

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM21392 (3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity against Rat 5-hydroxytryptamine 7 receptor using [3H]-5-HT | J Med Chem 43: 1339-49 (2001) BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123410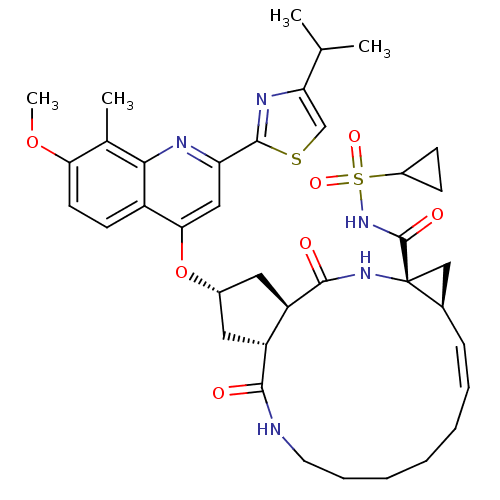 (US8741926, 82 | US8754106, 82 | US8754106, 91) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123407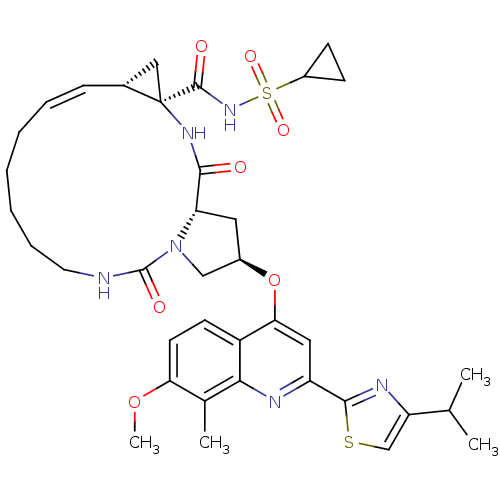 (US8741926, 91) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | -59.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123415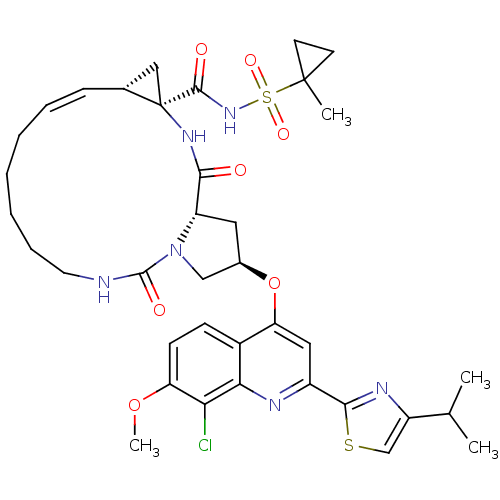 (US8741926, 95 | US8754106, 95) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123415 (US8741926, 95 | US8754106, 95) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123413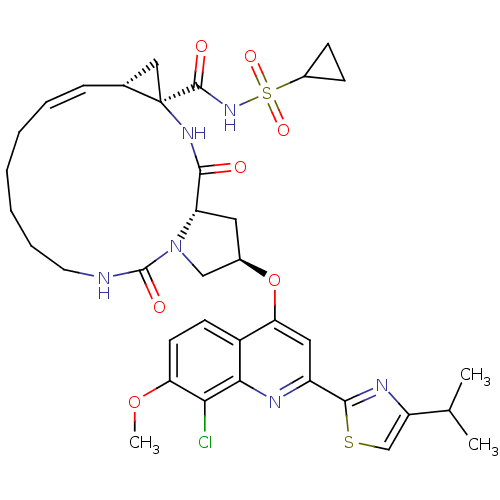 (US8741926, 94 | US8754106, 94) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123411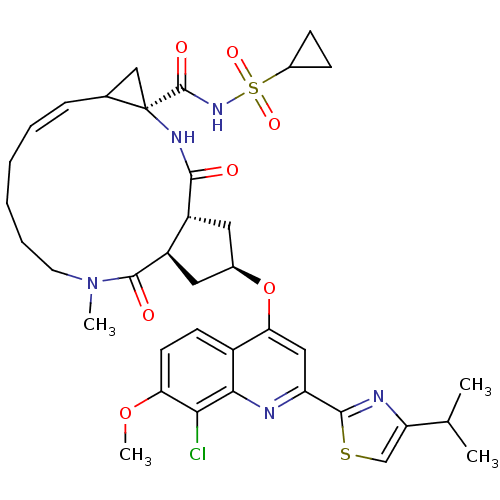 (US8741926, 56) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124106 (US8754106, 56) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123413 (US8741926, 94 | US8754106, 94) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]-quinpirole from human dopamine D2A receptors expressed in LtK cells | Bioorg Med Chem Lett 9: 2167-72 (1999) BindingDB Entry DOI: 10.7270/Q2CF9P97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]-quinpirole from Dopamine receptor D2 | J Med Chem 39: 4421-9 (1996) Article DOI: 10.1021/jm960350p BindingDB Entry DOI: 10.7270/Q21G0KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50054342 (8-{4-[((R)-5-Fluoro-1,2,3,4-tetrahydro-naphthalen-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor | J Med Chem 39: 4421-9 (1996) Article DOI: 10.1021/jm960350p BindingDB Entry DOI: 10.7270/Q21G0KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123414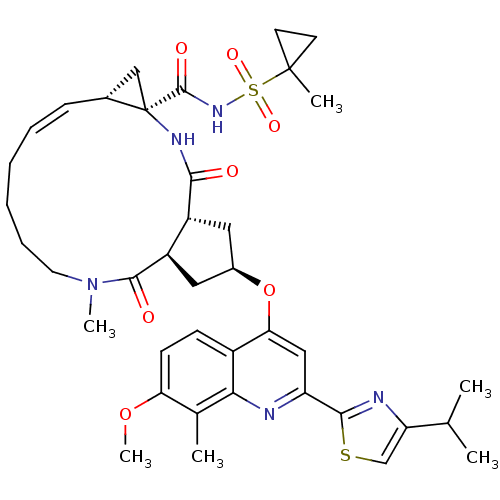 (US8741926, 48 | US8754106, 48) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123414 (US8741926, 48 | US8754106, 48) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | -55.