 Found 225 hits with Last Name = 'jenkins' and Initial = 'je'
Found 225 hits with Last Name = 'jenkins' and Initial = 'je' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50234380

(CHEMBL245876 | quinolin-8-yl 4-methyl-3-(piperidin...)Show SMILES Cc1ccc(cc1S(=O)(=O)N1CCCCC1)C(=O)Oc1cccc2cccnc12 Show InChI InChI=1S/C22H22N2O4S/c1-16-10-11-18(15-20(16)29(26,27)24-13-3-2-4-14-24)22(25)28-19-9-5-7-17-8-6-12-23-21(17)19/h5-12,15H,2-4,13-14H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK cells |
Bioorg Med Chem Lett 18: 1725-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.042
BindingDB Entry DOI: 10.7270/Q2X92B1F |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50234380

(CHEMBL245876 | quinolin-8-yl 4-methyl-3-(piperidin...)Show SMILES Cc1ccc(cc1S(=O)(=O)N1CCCCC1)C(=O)Oc1cccc2cccnc12 Show InChI InChI=1S/C22H22N2O4S/c1-16-10-11-18(15-20(16)29(26,27)24-13-3-2-4-14-24)22(25)28-19-9-5-7-17-8-6-12-23-21(17)19/h5-12,15H,2-4,13-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK cells |
Bioorg Med Chem Lett 18: 1725-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.042
BindingDB Entry DOI: 10.7270/Q2X92B1F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50042590
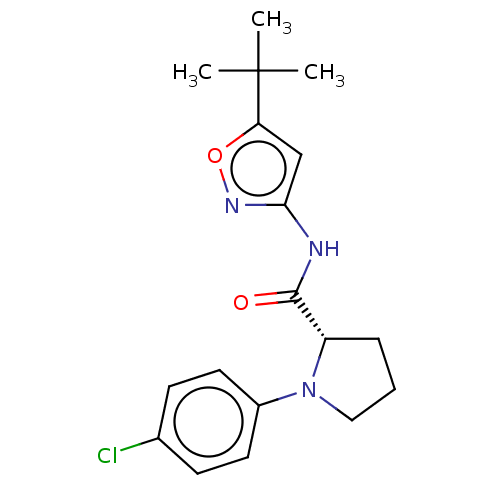
(CHEMBL3353863)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(Cl)cc2)no1 |r| Show InChI InChI=1S/C18H22ClN3O2/c1-18(2,3)15-11-16(21-24-15)20-17(23)14-5-4-10-22(14)13-8-6-12(19)7-9-13/h6-9,11,14H,4-5,10H2,1-3H3,(H,20,21,23)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50042591

(CHEMBL3353862)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(cc2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H22F3N3O2/c1-18(2,3)15-11-16(24-27-15)23-17(26)14-5-4-10-25(14)13-8-6-12(7-9-13)19(20,21)22/h6-9,11,14H,4-5,10H2,1-3H3,(H,23,24,26)/t14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50042590

(CHEMBL3353863)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(Cl)cc2)no1 |r| Show InChI InChI=1S/C18H22ClN3O2/c1-18(2,3)15-11-16(21-24-15)20-17(23)14-5-4-10-22(14)13-8-6-12(19)7-9-13/h6-9,11,14H,4-5,10H2,1-3H3,(H,20,21,23)/t14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50042700
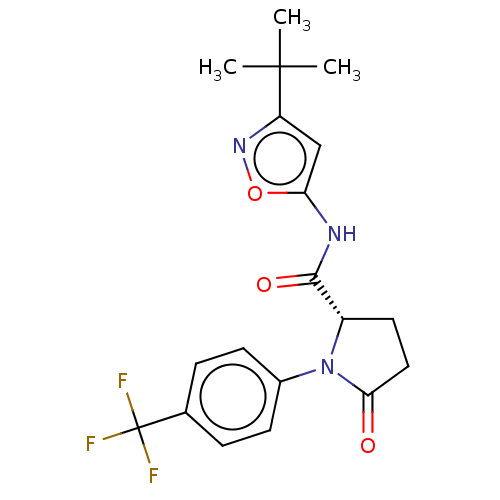
(CHEMBL3353881)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCC(=O)N2c2ccc(cc2)C(F)(F)F)on1 |r| Show InChI InChI=1S/C19H20F3N3O3/c1-18(2,3)14-10-15(28-24-14)23-17(27)13-8-9-16(26)25(13)12-6-4-11(5-7-12)19(20,21)22/h4-7,10,13H,8-9H2,1-3H3,(H,23,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50042700

