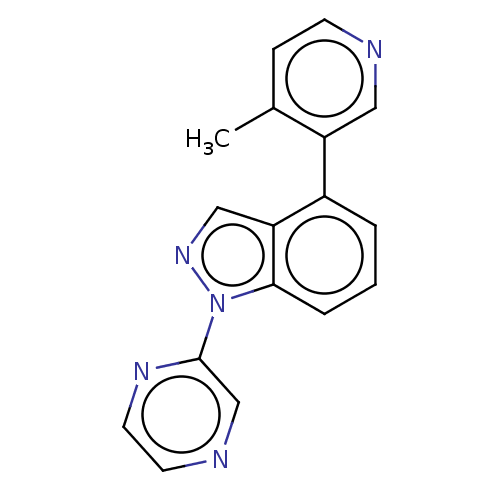
(CHEMBL3780226)Show InChI InChI=1S/C17H13N5/c1-12-5-6-18-9-14(12)13-3-2-4-16-15(13)10-21-22(16)17-11-19-7-8-20-17/h2-11H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156236
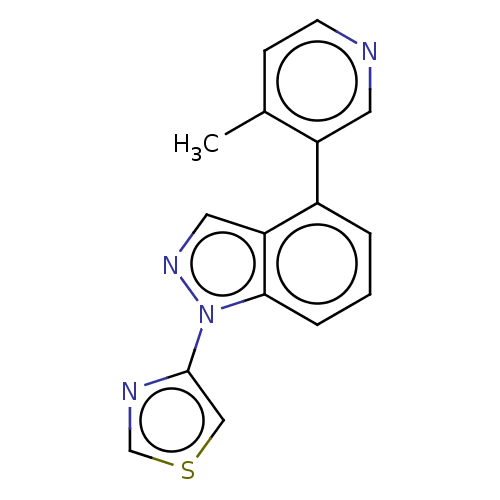
(CHEMBL3780048)Show InChI InChI=1S/C16H12N4S/c1-11-5-6-17-7-13(11)12-3-2-4-15-14(12)8-19-20(15)16-9-21-10-18-16/h2-10H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50404834
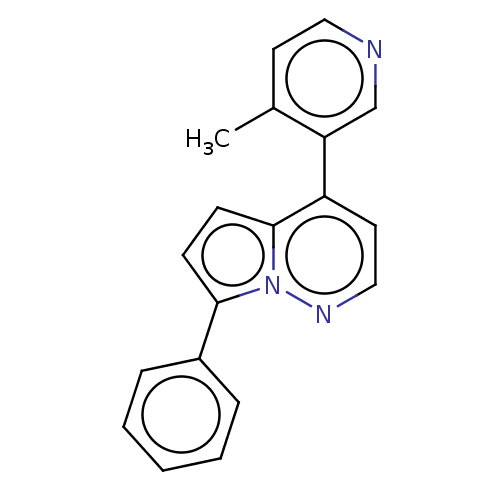
(CHEMBL5280658)Show InChI InChI=1S/C10H16O3S/c11-9(5-6-14)7-3-1-2-4-8(7)10(12)13/h7-8,14H,1-6H2,(H,12,13)/t7-,8+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50404843
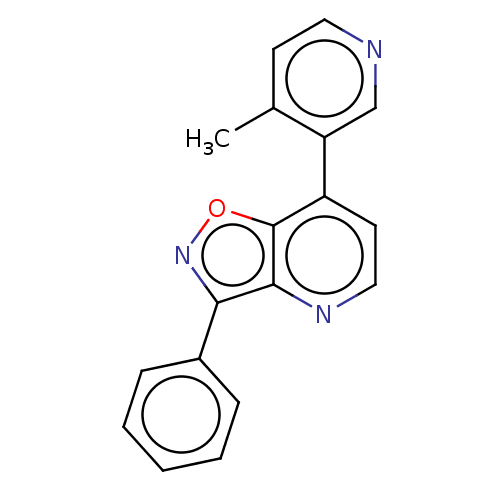
(CHEMBL5284207)Show InChI InChI=1S/C11H18O3S/c1-7(6-15)10(12)8-4-2-3-5-9(8)11(13)14/h7-9,15H,2-6H2,1H3,(H,13,14)/t7-,8+,9+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614703
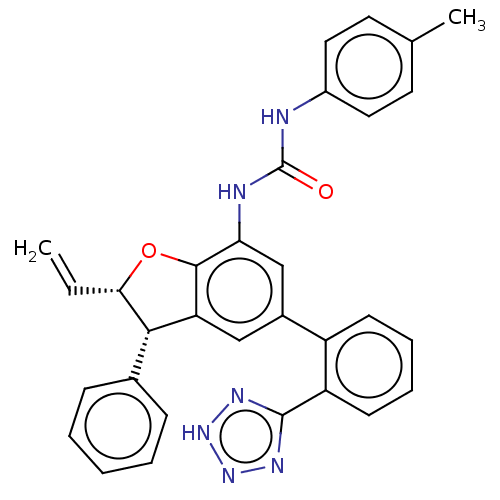
(CHEMBL5269992)Show SMILES Cc1ccc(NC(=O)Nc2cc(cc3[C@@H]([C@@H](Oc23)C=C)c2ccccc2)-c2ccccc2-c2nn[nH]n2)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50391839
