

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110268 (CHEMBL2369895 | CSNLSTCVLGKLSQELc[DKLQK]YPRTNTGSGT...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50024170 (CHEMBL2369912) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110272 (CHEMBL2369907 | CSNLSTCVLGKLSQELc[DKLHK]YPRTNTGSGT...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110265 (CHEMBL2369886 | CSNLSTCVLGKLSQELc[DKLHO]YPRTNTGSGT...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110275 (CGNLSTCBLGTYTQDF[DKFHO]YPQTAIGVGAP-amide | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50331916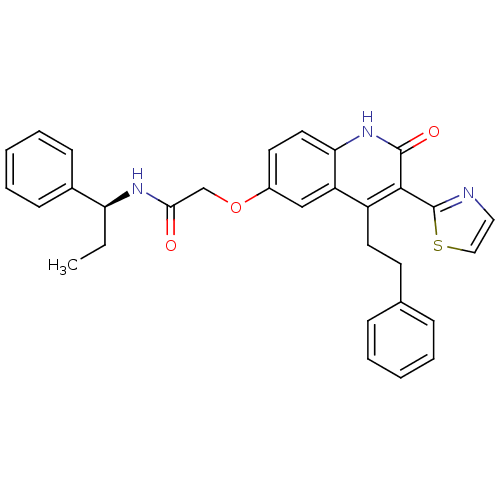 ((S)-2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at adenosien A2B receptor in human HMC-1 cells assessed as inhibition of NECA-induced IL-8 release after 6 hr by ELISA | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50331917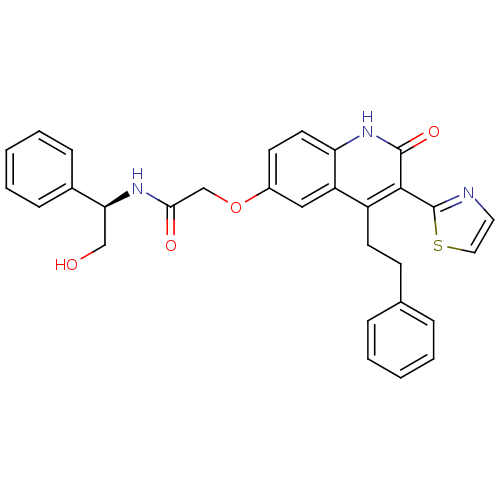 ((R)-N-(2-hydroxy-1-phenylethyl)-2-(2-oxo-4-pheneth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at adenosien A2B receptor in human HMC-1 cells assessed as inhibition of NECA-induced IL-8 release after 6 hr by ELISA | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110273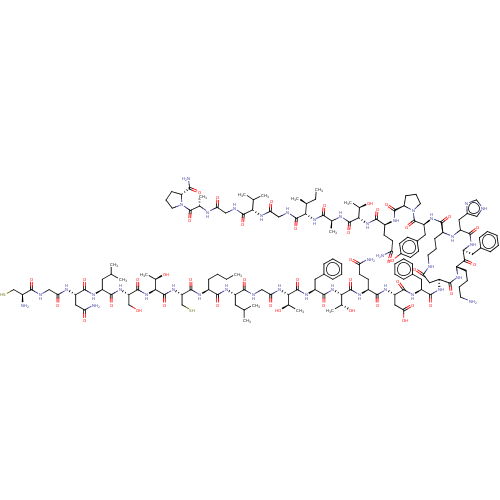 (CGNLSTCMLGTYTQDFc[DKFHK]FPQTAIGVGAP-amide | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20286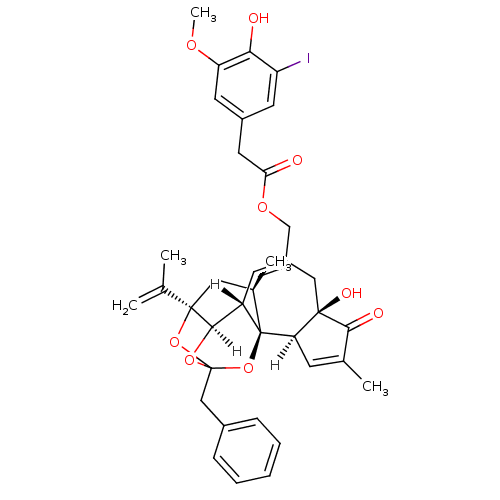 (5-I-RTX | 5-iodoresiniferatoxin | [(1R,2R,6R,10S,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.610 | -54.7 | n/a | n/a | 12.2 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50331914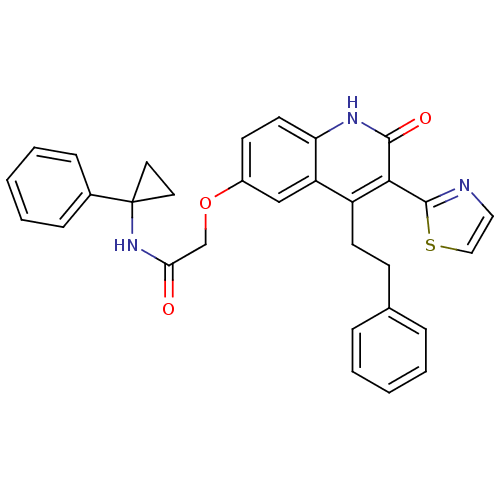 (2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihydroq...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at adenosien A2B receptor in human HMC-1 cells assessed as inhibition of NECA-induced IL-8 release after 6 hr by ELISA | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50331913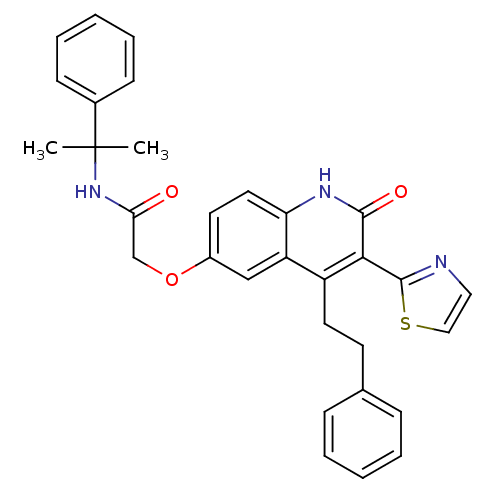 (2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihydroq...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at adenosien A2B receptor in human HMC-1 cells assessed as inhibition of NECA-induced IL-8 release after 6 hr by ELISA | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50331911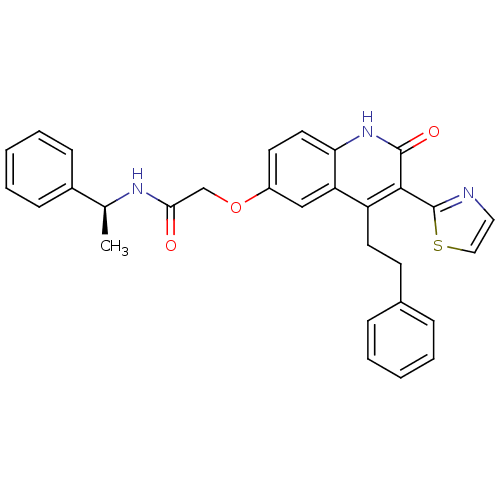 ((S)-2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at adenosien A2B receptor in human HMC-1 cells assessed as inhibition of NECA-induced IL-8 release after 6 hr by ELISA | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20314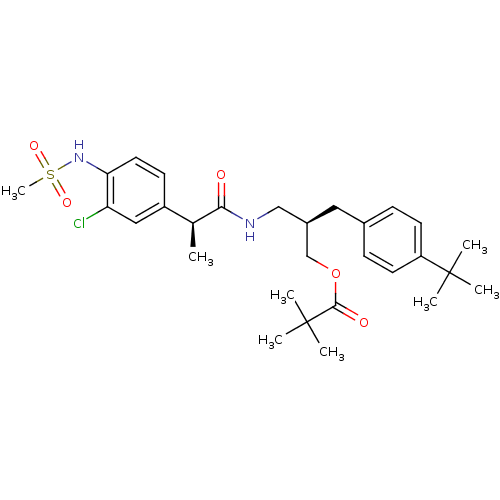 ((2R)-2-[(4-tert-butylphenyl)methyl]-3-[(2S)-2-(3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.83 | -51.9 | n/a | n/a | 5.20 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50017594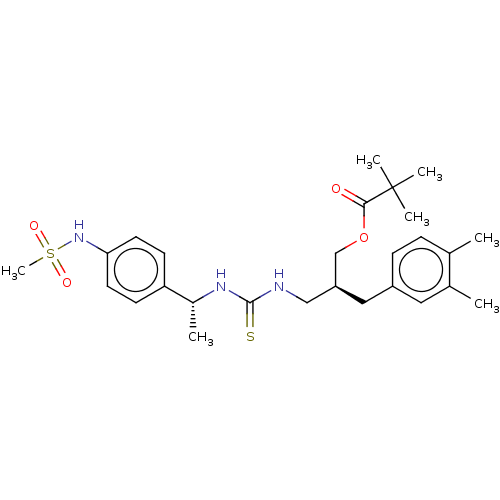 (CHEMBL3288626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from rat TRPV1 after 60 mins by competitive binding assay | Bioorg Med Chem Lett 24: 2685-8 (2014) Article DOI: 10.1016/j.bmcl.2014.04.054 BindingDB Entry DOI: 10.7270/Q26111VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50331917 ((R)-N-(2-hydroxy-1-phenylethyl)-2-(2-oxo-4-pheneth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2B receptor transfected in CHO cells assessed as inhibition of NECA-induced cAMP accumulation treated 15 mins... | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20311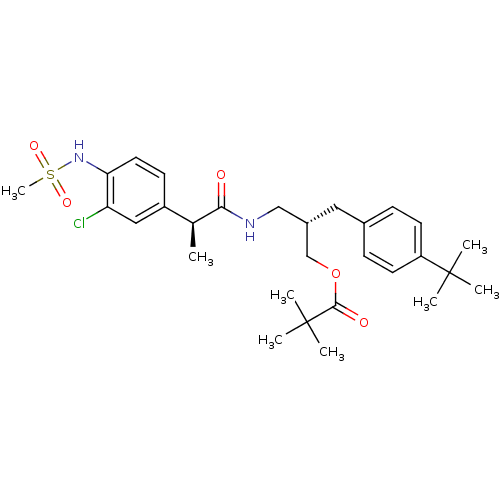 ((2S)-2-[(4-tert-butylphenyl)methyl]-3-[(2S)-2-(3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.29 | -50.4 | n/a | n/a | 12.1 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20330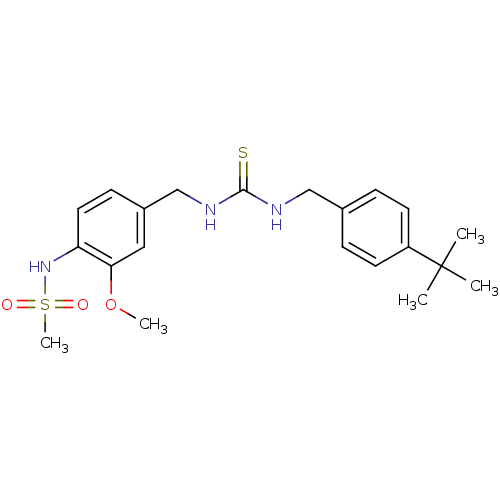 (3-[(4-tert-butylphenyl)methyl]-1-[(4-methanesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity towards rat TRPV1 expressed in CHO cells | Bioorg Med Chem Lett 15: 4143-50 (2005) Article DOI: 10.1016/j.bmcl.2005.06.006 BindingDB Entry DOI: 10.7270/Q2JH3KQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20330 (3-[(4-tert-butylphenyl)methyl]-1-[(4-methanesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells assessed as capsaicin-stimulated 45Ca2+ uptake | Eur J Med Chem 44: 322-31 (2008) Article DOI: 10.1016/j.ejmech.2008.02.026 BindingDB Entry DOI: 10.7270/Q2R49QJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20330 (3-[(4-tert-butylphenyl)methyl]-1-[(4-methanesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity for rat TRPV1 expressed in CHO cells | Bioorg Med Chem Lett 15: 4136-42 (2005) Article DOI: 10.1016/j.bmcl.2005.06.009 BindingDB Entry DOI: 10.7270/Q23B5ZN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20291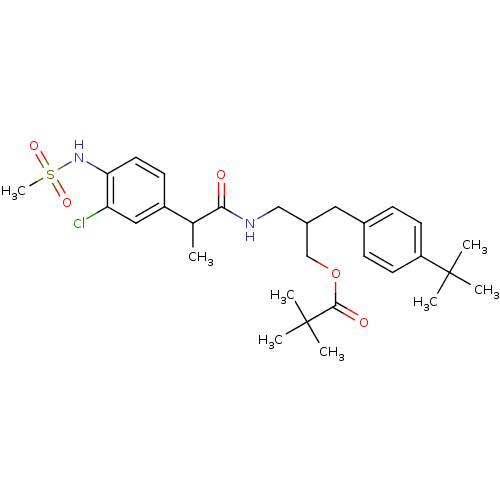 (2-[(4-tert-butylphenyl)methyl]-3-[2-(3-chloro-4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60 | -50.1 | n/a | n/a | 12.3 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20313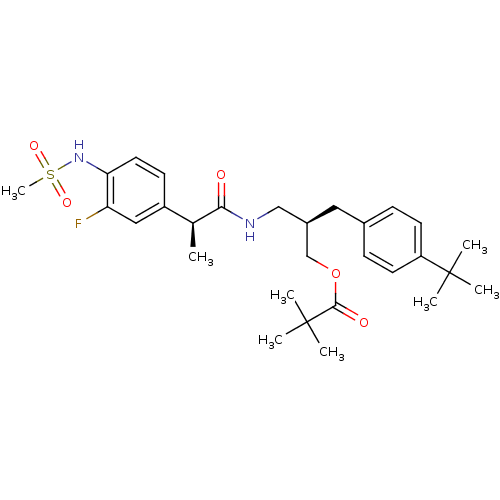 ((2R)-2-[(4-tert-butylphenyl)methyl]-3-[(2S)-2-(3-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.12 | -49.8 | n/a | n/a | 0.580 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50331914 (2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihydroq...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2B receptor transfected in CHO cells assessed as inhibition of NECA-induced cAMP accumulation treated 15 mins... | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50331913 (2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihydroq...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2B receptor transfected in CHO cells assessed as inhibition of NECA-induced cAMP accumulation treated 15 mins... | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110267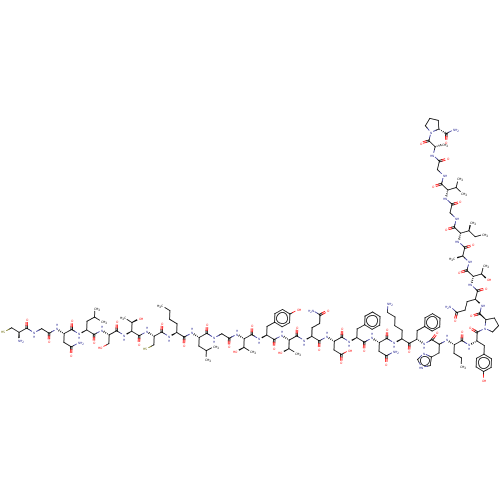 (CGNLSTCBLGTYTQDFNKFHZYPQTAIGVGAP-amide | CHEMBL236...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50017592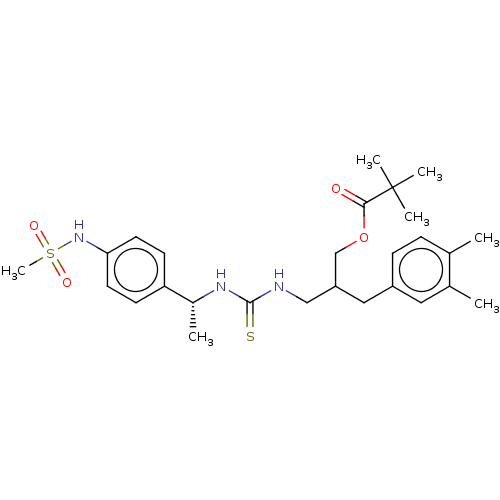 (CHEMBL3288624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from rat TRPV1 after 60 mins by competitive binding assay | Bioorg Med Chem Lett 24: 2685-8 (2014) Article DOI: 10.1016/j.bmcl.2014.04.054 BindingDB Entry DOI: 10.7270/Q26111VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50331917 ((R)-N-(2-hydroxy-1-phenylethyl)-2-(2-oxo-4-pheneth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO-K1 cells after 2 hrs | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20310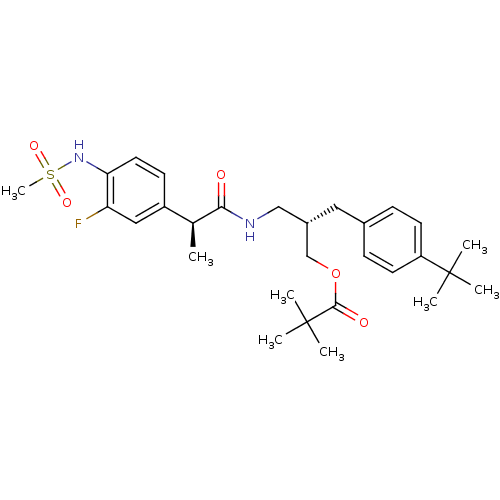 ((2S)-2-[(4-tert-butylphenyl)methyl]-3-[(2S)-2-(3-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.26 | -48.7 | n/a | n/a | 10.9 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50331917 ((R)-N-(2-hydroxy-1-phenylethyl)-2-(2-oxo-4-pheneth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2B receptor transfected in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50017581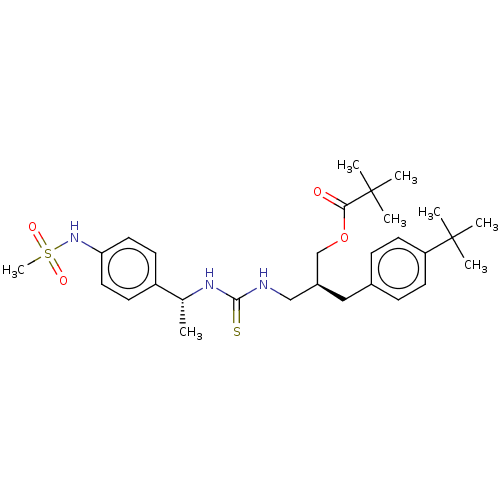 (CHEMBL3288631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from rat TRPV1 after 60 mins by competitive binding assay | Bioorg Med Chem Lett 24: 2685-8 (2014) Article DOI: 10.1016/j.bmcl.2014.04.054 BindingDB Entry DOI: 10.7270/Q26111VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50017592 (CHEMBL3288624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 heterologously expressed in CHO cells assessed as inhibition of capsaicin-induced [45Ca2+] uptake by liquid scintill... | Bioorg Med Chem Lett 24: 2685-8 (2014) Article DOI: 10.1016/j.bmcl.2014.04.054 BindingDB Entry DOI: 10.7270/Q26111VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50017593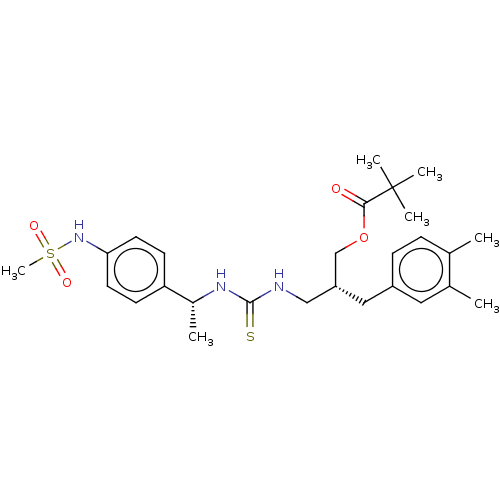 (CHEMBL3288625) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 heterologously expressed in CHO cells assessed as inhibition of capsaicin-induced [45Ca2+] uptake by liquid scintill... | Bioorg Med Chem Lett 24: 2685-8 (2014) Article DOI: 10.1016/j.bmcl.2014.04.054 BindingDB Entry DOI: 10.7270/Q26111VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50331916 ((S)-2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2B receptor transfected in CHO cells assessed as inhibition of NECA-induced cAMP accumulation treated 15 mins... | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50331916 ((S)-2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO-K1 cells after 2 hrs | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20321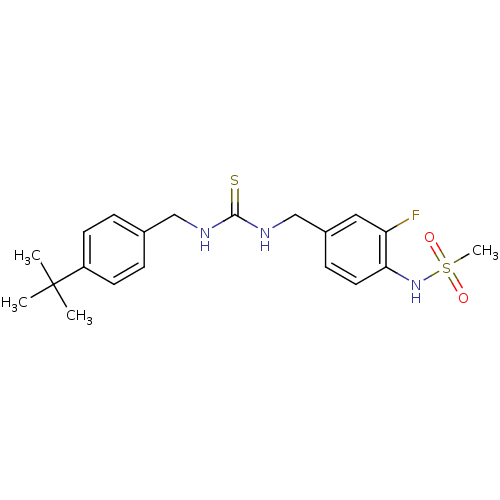 (3-[(4-tert-butylphenyl)methyl]-1-[(3-fluoro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity towards rat TRPV1 expressed in CHO cells | Bioorg Med Chem Lett 15: 4143-50 (2005) Article DOI: 10.1016/j.bmcl.2005.06.006 BindingDB Entry DOI: 10.7270/Q2JH3KQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20321 (3-[(4-tert-butylphenyl)methyl]-1-[(3-fluoro-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity for rat TRPV1 expressed in CHO cells | Bioorg Med Chem Lett 15: 4136-42 (2005) Article DOI: 10.1016/j.bmcl.2005.06.009 BindingDB Entry DOI: 10.7270/Q23B5ZN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50331920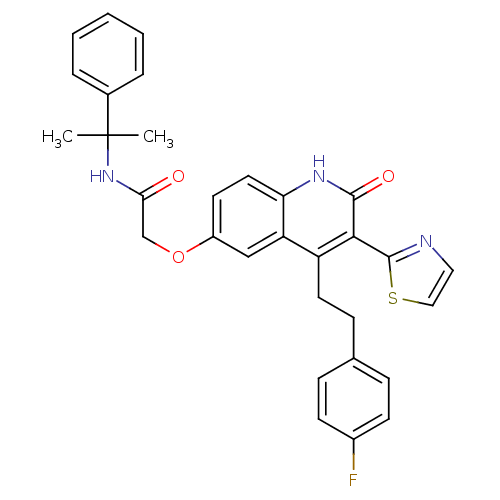 (2-(4-(4-fluorophenethyl)-2-oxo-3-(thiazol-2-yl)-1,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at adenosien A2B receptor in human HMC-1 cells assessed as inhibition of NECA-induced IL-8 