(CHEMBL3353881)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCC(=O)N2c2ccc(cc2)C(F)(F)F)on1 |r| Show InChI InChI=1S/C19H20F3N3O3/c1-18(2,3)14-10-15(28-24-14)23-17(27)13-8-9-16(26)25(13)12-6-4-11(5-7-12)19(20,21)22/h4-7,10,13H,8-9H2,1-3H3,(H,23,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50042591

(CHEMBL3353862)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(cc2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H22F3N3O2/c1-18(2,3)15-11-16(24-27-15)23-17(26)14-5-4-10-25(14)13-8-6-12(7-9-13)19(20,21)22/h6-9,11,14H,4-5,10H2,1-3H3,(H,23,24,26)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50042700

(CHEMBL3353881)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCC(=O)N2c2ccc(cc2)C(F)(F)F)on1 |r| Show InChI InChI=1S/C19H20F3N3O3/c1-18(2,3)14-10-15(28-24-14)23-17(27)13-8-9-16(26)25(13)12-6-4-11(5-7-12)19(20,21)22/h4-7,10,13H,8-9H2,1-3H3,(H,23,27)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50042591

(CHEMBL3353862)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(cc2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H22F3N3O2/c1-18(2,3)15-11-16(24-27-15)23-17(26)14-5-4-10-25(14)13-8-6-12(7-9-13)19(20,21)22/h6-9,11,14H,4-5,10H2,1-3H3,(H,23,24,26)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50341023
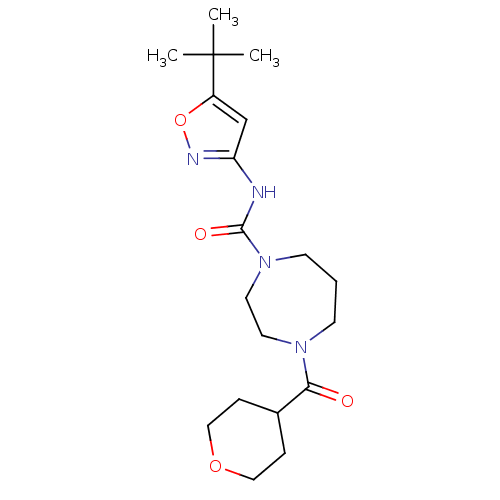
(CHEMBL1762293 | N-(5-tert-butylisoxazol-3-yl)-4-(t...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)C2CCOCC2)no1 Show InChI InChI=1S/C19H30N4O4/c1-19(2,3)15-13-16(21-27-15)20-18(25)23-8-4-7-22(9-10-23)17(24)14-5-11-26-12-6-14/h13-14H,4-12H2,1-3H3,(H,20,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 using flourescent probe 7-methoxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50341008
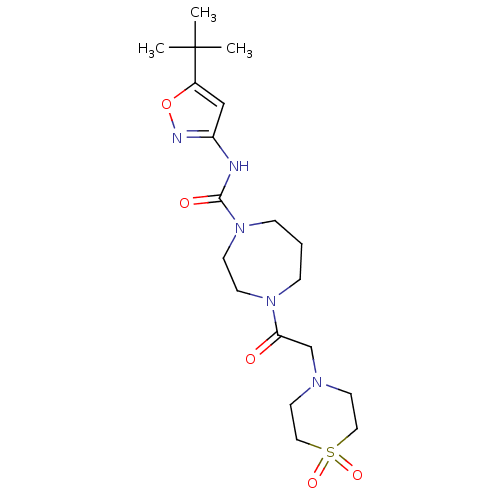
(4-[2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-ace...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)CN2CCS(=O)(=O)CC2)no1 Show InChI InChI=1S/C19H31N5O5S/c1-19(2,3)15-13-16(21-29-15)20-18(26)24-6-4-5-23(7-8-24)17(25)14-22-9-11-30(27,28)12-10-22/h13H,4-12,14H2,1-3H3,(H,20,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using flourescent probe 7-benzyloxyquinoline and 7-benzyloxy-4-(trifluoromethyl)-coumarin) |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50341023

