 Found 138 hits with Last Name = 'johnstone' and Initial = 'kd'
Found 138 hits with Last Name = 'johnstone' and Initial = 'kd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Heparanase
(Homo sapiens (Human)) | BDBM50388329
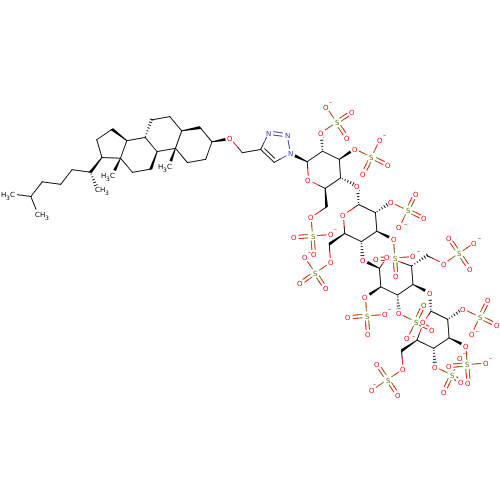
(CHEMBL2059500)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OCc1cn(nn1)[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]4OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C54H91N3O60S13/c1-24(2)7-6-8-25(3)30-11-12-31-29-10-9-26-17-28(13-15-53(26,4)32(29)14-16-54(30,31)5)97-19-27-18-57(56-55-27)49-45(114-127(85,86)87)41(110-123(73,74)75)37(33(102-49)20-98-118(58,59)60)106-50-46(115-128(88,89)90)42(111-124(76,77)78)38(34(103-50)21-99-119(61,62)63)107-51-47(116-129(91,92)93)43(112-125(79,80)81)39(35(104-51)22-100-120(64,65)66)108-52-48(117-130(94,95)96)44(113-126(82,83)84)40(109-122(70,71)72)36(105-52)23-101-121(67,68)69/h18,24-26,28-52H,6-17,19-23H2,1-5H3,(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)(H,85,86,87)(H,88,89,90)(H,91,92,93)(H,94,95,96)/p-13/t25-,26+,28+,29+,30-,31+,32+,33-,34-,35-,36-,37-,38-,39-,40-,41+,42+,43+,44+,45-,46-,47-,48-,49-,50-,51-,52-,53+,54-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388343
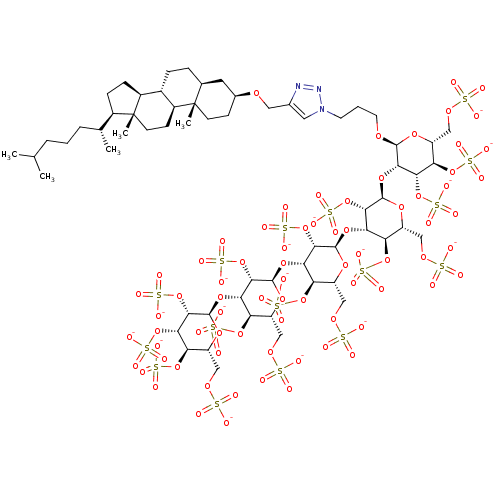
(CHEMBL2059243)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OCc1cn(CCCO[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]2O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]5O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)nn1 |r| Show InChI InChI=1S/C63H107N3O75S16/c1-28(2)8-6-9-29(3)34-12-13-35-33-11-10-30-20-32(14-16-62(30,4)36(33)15-17-63(34,35)5)116-22-31-21-66(65-64-31)18-7-19-115-57-52(50(136-152(97,98)99)45(134-150(91,92)93)40(122-57)26-120-145(76,77)78)130-60-55(140-156(109,110)111)49(44(133-149(88,89)90)39(125-60)25-119-144(73,74)75)128-58-53(138-154(103,104)105)47(42(131-147(82,83)84)37(123-58)23-117-142(67,68)69)127-59-54(139-155(106,107)108)48(43(132-148(85,86)87)38(124-59)24-118-143(70,71)72)129-61-56(141-157(112,113)114)51(137-153(100,101)102)46(135-151(94,95)96)41(126-61)27-121-146(79,80)81/h21,28-30,32-61H,6-20,22-27H2,1-5H3,(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)(H,85,86,87)(H,88,89,90)(H,91,92,93)(H,94,95,96)(H,97,98,99)(H,100,101,102)(H,103,104,105)(H,106,107,108)(H,109,110,111)(H,112,113,114)/p-16/t29-,30+,32+,33+,34-,35+,36+,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47+,48+,49+,50+,51+,52+,53+,54+,55+,56+,57+,58-,59-,60-,61-,62+,63-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388341
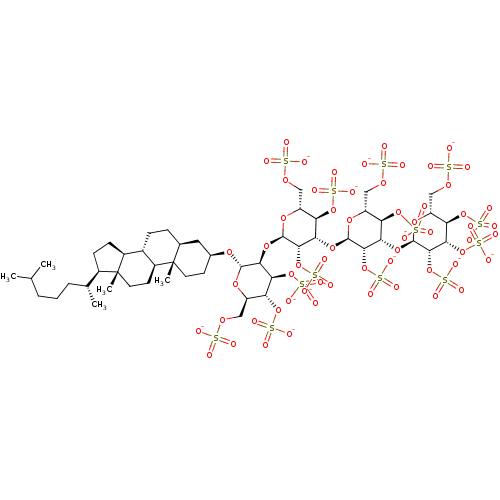
(CHEMBL2059241)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C51H88O60S13/c1-22(2)7-6-8-23(3)27-11-12-28-26-10-9-24-17-25(13-15-50(24,4)29(26)14-16-51(27,28)5)95-46-42(40(107-120(76,77)78)36(105-118(70,71)72)32(96-46)20-93-114(58,59)60)102-48-44(110-123(85,86)87)39(35(104-117(67,68)69)31(98-48)19-92-113(55,56)57)100-47-43(109-122(82,83)84)38(34(103-116(64,65)66)30(97-47)18-91-112(52,53)54)101-49-45(111-124(88,89)90)41(108-121(79,80)81)37(106-119(73,74)75)33(99-49)21-94-115(61,62)63/h22-49H,6-21H2,1-5H3,(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)(H,85,86,87)(H,88,89,90)/p-13/t23-,24+,25+,26+,27-,28+,29+,30-,31-,32-,33-,34-,35-,36-,37-,38+,39+,40+,41+,42+,43+,44+,45+,46+,47-,48-,49-,50+,51-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388342
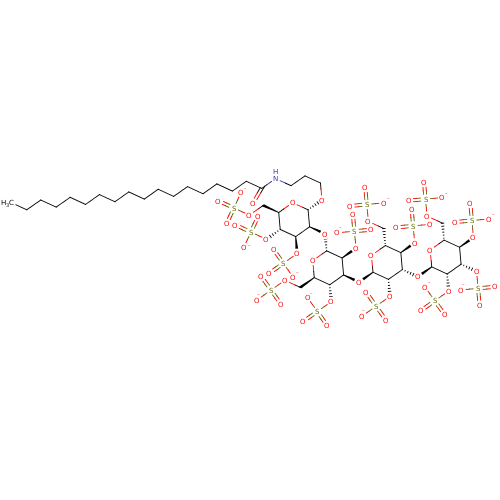
(CHEMBL2059242)Show SMILES CCCCCCCCCCCCCCCCCC(=O)NCCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C45H83NO61S13/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18-29(47)46-19-17-20-87-42-38(36(103-116(72,73)74)32(101-114(66,67)68)27(92-42)23-90-110(54,55)56)98-44-40(106-119(81,82)83)35(31(100-113(63,64)65)26(94-44)22-89-109(51,52)53)96-43-39(105-118(78,79)80)34(30(99-112(60,61)62)25(93-43)21-88-108(48,49)50)97-45-41(107-120(84,85)86)37(104-117(75,76)77)33(102-115(69,70)71)28(95-45)24-91-111(57,58)59/h25-28,30-45H,2-24H2,1H3,(H,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)/p-13/t25-,26-,27-,28-,30-,31-,32-,33-,34+,35+,36+,37+,38+,39+,40+,41+,42+,43-,44-,45-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388331
(CHEMBL2059499)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]4OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C51H88O60S13/c1-22(2)7-6-8-23(3)27-11-12-28-26-10-9-24-17-25(13-15-50(24,4)29(26)14-16-51(27,28)5)95-46-42(108-121(79,80)81)38(104-117(67,68)69)34(30(96-46)18-91-112(52,53)54)100-47-43(109-122(82,83)84)39(105-118(70,71)72)35(31(97-47)19-92-113(55,56)57)101-48-44(110-123(85,86)87)40(106-119(73,74)75)36(32(98-48)20-93-114(58,59)60)102-49-45(111-124(88,89)90)41(107-120(76,77)78)37(103-116(64,65)66)33(99-49)21-94-115(61,62)63/h22-49H,6-21H2,1-5H3,(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)(H,85,86,87)(H,88,89,90)/p-13/t23-,24+,25+,26+,27-,28+,29+,30-,31-,32-,33-,34-,35-,36-,37-,38+,39+,40+,41+,42-,43-,44-,45-,46-,47-,48-,49-,50+,51-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388345
(CHEMBL2059245)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C45H78O46S10/c1-21(2)7-6-8-22(3)26-11-12-27-25-10-9-23-17-24(13-15-44(23,4)28(25)14-16-45(26,27)5)79-41-38(36(88-98(64,65)66)33(86-96(58,59)60)30(80-41)19-77-93(49,50)51)84-42-39(90-100(70,71)72)35(32(85-95(55,56)57)29(81-42)18-76-92(46,47)48)83-43-40(91-101(73,74)75)37(89-99(67,68)69)34(87-97(61,62)63)31(82-43)20-78-94(52,53)54/h21-43H,6-20H2,1-5H3,(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)/p-10/t22-,23+,24+,25+,26-,27+,28+,29-,30-,31-,32-,33-,34-,35+,36+,37+,38+,39+,40+,41+,42-,43-,44+,45-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50378647
(CHEMBL1627122 | PI-88)Show SMILES [O-]P([O-])(=O)OC[C@H]1O[C@H](O[C@H]2[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@H]3[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@H]4[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@@H]5[C@@H](OS([O-])(=O)=O)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@H]4OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C30H53O77PS16/c31-108(32,33)82-1-6-14(99-116(55,56)57)20(102-119(64,65)66)25(106-123(76,77)78)29(87-6)94-17-12(97-114(49,50)51)8(3-84-110(37,38)39)89-27(23(17)104-121(70,71)72)92-16-11(96-113(46,47)48)7(2-83-109(34,35)36)88-26(22(16)103-120(67,68)69)93-18-13(98-115(52,53)54)9(4-85-111(40,41)42)90-28(24(18)105-122(73,74)75)95-21-19(101-118(61,62)63)15(100-117(58,59)60)10(5-86-112(43,44)45)91-30(21)107-124(79,80)81/h6-30H,1-5H2,(H2,31,32,33)(H,34,35,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)/p-18/t6-,7-,8-,9-,10-,11-,12-,13-,14-,15-,16+,17+,18+,19+,20+,21+,22+,23+,24+,25+,26-,27-,28-,29-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388332
(CHEMBL2059498)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OCc1cn(CCCO[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)nn1 |r| Show InChI InChI=1S/C51H87N3O47S10/c1-26(2)8-6-9-27(3)32-12-13-33-31-11-10-28-20-30(14-16-50(28,4)34(31)15-17-51(32,33)5)86-22-29-21-54(53-52-29)18-7-19-85-47-44(99-109(76,77)78)41(38(95-105(64,65)66)35(90-47)23-87-102(55,56)57)93-48-45(100-110(79,80)81)42(39(96-106(67,68)69)36(91-48)24-88-103(58,59)60)94-49-46(101-111(82,83)84)43(98-108(73,74)75)40(97-107(70,71)72)37(92-49)25-89-104(61,62)63/h21,26-28,30-49H,6-20,22-25H2,1-5H3,(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)/p-10/t27-,28+,30+,31+,32-,33+,34+,35-,36-,37-,38-,39-,40-,41+,42+,43+,44+,45+,46+,47+,48-,49-,50+,51-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388346
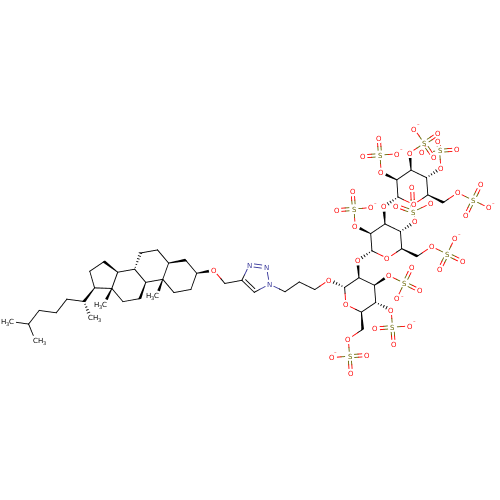
(CHEMBL2059246)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OCc1cn(CCCO[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]2O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)nn1 |r| Show InChI InChI=1S/C51H87N3O47S10/c1-26(2)8-6-9-27(3)32-12-13-33-31-11-10-28-20-30(14-16-50(28,4)34(31)15-17-51(32,33)5)86-22-29-21-54(53-52-29)18-7-19-85-47-44(42(98-108(73,74)75)39(96-106(67,68)69)36(90-47)24-88-103(58,59)60)94-48-45(100-110(79,80)81)41(38(95-105(64,65)66)35(91-48)23-87-102(55,56)57)93-49-46(101-111(82,83)84)43(99-109(76,77)78)40(97-107(70,71)72)37(92-49)25-89-104(61,62)63/h21,26-28,30-49H,6-20,22-25H2,1-5H3,(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)/p-10/t27-,28+,30+,31+,32-,33+,34+,35-,36-,37-,38-,39-,40-,41+,42+,43+,44+,45+,46+,47+,48-,49-,50+,51-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388335