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50054342 (8-{4-[((R)-5-Fluoro-1,2,3,4-tetrahydro-naphthalen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from Dopamine receptor D3 | J Med Chem 39: 4421-9 (1996) Article DOI: 10.1021/jm960350p BindingDB Entry DOI: 10.7270/Q21G0KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123412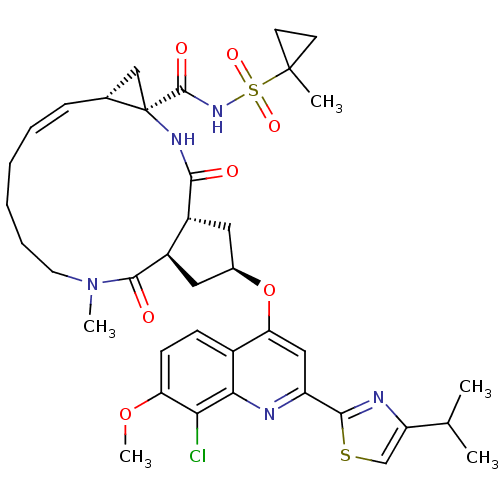 (US8741926, 57 | US8754106, 57) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123412 (US8741926, 57 | US8754106, 57) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2A receptors expressed in LtK cells | Bioorg Med Chem Lett 9: 2167-72 (1999) BindingDB Entry DOI: 10.7270/Q2CF9P97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50036286 ((R)-(-)-10-methyl-11-hydroxyaporphine | (R)-6,10-d...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro affinity at 5-hydroxytryptamine 1A receptor of rat hippocampus by [3H]-8-OH-DPAT displacement. | J Med Chem 39: 3491-502 (1996) Article DOI: 10.1021/jm960188q BindingDB Entry DOI: 10.7270/Q2XW4HXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50036286 ((R)-(-)-10-methyl-11-hydroxyaporphine | (R)-6,10-d...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Ability to displace [3H]-8-OH-DPAT binding to rat hippocampal 5-hydroxytryptamine 1A receptor | J Med Chem 38: 647-58 (1995) BindingDB Entry DOI: 10.7270/Q2HX1BQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50336504 ((2R,3aR,10Z,11aS,12aR,14aR)-N-(cyclopropylsulfonyl...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50336504 ((2R,3aR,10Z,11aS,12aR,14aR)-N-(cyclopropylsulfonyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of [3H]-raclopride binding to Dopamine receptor D2 | J Med Chem 39: 4421-9 (1996) Article DOI: 10.1021/jm960350p BindingDB Entry DOI: 10.7270/Q21G0KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50030004 (1-((S)-7-Dipropylamino-5,6,7,8-tetrahydro-naphthal...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity against rat hippocampal 5-hydroxytryptamine 1A (5-HT1A) receptor determined using [3H]8-OH-DPAT as radioligand | J Med Chem 39: 4036-43 (1996) Article DOI: 10.1021/jm960329o BindingDB Entry DOI: 10.7270/Q2XK8G6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50150685 (((S)-5-Phenyl-1,2,3,4-tetrahydro-naphthalen-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of [3H]-8-OH-DPAT binding to rat 5-hydroxytryptamine 1A receptor | J Med Chem 47: 3927-30 (2004) Article DOI: 10.1021/jm0498102 BindingDB Entry DOI: 10.7270/Q2FQ9W2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50053667 (CHEMBL552643 | Trifluoro-methanesulfonic acid (S)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity against rat hippocampal 5-hydroxytryptamine 1A (5-HT1A) receptor determined using [3H]8-OH-DPAT as radioligand | J Med Chem 39: 4036-43 (1996) Article DOI: 10.1021/jm960329o BindingDB Entry DOI: 10.7270/Q2XK8G6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50053661 (8-{4-[((R)-5-Fluoro-8-furan-2-yl-1,2,3,4-tetrahydr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity against rat hippocampal 5-hydroxytryptamine 1A (5-HT1A) receptor determined using [3H]8-OH-DPAT as radioligand | J Med Chem 39: 4036-43 (1996) Article DOI: 10.1021/jm960329o BindingDB Entry DOI: 10.7270/Q2XK8G6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078242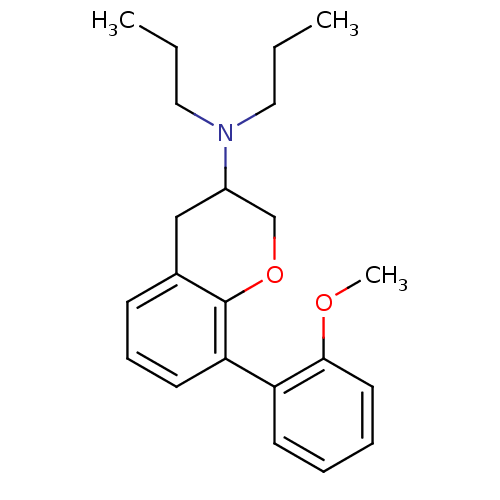 (CHEMBL41491 | [8-(2-Methoxy-phenyl)-chroman-3-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078250 (CHEMBL39847 | Dipropyl-(8-thiophen-3-yl-chroman-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078243 ((8-Phenyl-chroman-3-yl)-dipropyl-amine | CHEMBL411...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM50087029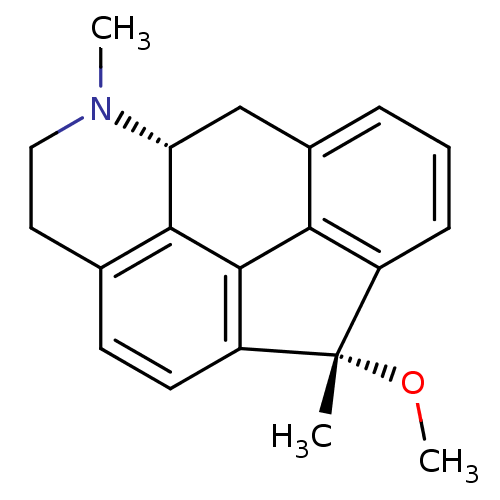 ((1S,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]-5-HT from over-expressed rat 5-hydroxytryptamine 7 receptor | J Med Chem 43: 1339-49 (2001) BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM50087033 ((1R,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]-5-HT from over-expressed rat 5-hydroxytryptamine 7 receptor | J Med Chem 43: 1339-49 (2001) BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50150676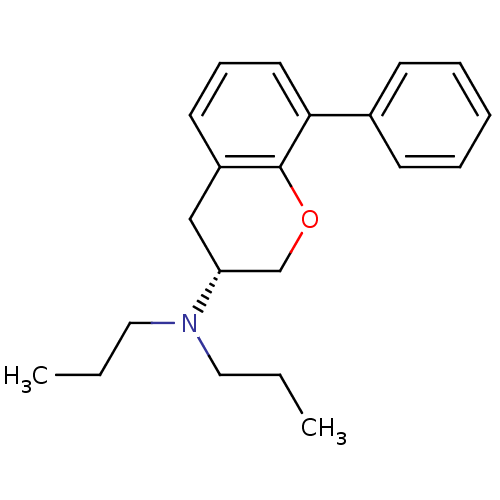 (((R)-8-Phenyl-chroman-3-yl)-dipropyl-amine | (R)-8...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of [3H]-8-OH-DPAT binding to rat 5-hydroxytryptamine 1A receptor | J Med Chem 47: 3927-30 (2004) Article DOI: 10.1021/jm0498102 BindingDB Entry DOI: 10.7270/Q2FQ9W2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM50098551 ((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]-5-HT from rat 5-hydroxytryptamine 7 receptor expressed in CHO cells | J Med Chem 44: 1337-40 (2001) BindingDB Entry DOI: 10.7270/Q23X85WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50016777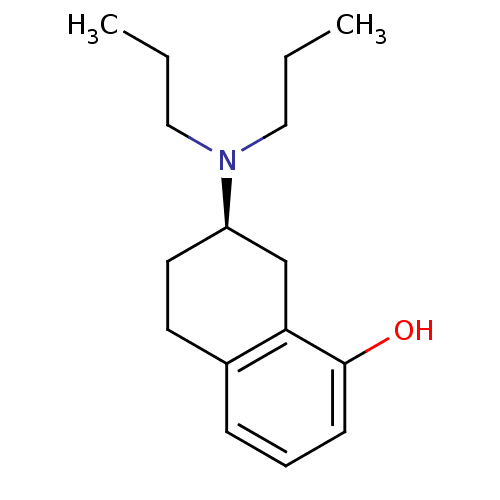 (((R)-8-Hydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity was measured on cloned