(CHEMBL1762293 | N-(5-tert-butylisoxazol-3-yl)-4-(t...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)C2CCOCC2)no1 Show InChI InChI=1S/C19H30N4O4/c1-19(2,3)15-13-16(21-27-15)20-18(25)23-8-4-7-22(9-10-23)17(24)14-5-11-26-12-6-14/h13-14H,4-12H2,1-3H3,(H,20,21,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 flourescent probe 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50341023

(CHEMBL1762293 | N-(5-tert-butylisoxazol-3-yl)-4-(t...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)C2CCOCC2)no1 Show InChI InChI=1S/C19H30N4O4/c1-19(2,3)15-13-16(21-27-15)20-18(25)23-8-4-7-22(9-10-23)17(24)14-5-11-26-12-6-14/h13-14H,4-12H2,1-3H3,(H,20,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using flourescent probe 7-benzyloxyquinoline and 7-benzyloxy-4-(trifluoromethyl)-coumarin) |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50341008

(4-[2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-ace...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)CN2CCS(=O)(=O)CC2)no1 Show InChI InChI=1S/C19H31N5O5S/c1-19(2,3)15-13-16(21-29-15)20-18(26)24-6-4-5-23(7-8-24)17(25)14-22-9-11-30(27,28)12-10-22/h13H,4-12,14H2,1-3H3,(H,20,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 using flourescent probe 7-methoxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50341008

(4-[2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-ace...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)CN2CCS(=O)(=O)CC2)no1 Show InChI InChI=1S/C19H31N5O5S/c1-19(2,3)15-13-16(21-29-15)20-18(26)24-6-4-5-23(7-8-24)17(25)14-22-9-11-30(27,28)12-10-22/h13H,4-12,14H2,1-3H3,(H,20,21,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 flourescent probe 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50340991
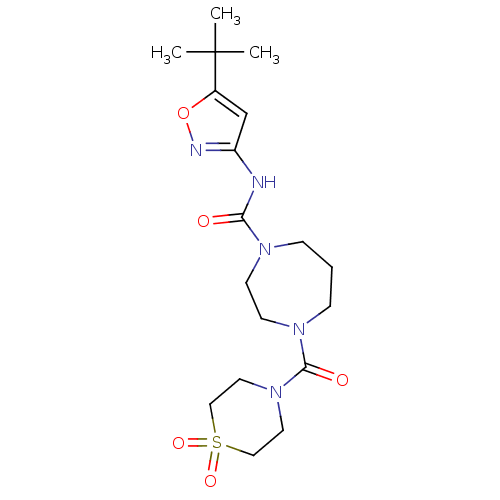
(4-(1,1-Dioxo-1lambda*6*-thiomorpholine-4-carbonyl)...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)N2CCS(=O)(=O)CC2)no1 Show InChI InChI=1S/C18H29N5O5S/c1-18(2,3)14-13-15(20-28-14)19-16(24)21-5-4-6-22(8-7-21)17(25)23-9-11-29(26,27)12-10-23/h13H,4-12H2,1-3H3,(H,19,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using flourescent probe 7-benzyloxyquinoline and 7-benzyloxy-4-(trifluoromethyl)-coumarin) |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50340991

(4-(1,1-Dioxo-1lambda*6*-thiomorpholine-4-carbonyl)...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)N2CCS(=O)(=O)CC2)no1 Show InChI InChI=1S/C18H29N5O5S/c1-18(2,3)14-13-15(20-28-14)19-16(24)21-5-4-6-22(8-7-21)17(25)23-9-11-29(26,27)12-10-23/h13H,4-12H2,1-3H3,(H,19,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 using flourescent probe 7-methoxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50340991

(4-(1,1-Dioxo-1lambda*6*-thiomorpholine-4-carbonyl)...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)N2CCS(=O)(=O)CC2)no1 Show InChI InChI=1S/C18H29N5O5S/c1-18(2,3)14-13-15(20-28-14)19-16(24)21-5-4-6-22(8-7-21)17(25)23-9-11-29(26,27)12-10-23/h13H,4-12H2,1-3H3,(H,19,20,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 flourescent probe 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50042590