(CHEMBL2059503)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C45H78O46S10/c1-21(2)7-6-8-22(3)26-11-12-27-25-10-9-23-17-24(13-15-44(23,4)28(25)14-16-45(26,27)5)79-41-38(89-99(67,68)69)35(86-96(58,59)60)32(29(80-41)18-76-92(46,47)48)83-42-39(90-100(70,71)72)36(87-97(61,62)63)33(30(81-42)19-77-93(49,50)51)84-43-40(91-101(73,74)75)37(88-98(64,65)66)34(85-95(55,56)57)31(82-43)20-78-94(52,53)54/h21-43H,6-20H2,1-5H3,(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)/p-10/t22-,23+,24+,25+,26-,27+,28+,29-,30-,31-,32-,33-,34-,35+,36+,37+,38-,39-,40-,41-,42-,43-,44+,45-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388336
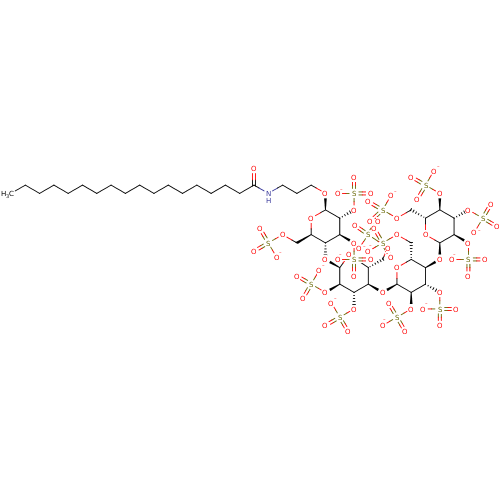
(CHEMBL2059504)Show SMILES CCCCCCCCCCCCCCCCCC(=O)NCCCO[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]4OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C45H83NO61S13/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18-29(47)46-19-17-20-87-42-38(104-117(75,76)77)34(100-113(63,64)65)30(25(92-42)21-88-108(48,49)50)96-43-39(105-118(78,79)80)35(101-114(66,67)68)31(26(93-43)22-89-109(51,52)53)97-44-40(106-119(81,82)83)36(102-115(69,70)71)32(27(94-44)23-90-110(54,55)56)98-45-41(107-120(84,85)86)37(103-116(72,73)74)33(99-112(60,61)62)28(95-45)24-91-111(57,58)59/h25-28,30-45H,2-24H2,1H3,(H,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)/p-13/t25-,26-,27-,28-,30-,31-,32-,33-,34+,35+,36+,37+,38-,39-,40-,41-,42-,43-,44-,45-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388330
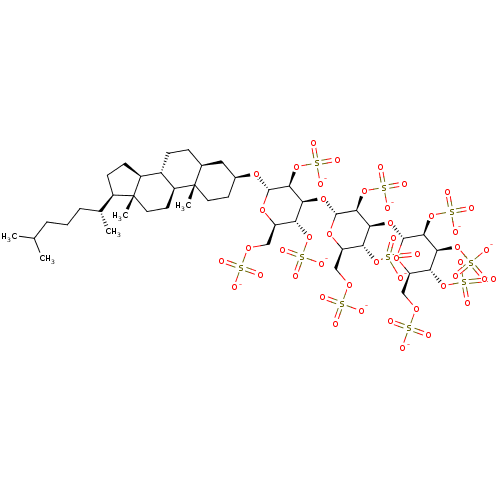
(CHEMBL2059247)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C45H78O46S10/c1-21(2)7-6-8-22(3)26-11-12-27-25-10-9-23-17-24(13-15-44(23,4)28(25)14-16-45(26,27)5)79-41-38(89-99(67,68)69)35(32(85-95(55,56)57)29(80-41)18-76-92(46,47)48)83-42-39(90-100(70,71)72)36(33(86-96(58,59)60)30(81-42)19-77-93(49,50)51)84-43-40(91-101(73,74)75)37(88-98(64,65)66)34(87-97(61,62)63)31(82-43)20-78-94(52,53)54/h21-43H,6-20H2,1-5H3,(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)/p-10/t22-,23+,24+,25+,26-,27+,28+,29-,30-,31-,32-,33-,34-,35+,36+,37+,38+,39+,40+,41+,42-,43-,44+,45-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388328
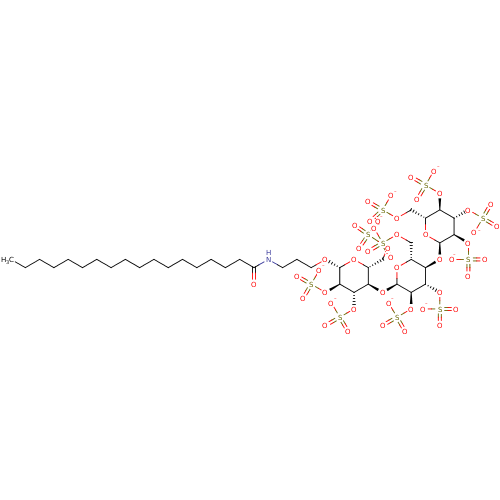
(CHEMBL2059505)Show SMILES CCCCCCCCCCCCCCCCCC(=O)NCCCO[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C39H73NO47S10/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18-27(41)40-19-17-20-72-37-34(85-95(63,64)65)31(82-92(54,55)56)28(24(76-37)21-73-88(42,43)44)79-38-35(86-96(66,67)68)32(83-93(57,58)59)29(25(77-38)22-74-89(45,46)47)80-39-36(87-97(69,70)71)33(84-94(60,61)62)30(81-91(51,52)53)26(78-39)23-75-90(48,49)50/h24-26,28-39H,2-23H2,1H3,(H,40,41)(H,42,43,44)(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)/p-10/t24-,25-,26-,28-,29-,30-,31+,32+,33+,34-,35-,36-,37-,38-,39-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388334
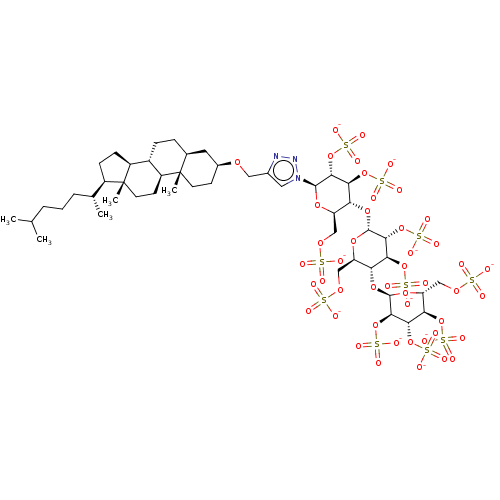
(CHEMBL2059501)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OCc1cn(nn1)[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C48H83N3O46S10/c1-23(2)7-6-8-24(3)29-11-12-30-28-10-9-25-17-27(13-15-47(25,4)31(28)14-16-48(29,30)5)82-19-26-18-51(50-49-26)44-41(95-105(73,74)75)38(92-102(64,65)66)35(32(86-44)20-83-98(52,53)54)89-45-42(96-106(76,77)78)39(93-103(67,68)69)36(33(87-45)21-84-99(55,56)57)90-46-43(97-107(79,80)81)40(94-104(70,71)72)37(91-101(61,62)63)34(88-46)22-85-100(58,59)60/h18,23-25,27-46,101H,6-17,19-22H2,1-5H3,(H,52,53,54)(H,55,56,57)(H,58,59,60)(H2,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)/p-10/t24-,25+,27+,28+,29-,30+,31+,32-,33-,34-,35-,36-,37-,38+,39+,40+,41-,42-,43-,44-,45-,46-,47+,48-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388333
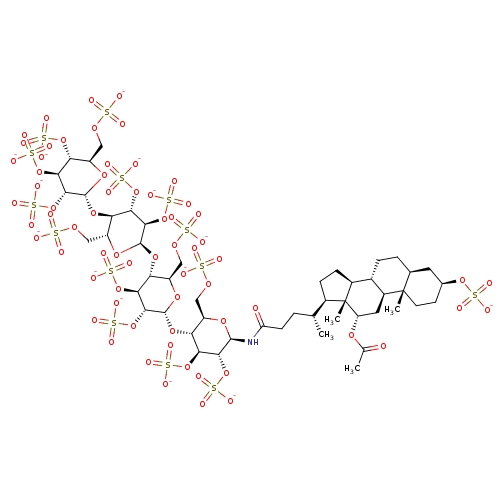
(CHEMBL2059502)Show SMILES C[C@H](CCC(=O)N[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]4OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3C[C@H](OC(C)=O)[C@]12C)OS([O-])(=O)=O |r| Show InChI InChI=1S/C50H83NO66S14/c1-19(24-8-9-25-23-7-6-21-13-22(108-122(66,67)68)11-12-49(21,3)26(23)14-31(50(24,25)4)100-20(2)52)5-10-32(53)51-45-41(114-128(84,85)86)37(110-124(72,73)74)33(27(101-45)15-96-118(54,55)56)105-46-42(115-129(87,88)89)38(111-125(75,76)77)34(28(102-46)16-97-119(57,58)59)106-47-43(116-130(90,91)92)39(112-126(78,79)80)35(29(103-47)17-98-120(60,61)62)107-48-44(117-131(93,94)95)40(113-127(81,82)83)36(109-123(69,70)71)30(104-48)18-99-121(63,64)65/h19,21-31,33-48H,5-18H2,1-4H3,(H,51,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)(H,87,88,89)(H,90,91,92)(H,93,94,95)/p-14/t19-,21+,22+,23+,24-,25+,26+,27-,28-,29-,30-,31+,33-,34-,35-,36-,37+,38+,39+,40+,41-,42-,43-,44-,45-,46-,47-,48-,49+,50-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388344
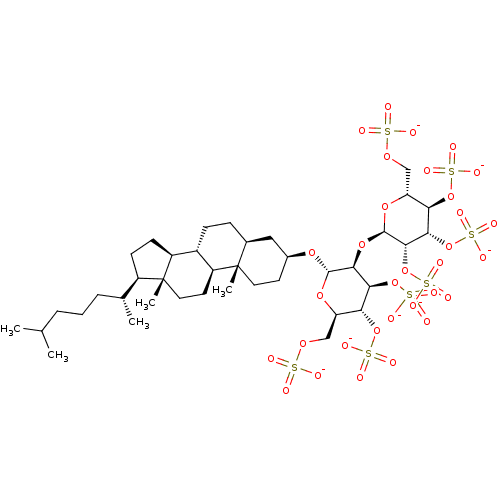
(CHEMBL2059244)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C39H68O32S7/c1-20(2)7-6-8-21(3)25-11-12-26-24-10-9-22-17-23(13-15-38(22,4)27(24)14-16-39(25,26)5)63-36-34(32(69-76(52,53)54)30(67-74(46,47)48)28(64-36)18-61-72(40,41)42)66-37-35(71-78(58,59)60)33(70-77(55,56)57)31(68-75(49,50)51)29(65-37)19-62-73(43,44)45/h20-37H,6-19H2,1-5H3,(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)/p-7/t21-,22+,23+,24+,25-,26+,27+,28-,29-,30-,31-,32+,33+,34+,35+,36+,37-,38+,39-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388340
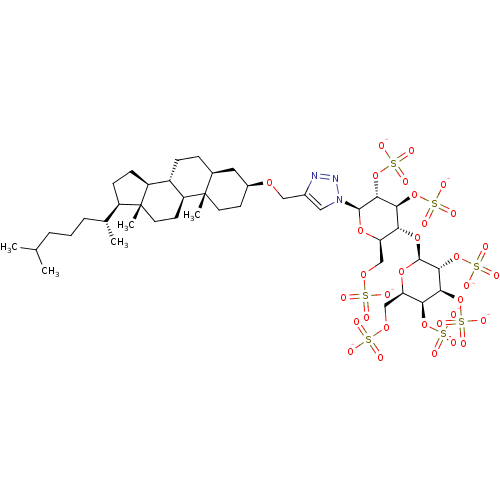