Human 5-hydroxytryptamine 1A receptor which is labeled by [3H]-8-OH-DPAT | J Med Chem 43: 1339-49 (2001) BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50078240 (CHEMBL41200 | [8-(4-Methoxy-phenyl)-chroman-3-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016777 (((R)-8-Hydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity against rat hippocampal 5-hydroxytryptamine 1A (5-HT1A) receptor determined using [3H]8-OH-DPAT as radioligand | J Med Chem 39: 4036-43 (1996) Article DOI: 10.1021/jm960329o BindingDB Entry DOI: 10.7270/Q2XK8G6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50150678 (CHEMBL186758 | [(R)-8-(4-Methoxy-phenyl)-chroman-3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of [3H]-8-OH-DPAT binding to rat 5-hydroxytryptamine 1A receptor | J Med Chem 47: 3927-30 (2004) Article DOI: 10.1021/jm0498102 BindingDB Entry DOI: 10.7270/Q2FQ9W2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50150677 (CHEMBL188176 | [(S)-5-(4-Methoxy-phenyl)-1,2,3,4-t...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of [3H]-8-OH-DPAT binding to rat 5-hydroxytryptamine 1A receptor | J Med Chem 47: 3927-30 (2004) Article DOI: 10.1021/jm0498102 BindingDB Entry DOI: 10.7270/Q2FQ9W2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM50150680 (CHEMBL184535 | [(S)-5-(2-Methoxy-phenyl)-1,2,3,4-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of [3H]-5-HT binding to rat 5-hydroxytryptamine 7 receptor | J Med Chem 47: 3927-30 (2004) Article DOI: 10.1021/jm0498102 BindingDB Entry DOI: 10.7270/Q2FQ9W2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50052869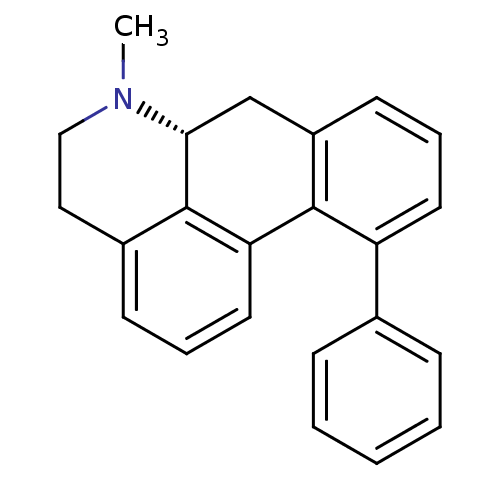 ((R)-6-Methyl-11-phenyl-5,6,6a,7-tetrahydro-4H-dibe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro displacement of [3H]-8-OH-DPAT binding to rat hippocampal 5-hydroxytryptamine 1A receptor | J Med Chem 39: 3503-13 (1996) Article DOI: 10.1021/jm960189i BindingDB Entry DOI: 10.7270/Q2T43S6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50052869 ((R)-6-Methyl-11-phenyl-5,6,6a,7-tetrahydro-4H-dibe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cortical membranes | Bioorg Med Chem Lett 9: 1583-6 (1999) BindingDB Entry DOI: 10.7270/Q2T152TW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50052869 ((R)-6-Methyl-11-phenyl-5,6,6a,7-tetrahydro-4H-dibe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in CHO cells | J Med Chem 44: 1337-40 (2001) BindingDB Entry DOI: 10.7270/Q23X85WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50011247 (7-Dipropylamino-5,6,7,8-tetrahydro-naphthalen-1-ol...