(CHEMBL3353863)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(Cl)cc2)no1 |r| Show InChI InChI=1S/C18H22ClN3O2/c1-18(2,3)15-11-16(21-24-15)20-17(23)14-5-4-10-22(14)13-8-6-12(19)7-9-13/h6-9,11,14H,4-5,10H2,1-3H3,(H,20,21,23)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50042590

(CHEMBL3353863)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(Cl)cc2)no1 |r| Show InChI InChI=1S/C18H22ClN3O2/c1-18(2,3)15-11-16(21-24-15)20-17(23)14-5-4-10-22(14)13-8-6-12(19)7-9-13/h6-9,11,14H,4-5,10H2,1-3H3,(H,20,21,23)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50042700

(CHEMBL3353881)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCC(=O)N2c2ccc(cc2)C(F)(F)F)on1 |r| Show InChI InChI=1S/C19H20F3N3O3/c1-18(2,3)14-10-15(28-24-14)23-17(27)13-8-9-16(26)25(13)12-6-4-11(5-7-12)19(20,21)22/h4-7,10,13H,8-9H2,1-3H3,(H,23,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50042591

(CHEMBL3353862)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(cc2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H22F3N3O2/c1-18(2,3)15-11-16(24-27-15)23-17(26)14-5-4-10-25(14)13-8-6-12(7-9-13)19(20,21)22/h6-9,11,14H,4-5,10H2,1-3H3,(H,23,24,26)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50042591

(CHEMBL3353862)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(cc2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H22F3N3O2/c1-18(2,3)15-11-16(24-27-15)23-17(26)14-5-4-10-25(14)13-8-6-12(7-9-13)19(20,21)22/h6-9,11,14H,4-5,10H2,1-3H3,(H,23,24,26)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50042590

(CHEMBL3353863)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(Cl)cc2)no1 |r| Show InChI InChI=1S/C18H22ClN3O2/c1-18(2,3)15-11-16(21-24-15)20-17(23)14-5-4-10-22(14)13-8-6-12(19)7-9-13/h6-9,11,14H,4-5,10H2,1-3H3,(H,20,21,23)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50042700

(CHEMBL3353881)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCC(=O)N2c2ccc(cc2)C(F)(F)F)on1 |r| Show InChI InChI=1S/C19H20F3N3O3/c1-18(2,3)14-10-15(28-24-14)23-17(27)13-8-9-16(26)25(13)12-6-4-11(5-7-12)19(20,21)22/h4-7,10,13H,8-9H2,1-3H3,(H,23,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042585
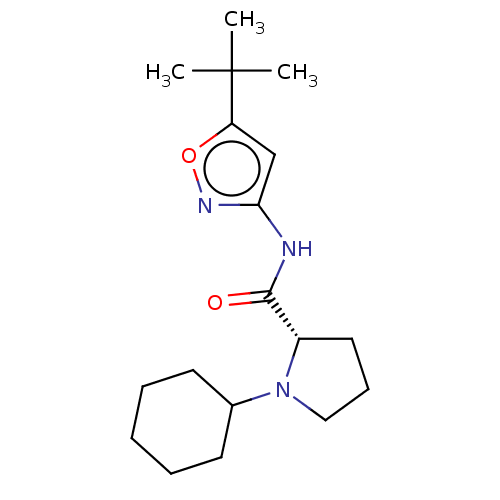
(CHEMBL3353864)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2C2CCCCC2)no1 |r| Show InChI InChI=1S/C18H29N3O2/c1-18(2,3)15-12-16(20-23-15)19-17(22)14-10-7-11-21(14)13-8-5-4-6-9-13/h12-14H,4-11H2,1-3H3,(H,19,20,22)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042590

(CHEMBL3353863)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(Cl)cc2)no1 |r| Show InChI InChI=1S/C18H22ClN3O2/c1-18(2,3)15-11-16(21-24-15)20-17(23)14-5-4-10-22(14)13-8-6-12(19)7-9-13/h6-9,11,14H,4-5,10H2,1-3H3,(H,20,21,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042591

(CHEMBL3353862)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(cc2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H22F3N3O2/c1-18(2,3)15-11-16(24-27-15)23-17(26)14-5-4-10-25(14)13-8-6-12(7-9-13)19(20,21)22/h6-9,11,14H,4-5,10H2,1-3H3,(H,23,24,26)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50042590