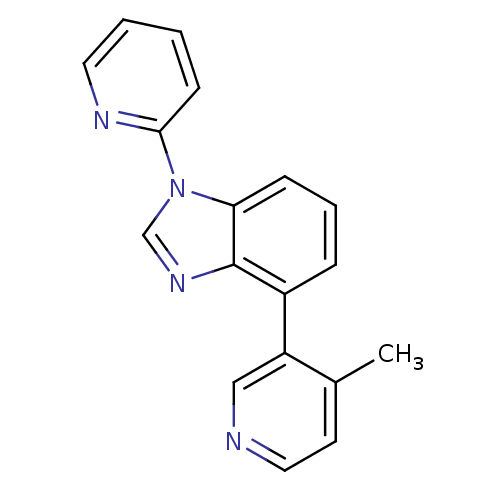
(CHEMBL2147034 | US9133160, 2)Show InChI InChI=1S/C18H14N4/c1-13-8-10-19-11-15(13)14-5-4-6-16-18(14)21-12-22(16)17-7-2-3-9-20-17/h2-12H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50405188
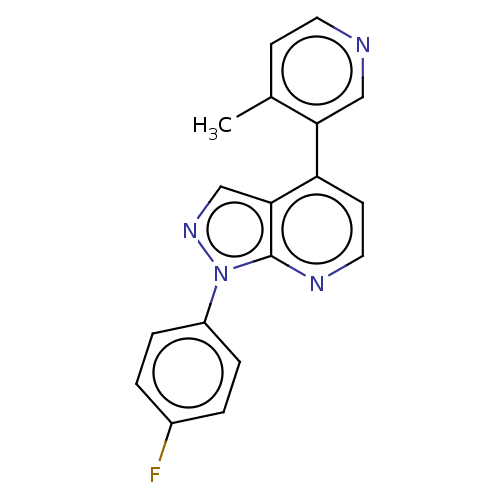
(CHEMBL5286511)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)CC[C@@H]4N)ccc5O |r| Show InChI InChI=1S/C17H22N2O3/c1-19-7-6-16-13-9-2-3-11(20)14(13)22-15(16)10(18)4-5-17(16,21)12(19)8-9/h2-3,10,12,15,20-21H,4-8,18H2,1H3/t10-,12+,15-,16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity by the displacement of [3H]NMS binding to muscarinic M2 receptor of rat heart |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Macaca fascicularis) | BDBM50405216

(CHEMBL5290029)Show InChI InChI=1S/C8H13N5O2/c1-3-13(4-2)6-5(12-15)7(14)11-8(9)10-6/h3-4H2,1-2H3,(H3,9,10,11,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Mycobacterium lufu |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614705
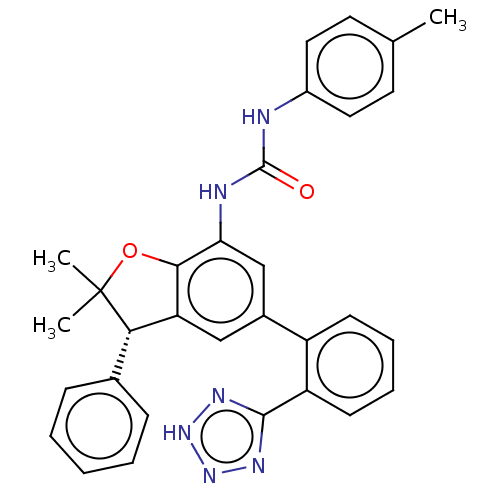