(CHEMBL2059510)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OCc1cn(nn1)[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](O[C@@H]2O[C@H](COS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C42H71N3O32S7/c1-22(2)7-6-8-23(3)28-11-12-29-27-10-9-24-17-26(13-15-41(24,4)30(27)14-16-42(28,29)5)67-19-25-18-45(44-43-25)39-37(76-83(61,62)63)35(74-81(55,56)57)33(31(70-39)20-68-78(46,47)48)72-40-38(77-84(64,65)66)36(75-82(58,59)60)34(73-80(52,53)54)32(71-40)21-69-79(49,50)51/h18,22-24,26-40H,6-17,19-21H2,1-5H3,(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)/p-7/t23-,24+,26+,27+,28-,29+,30+,31-,32-,33-,34+,35+,36+,37-,38-,39-,40+,41+,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388338
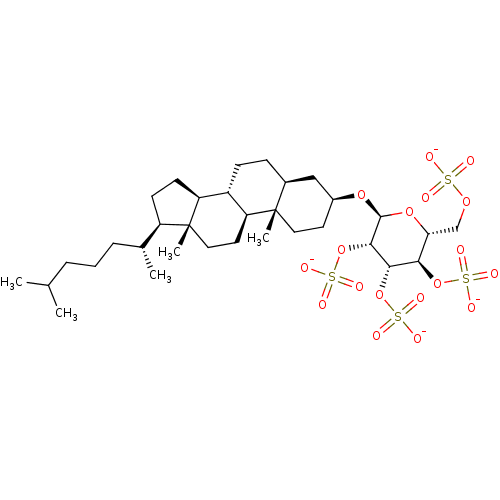
(CHEMBL2059508)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C33H58O18S4/c1-19(2)7-6-8-20(3)24-11-12-25-23-10-9-21-17-22(13-15-32(21,4)26(23)14-16-33(24,25)5)47-31-30(51-55(43,44)45)29(50-54(40,41)42)28(49-53(37,38)39)27(48-31)18-46-52(34,35)36/h19-31H,6-18H2,1-5H3,(H,34,35,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)/p-4/t20-,21+,22+,23+,24-,25+,26+,27-,28-,29+,30+,31+,32+,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay |
J Med Chem 55: 3804-13 (2012)
Article DOI: 10.1021/jm201708h
BindingDB Entry DOI: 10.7270/Q2G161WN |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50375292
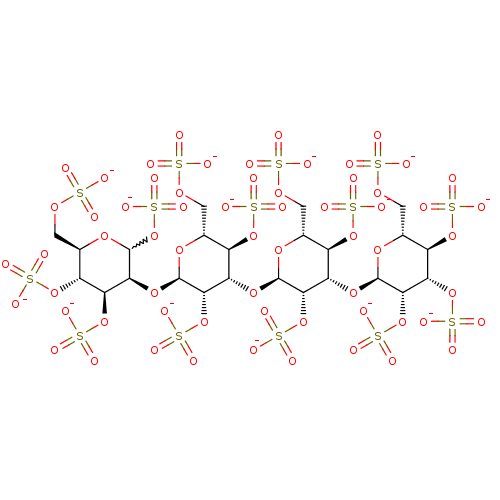
(CHEMBL407200)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](O[C@@H]2C(OS([O-])(=O)=O)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |w:11.11| Show InChI InChI=1S/C24H42O63S14/c25-88(26,27)67-1-5-9(78-92(37,38)39)13(76-23-20(86-100(61,62)63)16(83-97(52,53)54)12(81-95(46,47)48)7(73-23)3-69-90(31,32)33)18(84-98(55,56)57)21(71-5)75-14-10(79-93(40,41)42)6(2-68-89(28,29)30)72-22(19(14)85-99(58,59)60)77-17-15(82-96(49,50)51)11(80-94(43,44)45)8(4-70-91(34,35)36)74-24(17)87-101(64,65)66/h5-24H,1-4H2,(H,25,26,27)(H,28,29,30)(H,31,32,33)(H,34,35,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)/p-14/t5-,6-,7-,8-,9-,10-,11-,12-,13+,14+,15+,16+,17+,18+,19+,20+,21-,22-,23-,24?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human platelet heparanase by competitive binding assay |
Bioorg Med Chem 16: 699-709 (2008)
Article DOI: 10.1016/j.bmc.2007.10.044
BindingDB Entry DOI: 10.7270/Q2SQ918D |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50375290
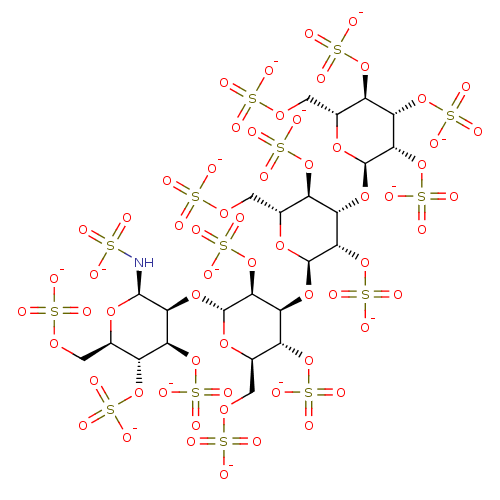
(CHEMBL279625)Show SMILES [O-]S(=O)(=O)N[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O Show InChI InChI=1S/C24H43NO62S14/c26-88(27,28)25-21-17(15(83-97(53,54)55)11(81-95(47,48)49)5(72-21)1-68-89(29,30)31)78-23-19(86-100(62,63)64)14(10(80-94(44,45)46)7(74-23)3-70-91(35,36)37)76-22-18(85-99(59,60)61)13(9(79-93(41,42)43)6(73-22)2-69-90(32,33)34)77-24-20(87-101(65,66)67)16(84-98(56,57)58)12(82-96(50,51)52)8(75-24)4-71-92(38,39)40/h5-25H,1-4H2,(H,26,27,28)(H,29,30,31)(H,32,33,34)(H,35,36,37)(H,38,39,40)(H,41,42,43)(H,44,45,46)(H,47,48,49)(H,50,51,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64)(H,65,66,67)/p-14/t5-,6-,7-,8-,9-,10-,11-,12-,13+,14+,15+,16+,17+,18+,19+,20+,21-,22-,23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human platelet heparanase by competitive binding assay |
Bioorg Med Chem 16: 699-709 (2008)
Article DOI: 10.1016/j.bmc.2007.10.044
BindingDB Entry DOI: 10.7270/Q2SQ918D |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50375291
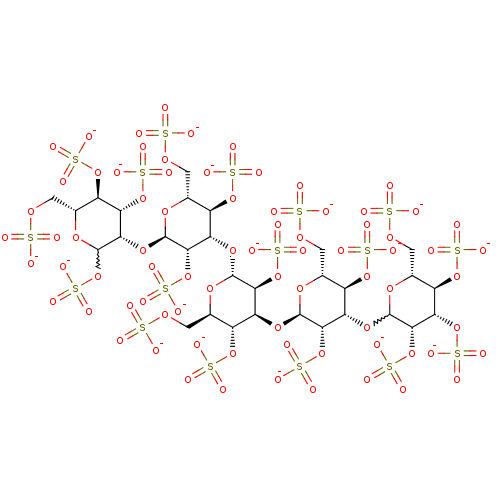
(CHEMBL439118)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](O[C@@H]2C(OS([O-])(=O)=O)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OC4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |w:11.11,79.79| Show InChI InChI=1S/C30H52O77S17/c31-108(32,33)82-1-6-11(96-113(46,47)48)16(92-27-23(104-121(70,71)72)17(12(97-114(49,50)51)7(88-27)2-83-109(34,35)36)94-29-25(106-123(76,77)78)20(102-119(64,65)66)15(100-117(58,59)60)9(90-29)4-85-111(40,41)42)22(103-120(67,68)69)26(87-6)93-18-13(98-115(52,53)54)8(3-84-110(37,38)39)89-28(24(18)105-122(73,74)75)95-21-19(101-118(61,62)63)14(99-116(55,56)57)10(5-86-112(43,44)45)91-30(21)107-124(79,80)81/h6-30H,1-5H2,(H,31,32,33)(H,34,35,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)/p-17/t6-,7-,8-,9-,10-,11-,12-,13-,14-,15-,16+,17+,18+,19+,20+,21+,22+,23+,24+,25+,26-,27-,28-,29?,30?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human platelet heparanase by competitive binding assay |
Bioorg Med Chem 16: 699-709 (2008)
Article DOI: 10.1016/j.bmc.2007.10.044
BindingDB Entry DOI: 10.7270/Q2SQ918D |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50375287
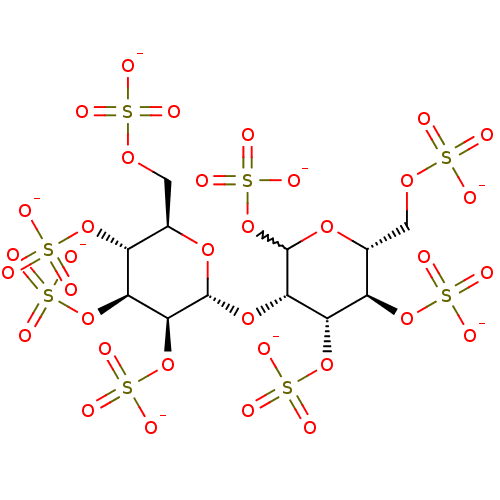
(CHEMBL260220)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](O[C@@H]2C(OS([O-])(=O)=O)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |w:11.11| Show InChI InChI=1S/C12H22O35S8/c13-48(14,15)37-1-3-6(43-51(22,23)24)8(45-53(28,29)30)10(46-54(31,32)33)11(39-3)41-9-7(44-52(25,26)27)5(42-50(19,20)21)4(2-38-49(16,17)18)40-12(9)47-55(34,35)36/h3-12H,1-2H2,(H,13,14,15)(H,16,17,18)(H,19,20,21)(H,22,23,24)(H,25,26,27)(H,28,29,30)(H,31,32,33)(H,34,35,36)/p-8/t3-,4-,5-,6-,7+,8+,9+,10+,11-,12?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human platelet heparanase by competitive binding assay |
Bioorg Med Chem 16: 699-709 (2008)
Article DOI: 10.1016/j.bmc.2007.10.044
BindingDB Entry DOI: 10.7270/Q2SQ918D |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50375287