(CHEMBL3353863)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(Cl)cc2)no1 |r| Show InChI InChI=1S/C18H22ClN3O2/c1-18(2,3)15-11-16(21-24-15)20-17(23)14-5-4-10-22(14)13-8-6-12(19)7-9-13/h6-9,11,14H,4-5,10H2,1-3H3,(H,20,21,23)/t14-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55,940 from human CB1 receptor expressed in HEK293 cell membranes cells by SPA |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042590

(CHEMBL3353863)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(Cl)cc2)no1 |r| Show InChI InChI=1S/C18H22ClN3O2/c1-18(2,3)15-11-16(21-24-15)20-17(23)14-5-4-10-22(14)13-8-6-12(19)7-9-13/h6-9,11,14H,4-5,10H2,1-3H3,(H,20,21,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-55,212-2 from human CB2 receptor expressed in HEK293 cell membranes cells by SPA |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50006233
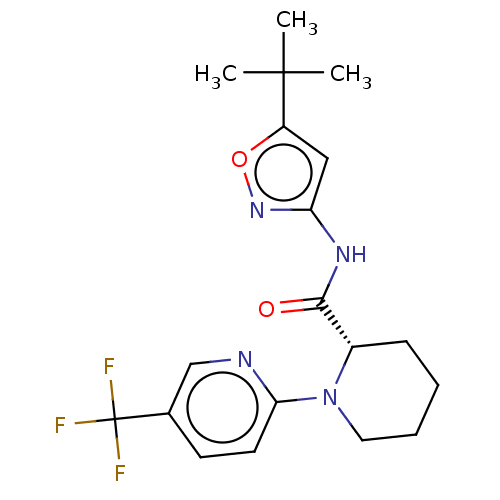
(CHEMBL3235059)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCCN2c2ccc(cn2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H23F3N4O2/c1-18(2,3)14-10-15(25-28-14)24-17(27)13-6-4-5-9-26(13)16-8-7-12(11-23-16)19(20,21)22/h7-8,10-11,13H,4-6,9H2,1-3H3,(H,24,25,27)/t13-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50006259

(CHEMBL3234706)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(cn2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C18H21F3N4O2/c1-17(2,3)13-9-14(24-27-13)23-16(26)12-5-4-8-25(12)15-7-6-11(10-22-15)18(19,20)21/h6-7,9-10,12H,4-5,8H2,1-3H3,(H,23,24,26)/t12-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50006232
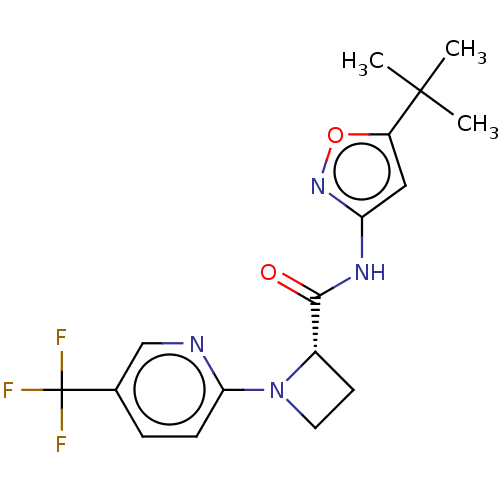
(CHEMBL3235058)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCN2c2ccc(cn2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C17H19F3N4O2/c1-16(2,3)12-8-13(23-26-12)22-15(25)11-6-7-24(11)14-5-4-10(9-21-14)17(18,19)20/h4-5,8-9,11H,6-7H2,1-3H3,(H,22,23,25)/t11-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50006233

(CHEMBL3235059)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCCN2c2ccc(cn2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H23F3N4O2/c1-18(2,3)14-10-15(25-28-14)24-17(27)13-6-4-5-9-26(13)16-8-7-12(11-23-16)19(20,21)22/h7-8,10-11,13H,4-6,9H2,1-3H3,(H,24,25,27)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0930 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50006259

(CHEMBL3234706)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(cn2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C18H21F3N4O2/c1-17(2,3)13-9-14(24-27-13)23-16(26)12-5-4-8-25(12)15-7-6-11(10-22-15)18(19,20)21/h6-7,9-10,12H,4-5,8H2,1-3H3,(H,23,24,26)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50006232