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity against rat hippocampal 5-hydroxytryptamine 1A (5-HT1A) receptor determined using [3H]8-OH-DPAT as radioligand | J Med Chem 39: 4036-43 (1996) Article DOI: 10.1021/jm960329o BindingDB Entry DOI: 10.7270/Q2XK8G6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50053678 (((S)-8-Furan-2-yl-1,2,3,4-tetrahydro-naphthalen-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity against rat hippocampal 5-hydroxytryptamine 1A (5-HT1A) receptor determined using [3H]8-OH-DPAT as radioligand | J Med Chem 39: 4036-43 (1996) Article DOI: 10.1021/jm960329o BindingDB Entry DOI: 10.7270/Q2XK8G6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50030025 (1-((R)-7-Dipropylamino-5,6,7,8-tetrahydro-naphthal...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity against rat hippocampal 5-hydroxytryptamine 1A (5-HT1A) receptor determined using [3H]8-OH-DPAT as radioligand | J Med Chem 39: 4036-43 (1996) Article DOI: 10.1021/jm960329o BindingDB Entry DOI: 10.7270/Q2XK8G6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50087037 (1-Ethoxy-1-ethyl-6-methyl-1,5,5a,6,7,8-hexahydro-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity was measured on cloned human 5-hydroxytryptamine 1A receptor which is labeled by [3H]-8-OH-DPAT | J Med Chem 43: 1339-49 (2001) BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]-quinpirole from human dopamine D2A receptors expressed in LtK cells | Bioorg Med Chem Lett 9: 2167-72 (1999) BindingDB Entry DOI: 10.7270/Q2CF9P97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]-quinpirole from Dopamine receptor D2 | J Med Chem 39: 4421-9 (1996) Article DOI: 10.1021/jm960350p BindingDB Entry DOI: 10.7270/Q21G0KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM50150681 (4-(4-Chloro-phenoxy)-1-{2-[(R)-1-(3H-indene-5-sulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of [3H]-5-HT binding to rat 5-hydroxytryptamine 7 receptor | J Med Chem 47: 3927-30 (2004) Article DOI: 10.1021/jm0498102 BindingDB Entry DOI: 10.7270/Q2FQ9W2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2352 total ) | Next | Last >> |