(CHEMBL260220)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](O[C@@H]2C(OS([O-])(=O)=O)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |w:11.11| Show InChI InChI=1S/C12H22O35S8/c13-48(14,15)37-1-3-6(43-51(22,23)24)8(45-53(28,29)30)10(46-54(31,32)33)11(39-3)41-9-7(44-52(25,26)27)5(42-50(19,20)21)4(2-38-49(16,17)18)40-12(9)47-55(34,35)36/h3-12H,1-2H2,(H,13,14,15)(H,16,17,18)(H,19,20,21)(H,22,23,24)(H,25,26,27)(H,28,29,30)(H,31,32,33)(H,34,35,36)/p-8/t3-,4-,5-,6-,7+,8+,9+,10+,11-,12?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human platelet heparanase by uncompetitive binding assay |
Bioorg Med Chem 16: 699-709 (2008)
Article DOI: 10.1016/j.bmc.2007.10.044
BindingDB Entry DOI: 10.7270/Q2SQ918D |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50375289

(CHEMBL258980)Show SMILES CO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O Show InChI InChI=1S/C13H24O32S7/c1-35-12-10(44-51(29,30)31)8(6(41-48(20,21)22)4(38-12)2-36-46(14,15)16)40-13-11(45-52(32,33)34)9(43-50(26,27)28)7(42-49(23,24)25)5(39-13)3-37-47(17,18)19/h4-13H,2-3H2,1H3,(H,14,15,16)(H,17,18,19)(H,20,21,22)(H,23,24,25)(H,26,27,28)(H,29,30,31)(H,32,33,34)/p-7/t4-,5-,6-,7-,8+,9+,10+,11+,12+,13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human platelet heparanase |
Bioorg Med Chem 16: 699-709 (2008)
Article DOI: 10.1016/j.bmc.2007.10.044
BindingDB Entry DOI: 10.7270/Q2SQ918D |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50375292