(CHEMBL3235058)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCN2c2ccc(cn2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C17H19F3N4O2/c1-16(2,3)12-8-13(23-26-12)22-15(25)11-6-7-24(11)14-5-4-10(9-21-14)17(18,19)20/h4-5,8-9,11H,6-7H2,1-3H3,(H,22,23,25)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042593
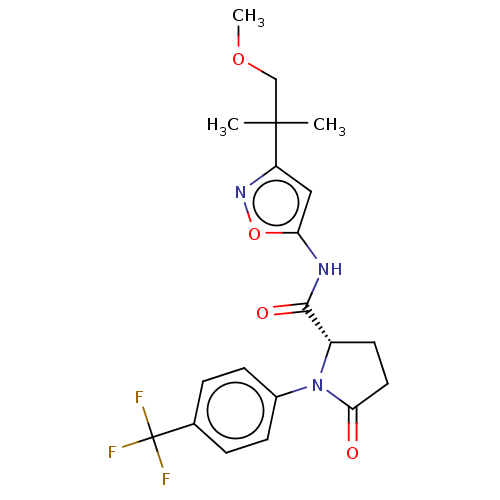
(CHEMBL3352832)Show SMILES COCC(C)(C)c1cc(NC(=O)[C@@H]2CCC(=O)N2c2ccc(cc2)C(F)(F)F)on1 |r| Show InChI InChI=1S/C20H22F3N3O4/c1-19(2,11-29-3)15-10-16(30-25-15)24-18(28)14-8-9-17(27)26(14)13-6-4-12(5-7-13)20(21,22)23/h4-7,10,14H,8-9,11H2,1-3H3,(H,24,28)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042595
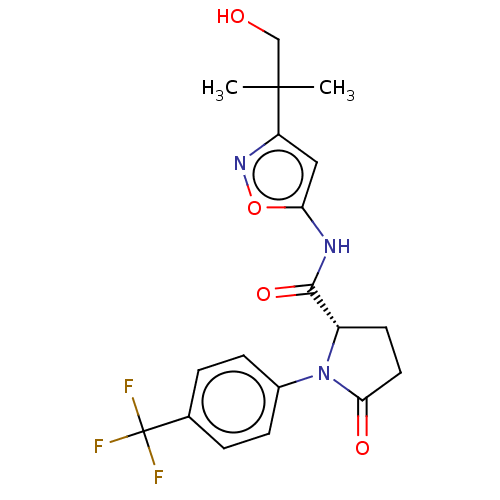
(CHEMBL3353888)Show SMILES CC(C)(CO)c1cc(NC(=O)[C@@H]2CCC(=O)N2c2ccc(cc2)C(F)(F)F)on1 |r| Show InChI InChI=1S/C19H20F3N3O4/c1-18(2,10-26)14-9-15(29-24-14)23-17(28)13-7-8-16(27)25(13)12-5-3-11(4-6-12)19(20,21)22/h3-6,9,13,26H,7-8,10H2,1-2H3,(H,23,28)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042596
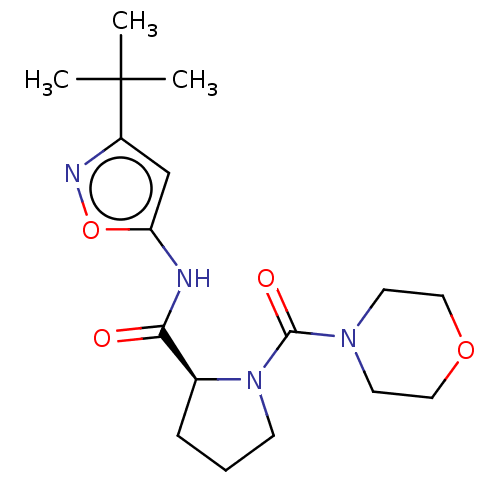
(CHEMBL3353889)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2C(=O)N2CCOCC2)on1 |r| Show InChI InChI=1S/C17H26N4O4/c1-17(2,3)13-11-14(25-19-13)18-15(22)12-5-4-6-21(12)16(23)20-7-9-24-10-8-20/h11-12H,4-10H2,1-3H3,(H,18,22)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042597