release after 6 hr by ELISA | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50017579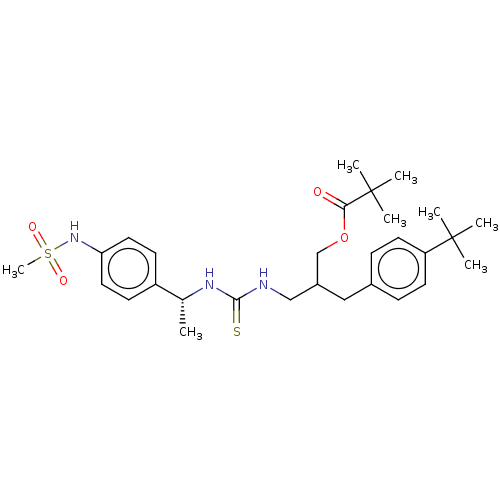 (CHEMBL3288629) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from rat TRPV1 after 60 mins by competitive binding assay | Bioorg Med Chem Lett 24: 2685-8 (2014) Article DOI: 10.1016/j.bmcl.2014.04.054 BindingDB Entry DOI: 10.7270/Q26111VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20297 (3-[2-(3-chloro-4-methanesulfonamidophenyl)propanam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11.3 | -47.2 | n/a | n/a | 36 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50331914 (2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihydroq...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2B receptor transfected in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50331933 (2-(3-(3-methylisoxazol-5-yl)-2-oxo-4-phenethyl-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO-K1 cells after 2 hrs | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50017594 (CHEMBL3288626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 heterologously expressed in CHO cells assessed as inhibition of capsaicin-induced [45Ca2+] uptake by liquid scintill... | Bioorg Med Chem Lett 24: 2685-8 (2014) Article DOI: 10.1016/j.bmcl.2014.04.054 BindingDB Entry DOI: 10.7270/Q26111VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50331914 (2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihydroq...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO-K1 cells after 2 hrs | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20290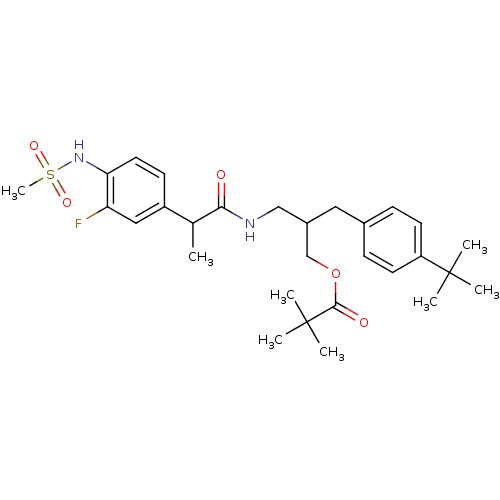 (2-[(4-tert-butylphenyl)methyl]-3-[2-(3-fluoro-4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13.6 | -46.7 | n/a | n/a | 3.24 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50331911 ((S)-2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2B receptor transfected in CHO cells assessed as inhibition of NECA-induced cAMP accumulation treated 15 mins... | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50017593 (CHEMBL3288625) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from rat TRPV1 after 60 mins by competitive binding assay | Bioorg Med Chem Lett 24: 2685-8 (2014) Article DOI: 10.1016/j.bmcl.2014.04.054 BindingDB Entry DOI: 10.7270/Q26111VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50331911 ((S)-2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO-K1 cells after 2 hrs | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20306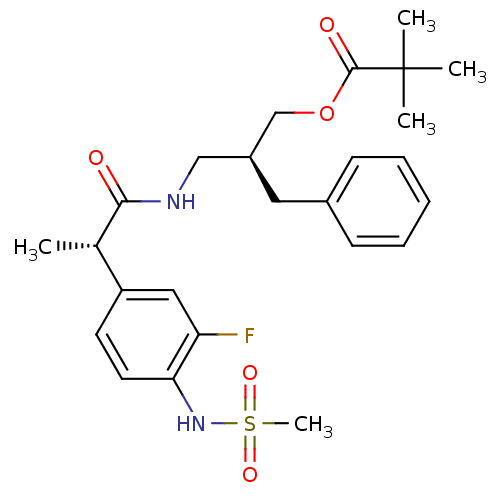 ((2S)-2-benzyl-3-[(2S)-2-(3-fluoro-4-methanesulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16.5 | -46.2 | n/a | n/a | 10 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50331929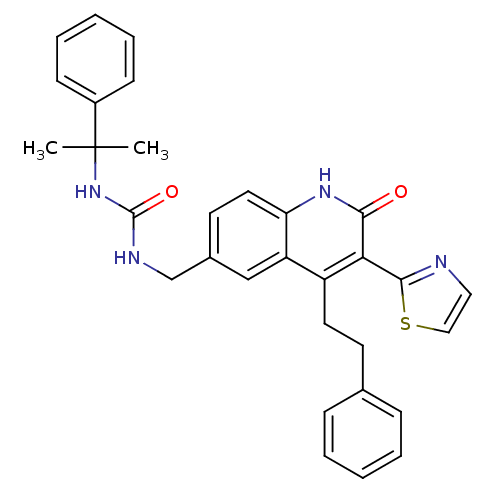 (1-((2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2B receptor transfected in CHO cells assessed as inhibition of NECA-induced cAMP accumulation treated 15 mins... | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50017580 (CHEMBL3288630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from rat TRPV1 after 60 mins by competitive binding assay | Bioorg Med Chem Lett 24: 2685-8 (2014) Article DOI: 10.1016/j.bmcl.2014.04.054 BindingDB Entry DOI: 10.7270/Q26111VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50331910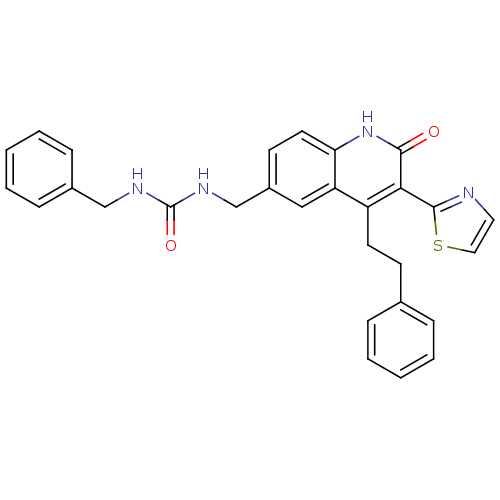 (1-benzyl-3-((2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2B receptor transfected in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 7414-20 (2010) Article DOI: 10.1016/j.bmcl.2010.10.030 BindingDB Entry DOI: 10.7270/Q2WH2Q60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2140 total ) | Next | Last >> |