(CHEMBL407200)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](O[C@@H]2C(OS([O-])(=O)=O)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |w:11.11| Show InChI InChI=1S/C24H42O63S14/c25-88(26,27)67-1-5-9(78-92(37,38)39)13(76-23-20(86-100(61,62)63)16(83-97(52,53)54)12(81-95(46,47)48)7(73-23)3-69-90(31,32)33)18(84-98(55,56)57)21(71-5)75-14-10(79-93(40,41)42)6(2-68-89(28,29)30)72-22(19(14)85-99(58,59)60)77-17-15(82-96(49,50)51)11(80-94(43,44)45)8(4-70-91(34,35)36)74-24(17)87-101(64,65)66/h5-24H,1-4H2,(H,25,26,27)(H,28,29,30)(H,31,32,33)(H,34,35,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)/p-14/t5-,6-,7-,8-,9-,10-,11-,12-,13+,14+,15+,16+,17+,18+,19+,20+,21-,22-,23-,24?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human platelet heparanase |
Bioorg Med Chem 16: 699-709 (2008)
Article DOI: 10.1016/j.bmc.2007.10.044
BindingDB Entry DOI: 10.7270/Q2SQ918D |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50375290

(CHEMBL279625)Show SMILES [O-]S(=O)(=O)N[C@@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O Show InChI InChI=1S/C24H43NO62S14/c26-88(27,28)25-21-17(15(83-97(53,54)55)11(81-95(47,48)49)5(72-21)1-68-89(29,30)31)78-23-19(86-100(62,63)64)14(10(80-94(44,45)46)7(74-23)3-70-91(35,36)37)76-22-18(85-99(59,60)61)13(9(79-93(41,42)43)6(73-22)2-69-90(32,33)34)77-24-20(87-101(65,66)67)16(84-98(56,57)58)12(82-96(50,51)52)8(75-24)4-71-92(38,39)40/h5-25H,1-4H2,(H,26,27,28)(H,29,30,31)(H,32,33,34)(H,35,36,37)(H,38,39,40)(H,41,42,43)(H,44,45,46)(H,47,48,49)(H,50,51,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64)(H,65,66,67)/p-14/t5-,6-,7-,8-,9-,10-,11-,12-,13+,14+,15+,16+,17+,18+,19+,20+,21-,22-,23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human platelet heparanase |
Bioorg Med Chem 16: 699-709 (2008)
Article DOI: 10.1016/j.bmc.2007.10.044
BindingDB Entry DOI: 10.7270/Q2SQ918D |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50375291

(CHEMBL439118)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](O[C@@H]2C(OS([O-])(=O)=O)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OC4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |w:11.11,79.79| Show InChI InChI=1S/C30H52O77S17/c31-108(32,33)82-1-6-11(96-113(46,47)48)16(92-27-23(104-121(70,71)72)17(12(97-114(49,50)51)7(88-27)2-83-109(34,35)36)94-29-25(106-123(76,77)78)20(102-119(64,65)66)15(100-117(58,59)60)9(90-29)4-85-111(40,41)42)22(103-120(67,68)69)26(87-6)93-18-13(98-115(52,53)54)8(3-84-110(37,38)39)89-28(24(18)105-122(73,74)75)95-21-19(101-118(61,62)63)14(99-116(55,56)57)10(5-86-112(43,44)45)91-30(21)107-124(79,80)81/h6-30H,1-5H2,(H,31,32,33)(H,34,35,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)/p-17/t6-,7-,8-,9-,10-,11-,12-,13-,14-,15-,16+,17+,18+,19+,20+,21+,22+,23+,24+,25+,26-,27-,28-,29?,30?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human platelet heparanase |
Bioorg Med Chem 16: 699-709 (2008)
Article DOI: 10.1016/j.bmc.2007.10.044
BindingDB Entry DOI: 10.7270/Q2SQ918D |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50375287

(CHEMBL260220)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](O[C@@H]2C(OS([O-])(=O)=O)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |w:11.11| Show InChI InChI=1S/C12H22O35S8/c13-48(14,15)37-1-3-6(43-51(22,23)24)8(45-53(28,29)30)10(46-54(31,32)33)11(39-3)41-9-7(44-52(25,26)27)5(42-50(19,20)21)4(2-38-49(16,17)18)40-12(9)47-55(34,35)36/h3-12H,1-2H2,(H,13,14,15)(H,16,17,18)(H,19,20,21)(H,22,23,24)(H,25,26,27)(H,28,29,30)(H,31,32,33)(H,34,35,36)/p-8/t3-,4-,5-,6-,7+,8+,9+,10+,11-,12?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human platelet heparanase |
Bioorg Med Chem 16: 699-709 (2008)
Article DOI: 10.1016/j.bmc.2007.10.044
BindingDB Entry DOI: 10.7270/Q2SQ918D |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50375293
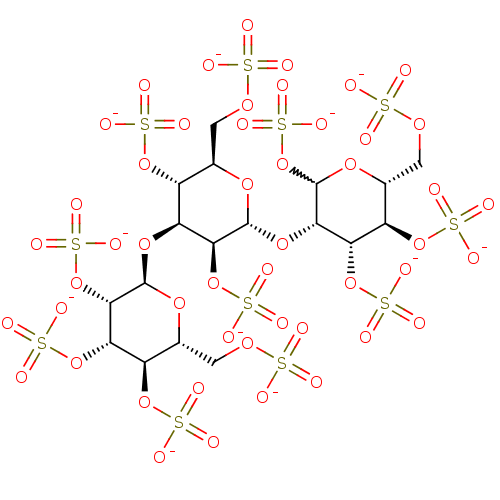
(CHEMBL1627086)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](O[C@@H]2C(OS([O-])(=O)=O)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |w:11.11| Show InChI InChI=1S/C18H32O49S11/c19-68(20,21)52-1-4-7(60-71(28,29)30)10(58-17-15(66-77(46,47)48)12(64-75(40,41)42)9(62-73(34,35)36)5(56-17)2-53-69(22,23)24)14(65-76(43,44)45)16(55-4)59-13-11(63-74(37,38)39)8(61-72(31,32)33)6(3-54-70(25,26)27)57-18(13)67-78(49,50)51/h4-18H,1-3H2,(H,19,20,21)(H,22,23,24)(H,25,26,27)(H,28,29,30)(H,31,32,33)(H,34,35,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)/p-11/t4-,5-,6-,7-,8-,9-,10+,11+,12+,13+,14+,15+,16-,17-,18?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human platelet heparanase |
Bioorg Med Chem 16: 699-709 (2008)
Article DOI: 10.1016/j.bmc.2007.10.044
BindingDB Entry DOI: 10.7270/Q2SQ918D |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50375288
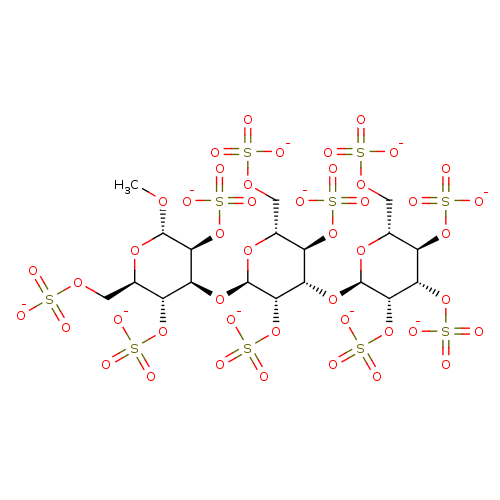
(CHEMBL258894)Show SMILES CO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O Show InChI InChI=1S/C19H34O46S10/c1-50-17-14(63-73(41,42)43)11(8(59-69(29,30)31)5(54-17)2-51-66(20,21)22)57-18-15(64-74(44,45)46)12(9(60-70(32,33)34)6(55-18)3-52-67(23,24)25)58-19-16(65-75(47,48)49)13(62-72(38,39)40)10(61-71(35,36)37)7(56-19)4-53-68(26,27)28/h5-19H,2-4H2,1H3,(H,20,21,22)(H,23,24,25)(H,26,27,28)(H,29,30,31)(H,32,33,34)(H,35,36,37)(H,38,39,40)(H,41,42,43)(H,44,45,46)(H,47,48,49)/p-10/t5-,6-,7-,8-,9-,10-,11+,12+,13+,14+,15+,16+,17+,18-,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human platelet heparanase |
Bioorg Med Chem 16: 699-709 (2008)
Article DOI: 10.1016/j.bmc.2007.10.044
BindingDB Entry DOI: 10.7270/Q2SQ918D |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50378647