(CHEMBL3353890)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2C(=O)N2CCS(=O)(=O)CC2)no1 |r| Show InChI InChI=1S/C17H26N4O5S/c1-17(2,3)13-11-14(19-26-13)18-15(22)12-5-4-6-21(12)16(23)20-7-9-27(24,25)10-8-20/h11-12H,4-10H2,1-3H3,(H,18,19,22)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042598
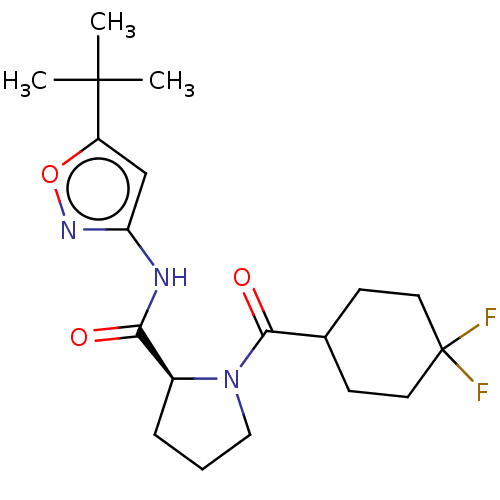
(CHEMBL3353891)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2C(=O)C2CCC(F)(F)CC2)no1 |r| Show InChI InChI=1S/C19H27F2N3O3/c1-18(2,3)14-11-15(23-27-14)22-16(25)13-5-4-10-24(13)17(26)12-6-8-19(20,21)9-7-12/h11-13H,4-10H2,1-3H3,(H,22,23,25)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50042591

(CHEMBL3353862)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(cc2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H22F3N3O2/c1-18(2,3)15-11-16(24-27-15)23-17(26)14-5-4-10-25(14)13-8-6-12(7-9-13)19(20,21)22/h6-9,11,14H,4-5,10H2,1-3H3,(H,23,24,26)/t14-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 307 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50042590

(CHEMBL3353863)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(Cl)cc2)no1 |r| Show InChI InChI=1S/C18H22ClN3O2/c1-18(2,3)15-11-16(21-24-15)20-17(23)14-5-4-10-22(14)13-8-6-12(19)7-9-13/h6-9,11,14H,4-5,10H2,1-3H3,(H,20,21,23)/t14-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50042585