(CHEMBL5287356)Show SMILES Cc1ccc(NC(=O)Nc2cc(cc3[C@H](c4ccccc4)C(C)(C)Oc23)-c2ccccc2-c2nn[nH]n2)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50404833
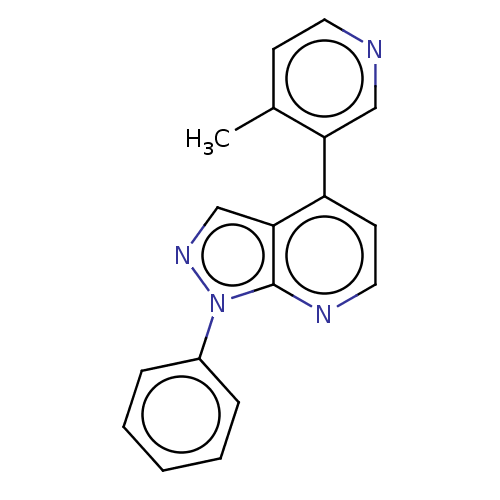
(CHEMBL5266728)Show InChI InChI=1S/C8H12O3S/c9-7(3-4-12)5-1-2-6(5)8(10)11/h5-6,12H,1-4H2,(H,10,11)/t5-,6-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of radioligand [3H]GR-65630. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50454785
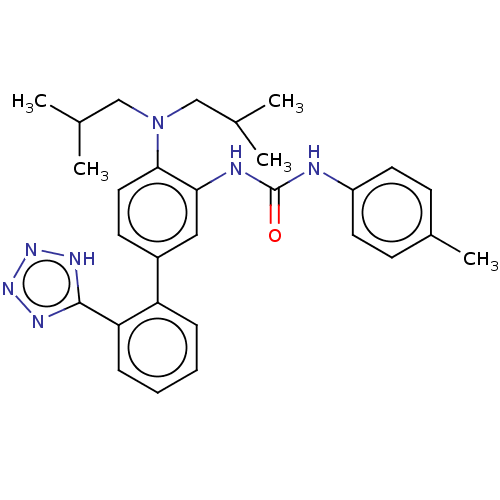
(CHEMBL4207581)Show SMILES CC(C)CN(CC(C)C)c1ccc(cc1NC(=O)Nc1ccc(C)cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C29H35N7O/c1-19(2)17-36(18-20(3)4)27-15-12-22(24-8-6-7-9-25(24)28-32-34-35-33-28)16-26(27)31-29(37)30-23-13-10-21(5)11-14-23/h6-16,19-20H,17-18H2,1-5H3,(H2,30,31,37)(H,32,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50405218
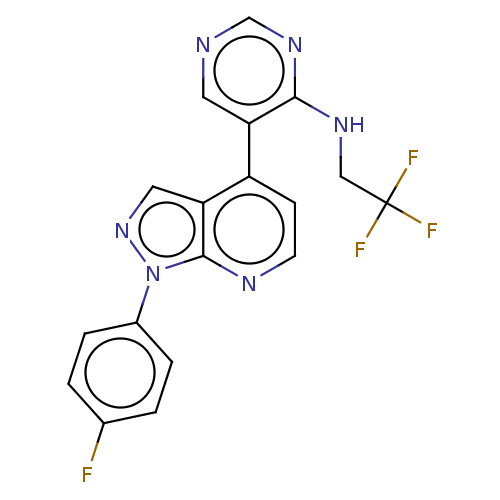
(CHEMBL5269068)Show InChI InChI=1S/C13H15N5O2/c14-13-16-11(10(18-20)12(19)17-13)15-8-4-7-9-5-2-1-3-6-9/h1-3,5-6H,4,7-8H2,(H4,14,15,16,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity by the displacement of [3H]NMS binding to muscarinic M1 receptor of rat brain |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156237