(CHEMBL1627122 | PI-88)Show SMILES [O-]P([O-])(=O)OC[C@H]1O[C@H](O[C@H]2[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@H]3[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@H]4[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@@H]5[C@@H](OS([O-])(=O)=O)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@H]4OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C30H53O77PS16/c31-108(32,33)82-1-6-14(99-116(55,56)57)20(102-119(64,65)66)25(106-123(76,77)78)29(87-6)94-17-12(97-114(49,50)51)8(3-84-110(37,38)39)89-27(23(17)104-121(70,71)72)92-16-11(96-113(46,47)48)7(2-83-109(34,35)36)88-26(22(16)103-120(67,68)69)93-18-13(98-115(52,53)54)9(4-85-111(40,41)42)90-28(24(18)105-122(73,74)75)95-21-19(101-118(61,62)63)15(100-117(58,59)60)10(5-86-112(43,44)45)91-30(21)107-124(79,80)81/h6-30H,1-5H2,(H2,31,32,33)(H,34,35,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)/p-18/t6-,7-,8-,9-,10-,11-,12-,13-,14-,15-,16+,17+,18+,19+,20+,21+,22+,23+,24+,25+,26-,27-,28-,29-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307398
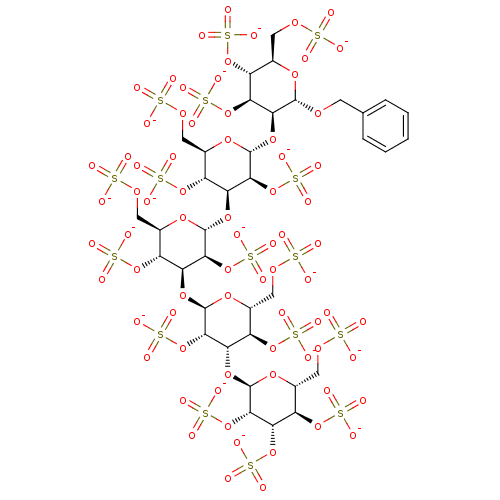
(CHEMBL603122 | Hexadecasodium [2-(benzyloxy)-3-({4...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCc2ccccc2)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]5O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C37H58O74S16/c38-112(39,40)87-7-13-18(101-117(53,54)55)23(97-35-30(109-125(77,78)79)24(19(102-118(56,57)58)14(94-35)8-88-113(41,42)43)99-37-32(111-127(83,84)85)27(107-123(71,72)73)22(105-121(65,66)67)17(96-37)11-91-116(50,51)52)29(108-124(74,75)76)34(93-13)98-25-20(103-119(59,60)61)15(9-89-114(44,45)46)95-36(31(25)110-126(80,81)82)100-28-26(106-122(68,69)70)21(104-120(62,63)64)16(10-90-115(47,48)49)92-33(28)86-6-12-4-2-1-3-5-12/h1-5,13-37H,6-11H2,(H,38,39,40)(H,41,42,43)(H,44,45,46)(H,47,48,49)(H,50,51,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64)(H,65,66,67)(H,68,69,70)(H,71,72,73)(H,74,75,76)(H,77,78,79)(H,80,81,82)(H,83,84,85)/p-16/t13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23+,24+,25+,26+,27+,28+,29+,30+,31+,32+,33+,34-,35-,36-,37-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307399
(Benzyl 2,3,4,6-Tetra-O-sulfo-alpha-D-mannopyranosy...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCc2ccccc2)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C31H48O60S13/c32-92(33,34)72-7-12-16(83-96(44,45)46)20(81-31-27(91-104(68,69)70)23(88-101(59,60)61)19(86-99(53,54)55)15(79-31)10-75-95(41,42)43)25(89-102(62,63)64)29(77-12)80-21-17(84-97(47,48)49)13(8-73-93(35,36)37)78-30(26(21)90-103(65,66)67)82-24-22(87-100(56,57)58)18(85-98(50,51)52)14(9-74-94(38,39)40)76-28(24)71-6-11-4-2-1-3-5-11/h1-5,12-31H,6-10H2,(H,32,33,34)(H,35,36,37)(H,38,39,40)(H,41,42,43)(H,44,45,46)(H,47,48,49)(H,50,51,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64)(H,65,66,67)(H,68,69,70)/p-13/t12-,13-,14-,15-,16-,17-,18-,19-,20+,21+,22+,23+,24+,25+,26+,27+,28+,29-,30-,31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307400

(CHEMBL604366 | hexadecasodium [3-({4-[(4-{[3,5-bis...)Show SMILES CCCCCCCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C38H68O74S16/c1-2-3-4-5-6-7-8-87-34-29(27(107-123(69,70)71)22(105-121(63,64)65)17(93-34)12-91-116(48,49)50)101-37-32(111-127(81,82)83)26(21(104-120(60,61)62)16(96-37)11-90-115(45,46)47)99-35-30(109-125(75,76)77)24(19(102-118(54,55)56)14(94-35)9-88-113(39,40)41)98-36-31(110-126(78,79)80)25(20(103-119(57,58)59)15(95-36)10-89-114(42,43)44)100-38-33(112-128(84,85)86)28(108-124(72,73)74)23(106-122(66,67)68)18(97-38)13-92-117(51,52)53/h14-38H,2-13H2,1H3,(H,39,40,41)(H,42,43,44)(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)/p-16/t14-,15-,16-,17-,18-,19-,20-,21-,22-,23-,24+,25+,26+,27+,28+,29+,30+,31+,32+,33+,34+,35-,36-,37-,38-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307401
(CHEMBL602113 | Octyl 2,3,4,6-Tetra-O-sulfo-alpha-D...)Show SMILES CCCCCCCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C32H58O60S13/c1-2-3-4-5-6-7-8-72-29-25(23(88-101(57,58)59)19(86-99(51,52)53)15(77-29)11-75-95(39,40)41)83-31-27(91-104(66,67)68)22(18(85-98(48,49)50)14(79-31)10-74-94(36,37)38)81-30-26(90-103(63,64)65)21(17(84-97(45,46)47)13(78-30)9-73-93(33,34)35)82-32-28(92-105(69,70)71)24(89-102(60,61)62)20(87-100(54,55)56)16(80-32)12-76-96(42,43)44/h13-32H,2-12H2,1H3,(H,33,34,35)(H,36,37,38)(H,39,40,41)(H,42,43,44)(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)/p-13/t13-,14-,15-,16-,17-,18-,19-,20-,21+,22+,23+,24+,25+,26+,27+,28+,29+,30-,31-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307402
(4-Butylbenzyl 2,3,4,6-Tetra-O-sulfo-alpha-D-mannop...)Show SMILES CCCCc1ccc(CO[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]2O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)cc1 |r| Show InChI InChI=1S/C35H56O60S13/c1-2-3-4-14-5-7-15(8-6-14)9-75-32-28(26(91-104(60,61)62)22(89-102(54,55)56)18(80-32)12-78-98(42,43)44)86-34-30(94-107(69,70)71)25(21(88-101(51,52)53)17(82-34)11-77-97(39,40)41)84-33-29(93-106(66,67)68)24(20(87-100(48,49)50)16(81-33)10-76-96(36,37)38)85-35-31(95-108(72,73)74)27(92-105(63,64)65)23(90-103(57,58)59)19(83-35)13-79-99(45,46)47/h5-8,16-35H,2-4,9-13H2,1H3,(H,36,37,38)(H,39,40,41)(H,42,43,44)(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)/p-13/t16-,17-,18-,19-,20-,21-,22-,23-,24+,25+,26+,27+,28+,29+,30+,31+,32+,33-,34-,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307386