(CHEMBL3353864)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2C2CCCCC2)no1 |r| Show InChI InChI=1S/C18H29N3O2/c1-18(2,3)15-12-16(20-23-15)19-17(22)14-10-7-11-21(14)13-8-5-4-6-9-13/h12-14H,4-11H2,1-3H3,(H,19,20,22)/t14-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50042599
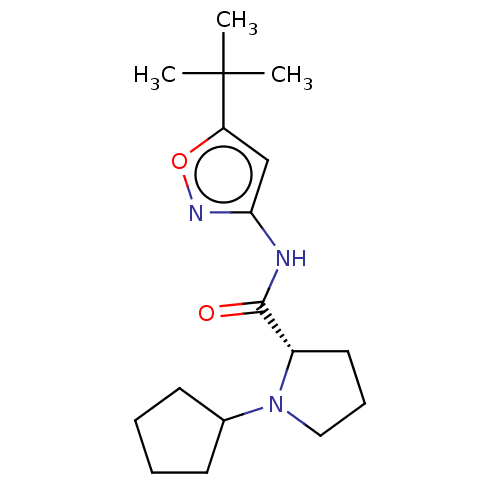
(CHEMBL3353865)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2C2CCCC2)no1 |r| Show InChI InChI=1S/C17H27N3O2/c1-17(2,3)14-11-15(19-22-14)18-16(21)13-9-6-10-20(13)12-7-4-5-8-12/h11-13H,4-10H2,1-3H3,(H,18,19,21)/t13-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50042600
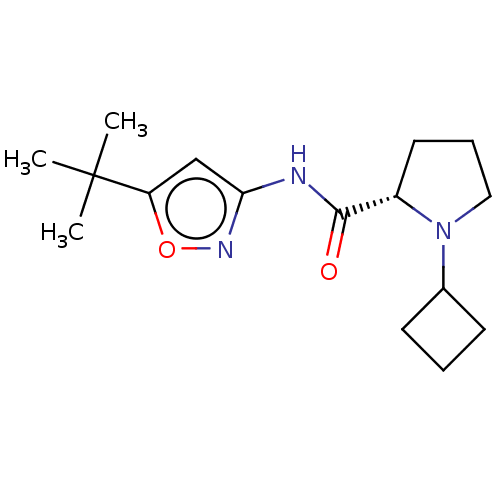
(CHEMBL3353866)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2C2CCC2)no1 |r| Show InChI InChI=1S/C16H25N3O2/c1-16(2,3)13-10-14(18-21-13)17-15(20)12-8-5-9-19(12)11-6-4-7-11/h10-12H,4-9H2,1-3H3,(H,17,18,20)/t12-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50042648
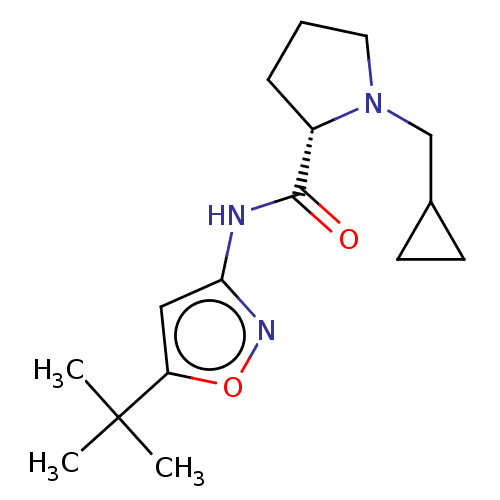
(CHEMBL3353867)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2CC2CC2)no1 |r| Show InChI InChI=1S/C16H25N3O2/c1-16(2,3)13-9-14(18-21-13)17-15(20)12-5-4-8-19(12)10-11-6-7-11/h9,11-12H,4-8,10H2,1-3H3,(H,17,18,20)/t12-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50042657
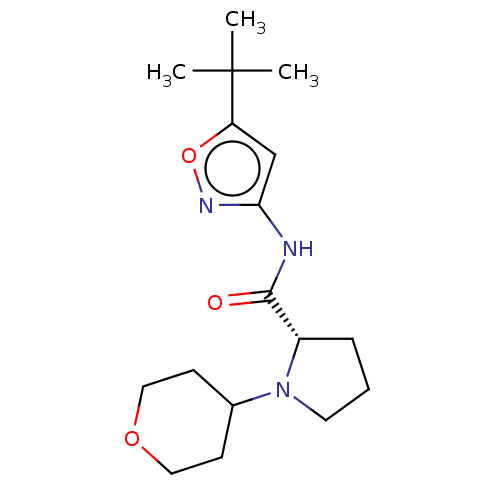
(CHEMBL3353868)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2C2CCOCC2)no1 |r| Show InChI InChI=1S/C17H27N3O3/c1-17(2,3)14-11-15(19-23-14)18-16(21)13-5-4-8-20(13)12-6-9-22-10-7-12/h11-13H,4-10H2,1-3H3,(H,18,19,21)/t13-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50042658
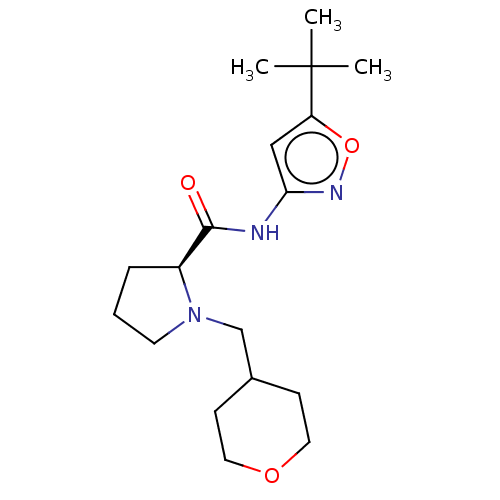
(CHEMBL3353869)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2CC2CCOCC2)no1 |r| Show InChI InChI=1S/C18H29N3O3/c1-18(2,3)15-11-16(20-24-15)19-17(22)14-5-4-8-21(14)12-13-6-9-23-10-7-13/h11,13-14H,4-10,12H2,1-3H3,(H,19,20,22)/t14-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data