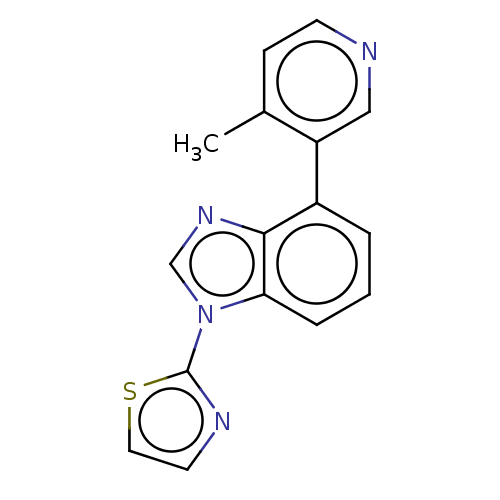
(CHEMBL3781487)Show InChI InChI=1S/C16H12N4S/c1-11-5-6-17-9-13(11)12-3-2-4-14-15(12)19-10-20(14)16-18-7-8-21-16/h2-10H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50404844
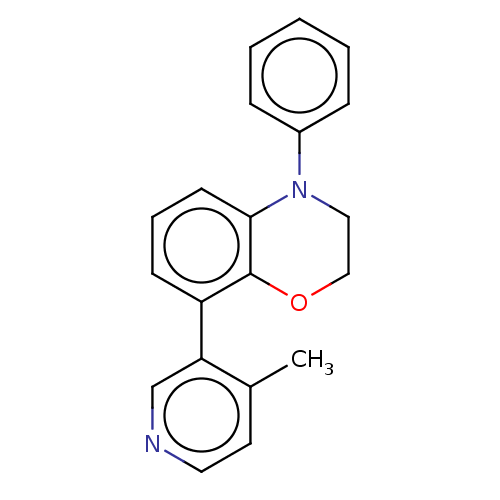
(CHEMBL5290740)Show InChI InChI=1S/C7H13NO4S2/c9-7(10)6-2-1-3-8(6)14(11,12)5-4-13/h6,13H,1-5H2,(H,9,10)/t6-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50405216

(CHEMBL5290029)Show InChI InChI=1S/C8H13N5O2/c1-3-13(4-2)6-5(12-15)7(14)11-8(9)10-6/h3-4H2,1-2H3,(H3,9,10,11,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydropteroic acid synthase (SYN) from Plasmodium berghei |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156238
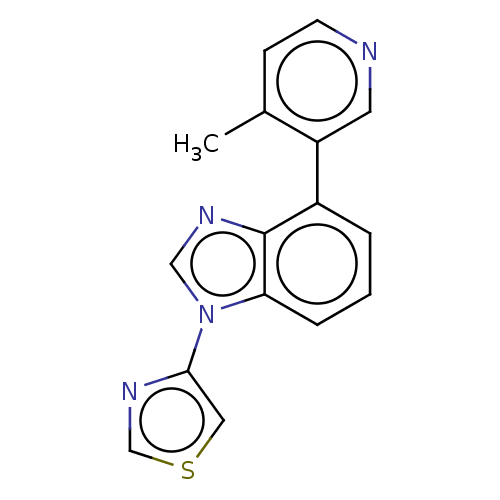
(CHEMBL3781910)Show InChI InChI=1S/C16H12N4S/c1-11-5-6-17-7-13(11)12-3-2-4-14-16(12)18-9-20(14)15-8-21-10-19-15/h2-10H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data