(CHEMBL604367 | Dodecyl 2,3,4,6-Tetra-O-sulfo-alpha...)Show SMILES CCCCCCCCCCCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C42H76O74S16/c1-2-3-4-5-6-7-8-9-10-11-12-91-38-33(31(111-127(73,74)75)26(109-125(67,68)69)21(97-38)16-95-120(52,53)54)105-41-36(115-131(85,86)87)30(25(108-124(64,65)66)20(100-41)15-94-119(49,50)51)103-39-34(113-129(79,80)81)28(23(106-122(58,59)60)18(98-39)13-92-117(43,44)45)102-40-35(114-130(82,83)84)29(24(107-123(61,62)63)19(99-40)14-93-118(46,47)48)104-42-37(116-132(88,89)90)32(112-128(76,77)78)27(110-126(70,71)72)22(101-42)17-96-121(55,56)57/h18-42H,2-17H2,1H3,(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)(H,85,86,87)(H,88,89,90)/p-16/t18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28+,29+,30+,31+,32+,33+,34+,35+,36+,37+,38+,39-,40-,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307387
(8-(4-Phenyl[1,2,3]triazol-1-yl)octyl 2,3,4,6-Tetra...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](O[C@@H]2[C@@H](OCCCCCCCCn3cc(nn3)-c3ccccc3)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C46H73N3O74S16/c50-124(51,52)99-15-22-27(113-129(65,66)67)32(109-44-39(121-137(89,90)91)33(28(114-130(68,69)70)23(106-44)16-100-125(53,54)55)111-46-41(123-139(95,96)97)36(119-135(83,84)85)31(117-133(77,78)79)26(108-46)19-103-128(62,63)64)38(120-136(86,87)88)43(105-22)110-34-29(115-131(71,72)73)24(17-101-126(56,57)58)107-45(40(34)122-138(92,93)94)112-37-35(118-134(80,81)82)30(116-132(74,75)76)25(18-102-127(59,60)61)104-42(37)98-13-9-4-2-1-3-8-12-49-14-21(47-48-49)20-10-6-5-7-11-20/h5-7,10-11,14,22-46H,1-4,8-9,12-13,15-19H2,(H,50,51,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64)(H,65,66,67)(H,68,69,70)(H,71,72,73)(H,74,75,76)(H,77,78,79)(H,80,81,82)(H,83,84,85)(H,86,87,88)(H,89,90,91)(H,92,93,94)(H,95,96,97)/p-16/t22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32+,33+,34+,35+,36+,37+,38+,39+,40+,41+,42+,43-,44-,45-,46-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307388
(8-(4-Phenyl[1,2,3]triazol-1-yl)octyl 2,3,4,6-Tetra...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](O[C@@H]2[C@@H](OCCCCCCCCn3cc(nn3)-c3ccccc3)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C40H63N3O60S13/c44-104(45,46)84-15-21-25(95-108(56,57)58)29(93-40-36(103-116(80,81)82)32(100-113(71,72)73)28(98-111(65,66)67)24(91-40)18-87-107(53,54)55)34(101-114(74,75)76)38(89-21)92-30-26(96-109(59,60)61)22(16-85-105(47,48)49)90-39(35(30)102-115(77,78)79)94-33-31(99-112(68,69)70)27(97-110(62,63)64)23(17-86-106(50,51)52)88-37(33)83-13-9-4-2-1-3-8-12-43-14-20(41-42-43)19-10-6-5-7-11-19/h5-7,10-11,14,21-40H,1-4,8-9,12-13,15-18H2,(H,44,45,46)(H,47,48,49)(H,50,51,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64)(H,65,66,67)(H,68,69,70)(H,71,72,73)(H,74,75,76)(H,77,78,79)(H,80,81,82)/p-13/t21-,22-,23-,24-,25-,26-,27-,28-,29+,30+,31+,32+,33+,34+,35+,36+,37+,38-,39-,40-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307389
(2,3,4,6-Tetra-O-sulfo-alpha-D-mannopyranosyl-(1->3...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCCCCCCCCn2cc(nn2)-c2cccc3ccccc23)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]5O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C50H75N3O74S16/c54-128(55,56)103-17-26-31(117-133(69,70)71)36(113-48-43(125-141(93,94)95)37(32(118-134(72,73)74)27(110-48)18-104-129(57,58)59)115-50-45(127-143(99,100)101)40(123-139(87,88)89)35(121-137(81,82)83)30(112-50)21-107-132(66,67)68)42(124-140(90,91)92)47(109-26)114-38-33(119-135(75,76)77)28(19-105-130(60,61)62)111-49(44(38)126-142(96,97)98)116-41-39(122-138(84,85)86)34(120-136(78,79)80)29(20-106-131(63,64)65)108-46(41)102-15-8-4-2-1-3-7-14-53-16-25(51-52-53)24-13-9-11-22-10-5-6-12-23(22)24/h5-6,9-13,16,26-50H,1-4,7-8,14-15,17-21H2,(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)(H,87,88,89)(H,90,91,92)(H,93,94,95)(H,96,97,98)(H,99,100,101)/p-16/t26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36+,37+,38+,39+,40+,41+,42+,43+,44+,45+,46+,47-,48-,49-,50-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307390
(8-(4-Naphthalen-1-yl[1,2,3]triazol-1-yl)octyl 2,3,...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCCCCCCCCn2cc(nn2)-c2cccc3ccccc23)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C44H65N3O60S13/c48-108(49,50)88-17-25-29(99-112(60,61)62)33(97-44-40(107-120(84,85)86)36(104-117(75,76)77)32(102-115(69,70)71)28(95-44)20-91-111(57,58)59)38(105-118(78,79)80)42(93-25)96-34-30(100-113(63,64)65)26(18-89-109(51,52)53)94-43(39(34)106-119(81,82)83)98-37-35(103-116(72,73)74)31(101-114(66,67)68)27(19-90-110(54,55)56)92-41(37)87-15-8-4-2-1-3-7-14-47-16-24(45-46-47)23-13-9-11-21-10-5-6-12-22(21)23/h5-6,9-13,16,25-44H,1-4,7-8,14-15,17-20H2,(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)/p-13/t25-,26-,27-,28-,29-,30-,31-,32-,33+,34+,35+,36+,37+,38+,39+,40+,41+,42-,43-,44-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307391
(8-(5-Dimethylaminonaphthalene-1-sulfonamido)octyl ...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)NCCCCCCCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C44H70N2O62S14/c1-46(2)23-13-9-12-22-21(23)11-10-14-28(22)109(47,48)45-15-7-5-3-4-6-8-16-88-41-37(35(104-118(73,74)75)31(102-116(67,68)69)26(93-41)19-91-112(55,56)57)99-43-39(107-121(82,83)84)34(30(101-115(64,65)66)25(95-43)18-90-111(52,53)54)97-42-38(106-120(79,80)81)33(29(100-114(61,62)63)24(94-42)17-89-110(49,50)51)98-44-40(108-122(85,86)87)36(105-119(76,77)78)32(103-117(70,71)72)27(96-44)20-92-113(58,59)60/h9-14,24-27,29-45H,3-8,15-20H2,1-2H3,(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)(H,85,86,87)/p-13/t24-,25-,26-,27-,29-,30-,31-,32-,33+,34+,35+,36+,37+,38+,39+,40+,41+,42-,43-,44-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307392
(3-(4-Phenyl[1,2,3]triazol-1-yl)propyl 2,3,4,6-Tetr...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCCCn2cc(nn2)-c2ccccc2)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]5O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C41H63N3O74S16/c45-119(46,47)94-10-17-22(108-124(60,61)62)27(104-39-34(116-132(84,85)86)28(23(109-125(63,64)65)18(101-39)11-95-120(48,49)50)106-41-36(118-134(90,91)92)31(114-130(78,79)80)26(112-128(72,73)74)21(103-41)14-98-123(57,58)59)33(115-131(81,82)83)38(100-17)105-29-24(110-126(66,67)68)19(12-96-121(51,52)53)102-40(35(29)117-133(87,88)89)107-32-30(113-129(75,76)77)25(111-127(69,70)71)20(13-97-122(54,55)56)99-37(32)93-8-4-7-44-9-16(42-43-44)15-5-2-1-3-6-15/h1-3,5-6,9,17-41H,4,7-8,10-14H2,(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)(H,87,88,89)(H,90,91,92)/p-16/t17-,18-,19-,20-,21-,22-,23-,24-,25-,26-,27+,28+,29+,30+,31+,32+,33+,34+,35+,36+,37+,38-,39-,40-,41-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307393
(3-(4-Phenyl[1,2,3]triazol-1-yl)propyl 2,3,4,6-Tetr...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCCCn2cc(nn2)-c2ccccc2)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C35H53N3O60S13/c39-99(40,41)79-10-16-20(90-103(51,52)53)24(88-35-31(98-111(75,76)77)27(95-108(66,67)68)23(93-106(60,61)62)19(86-35)13-82-102(48,49)50)29(96-109(69,70)71)33(84-16)87-25-21(91-104(54,55)56)17(11-80-100(42,43)44)85-34(30(25)97-110(72,73)74)89-28-26(94-107(63,64)65)22(92-105(57,58)59)18(12-81-101(45,46)47)83-32(28)78-8-4-7-38-9-15(36-37-38)14-5-2-1-3-6-14/h1-3,5-6,9,16-35H,4,7-8,10-13H2,(H,39,40,41)(H,42,43,44)(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)/p-13/t16-,17-,18-,19-,20-,21-,22-,23-,24+,25+,26+,27+,28+,29+,30+,31+,32+,33-,34-,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307395

(12-(4-Phenyl[1,2,3]triazol-1-yl)dodecyl 2,3,4,6-Te...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](O[C@@H]2[C@@H](OCCCCCCCCCCCCn3cc(nn3)-c3ccccc3)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C50H81N3O74S16/c54-128(55,56)103-19-26-31(117-133(69,70)71)36(113-48-43(125-141(93,94)95)37(32(118-134(72,73)74)27(110-48)20-104-129(57,58)59)115-50-45(127-143(99,100)101)40(123-139(87,88)89)35(121-137(81,82)83)30(112-50)23-107-132(66,67)68)42(124-140(90,91)92)47(109-26)114-38-33(119-135(75,76)77)28(21-105-130(60,61)62)111-49(44(38)126-142(96,97)98)116-41-39(122-138(84,85)86)34(120-136(78,79)80)29(22-106-131(63,64)65)108-46(41)102-17-13-8-6-4-2-1-3-5-7-12-16-53-18-25(51-52-53)24-14-10-9-11-15-24/h9-11,14-15,18,26-50H,1-8,12-13,16-17,19-23H2,(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)(H,87,88,89)(H,90,91,92)(H,93,94,95)(H,96,97,98)(H,99,100,101)/p-16/t26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36+,37+,38+,39+,40+,41+,42+,43+,44+,45+,46+,47-,48-,49-,50-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307396
(12-(4-Naphthalen-1-yl[1,2,3]triazol-1-yl)dodecyl 2...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCCCCCCCCCCCCn2cc(nn2)-c2cccc3ccccc23)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]5O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C54H83N3O74S16/c58-132(59,60)107-21-30-35(121-137(73,74)75)40(117-52-47(129-145(97,98)99)41(36(122-138(76,77)78)31(114-52)22-108-133(61,62)63)119-54-49(131-147(103,104)105)44(127-143(91,92)93)39(125-141(85,86)87)34(116-54)25-111-136(70,71)72)46(128-144(94,95)96)51(113-30)118-42-37(123-139(79,80)81)32(23-109-134(64,65)66)115-53(48(42)130-146(100,101)102)120-45-43(126-142(88,89)90)38(124-140(82,83)84)33(24-110-135(67,68)69)112-50(45)106-19-12-8-6-4-2-1-3-5-7-11-18-57-20-29(55-56-57)28-17-13-15-26-14-9-10-16-27(26)28/h9-10,13-17,20,30-54H,1-8,11-12,18-19,21-25H2,(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)(H,85,86,87)(H,88,89,90)(H,91,92,93)(H,94,95,96)(H,97,98,99)(H,100,101,102)(H,103,104,105)/p-16/t30-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40+,41+,42+,43+,44+,45+,46+,47+,48+,49+,50+,51-,52-,53-,54-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50378647

(CHEMBL1627122 | PI-88)Show SMILES [O-]P([O-])(=O)OC[C@H]1O[C@H](O[C@H]2[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@H]3[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@H]4[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@@H]5[C@@H](OS([O-])(=O)=O)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@H]4OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C30H53O77PS16/c31-108(32,33)82-1-6-14(99-116(55,56)57)20(102-119(64,65)66)25(106-123(76,77)78)29(87-6)94-17-12(97-114(49,50)51)8(3-84-110(37,38)39)89-27(23(17)104-121(70,71)72)92-16-11(96-113(46,47)48)7(2-83-109(34,35)36)88-26(22(16)103-120(67,68)69)93-18-13(98-115(52,53)54)9(4-85-111(40,41)42)90-28(24(18)105-122(73,74)75)95-21-19(101-118(61,62)63)15(100-117(58,59)60)10(5-86-112(43,44)45)91-30(21)107-124(79,80)81/h6-30H,1-5H2,(H2,31,32,33)(H,34,35,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)/p-18/t6-,7-,8-,9-,10-,11-,12-,13-,14-,15-,16+,17+,18+,19+,20+,21+,22+,23+,24+,25+,26-,27-,28-,29-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon-based solution affinity assay |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50307398

(CHEMBL603122 | Hexadecasodium [2-(benzyloxy)-3-({4...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCc2ccccc2)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]5O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C37H58O74S16/c38-112(39,40)87-7-13-18(101-117(53,54)55)23(97-35-30(109-125(77,78)79)24(19(102-118(56,57)58)14(94-35)8-88-113(41,42)43)99-37-32(111-127(83,84)85)27(107-123(71,72)73)22(105-121(65,66)67)17(96-37)11-91-116(50,51)52)29(108-124(74,75)76)34(93-13)98-25-20(103-119(59,60)61)15(9-89-114(44,45)46)95-36(31(25)110-126(80,81)82)100-28-26(106-122(68,69)70)21(104-120(62,63)64)16(10-90-115(47,48)49)92-33(28)86-6-12-4-2-1-3-5-12/h1-5,13-37H,6-11H2,(H,38,39,40)(H,41,42,43)(H,44,45,46)(H,47,48,49)(H,50,51,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64)(H,65,66,67)(H,68,69,70)(H,71,72,73)(H,74,75,76)(H,77,78,79)(H,80,81,82)(H,83,84,85)/p-16/t13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23+,24+,25+,26+,27+,28+,29+,30+,31+,32+,33+,34-,35-,36-,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon-based solution affinity assay |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50307399

(Benzyl 2,3,4,6-Tetra-O-sulfo-alpha-D-mannopyranosy...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCc2ccccc2)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C31H48O60S13/c32-92(33,34)72-7-12-16(83-96(44,45)46)20(81-31-27(91-104(68,69)70)23(88-101(59,60)61)19(86-99(53,54)55)15(79-31)10-75-95(41,42)43)25(89-102(62,63)64)29(77-12)80-21-17(84-97(47,48)49)13(8-73-93(35,36)37)78-30(26(21)90-103(65,66)67)82-24-22(87-100(56,57)58)18(85-98(50,51)52)14(9-74-94(38,39)40)76-28(24)71-6-11-4-2-1-3-5-11/h1-5,12-31H,6-10H2,(H,32,33,34)(H,35,36,37)(H,38,39,40)(H,41,42,43)(H,44,45,46)(H,47,48,49)(H,50,51,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64)(H,65,66,67)(H,68,69,70)/p-13/t12-,13-,14-,15-,16-,17-,18-,19-,20+,21+,22+,23+,24+,25+,26+,27+,28+,29-,30-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon-based solution affinity assay |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor 1
(Homo sapiens (Human)) | BDBM50307400

(CHEMBL604366 | hexadecasodium [3-({4-[(4-{[3,5-bis...)Show SMILES CCCCCCCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C38H68O74S16/c1-2-3-4-5-6-7-8-87-34-29(27(107-123(69,70)71)22(105-121(63,64)65)17(93-34)12-91-116(48,49)50)101-37-32(111-127(81,82)83)26(21(104-120(60,61)62)16(96-37)11-90-115(45,46)47)99-35-30(109-125(75,76)77)24(19(102-118(54,55)56)14(94-35)9-88-113(39,40)41)98-36-31(110-126(78,79)80)25(20(103-119(57,58)59)15(95-36)10-89-114(42,43)44)100-38-33(112-128(84,85)86)28(108-124(72,73)74)23(106-122(66,67)68)18(97-38)13-92-117(51,52)53/h14-38H,2-13H2,1H3,(H,39,40,41)(H,42,43,44)(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)/p-16/t14-,15-,16-,17-,18-,19-,20-,21-,22-,23-,24+,25+,26+,27+,28+,29+,30+,31+,32+,33+,34+,35-,36-,37-,38-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Binding affinity to FGF1 by surface plasmon-based solution affinity assay |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data