

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Translocator protein (Homo sapiens (Human)) | BDBM50159089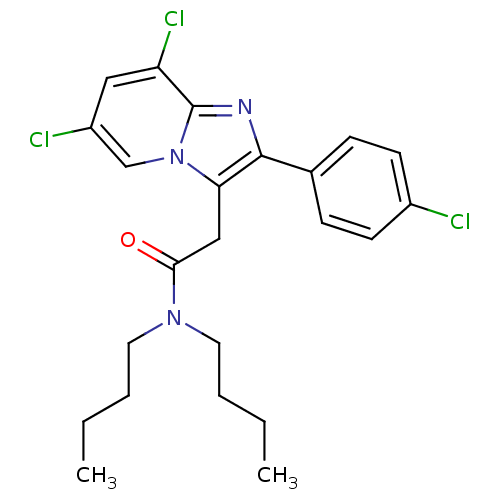 (CHEMBL180523 | N,N-Dibutyl-2-[6,8-dichloro-2-(4-ch...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of TSPO (unknown origin) | Eur J Med Chem 125: 1172-1192 (2017) Article DOI: 10.1016/j.ejmech.2016.11.017 BindingDB Entry DOI: 10.7270/Q25Q4Z83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Homo sapiens (Human)) | BDBM50045877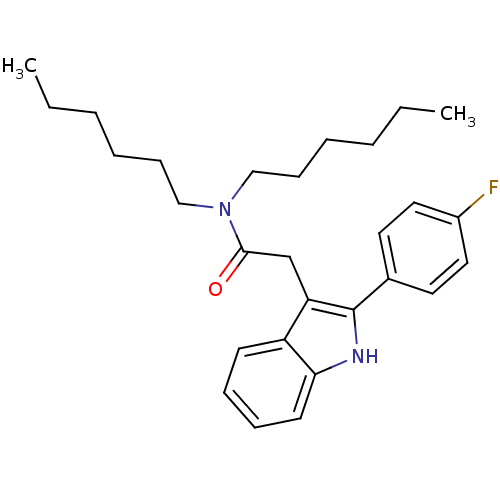 (2-(2-(4-fluorophenyl)-1H-indol-3-yl)-N,N-dihexylac...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of TSPO (unknown origin) | Eur J Med Chem 125: 1172-1192 (2017) Article DOI: 10.1016/j.ejmech.2016.11.017 BindingDB Entry DOI: 10.7270/Q25Q4Z83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM22032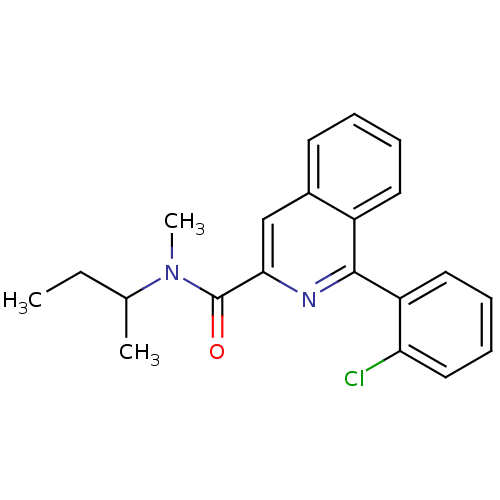 (1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoq...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from TSPO in Sprague-Dawley rat cerebral cortex membranes after 60 mins by microbeta liquid scintillation counting method | Eur J Med Chem 125: 1172-1192 (2017) Article DOI: 10.1016/j.ejmech.2016.11.017 BindingDB Entry DOI: 10.7270/Q25Q4Z83 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50210940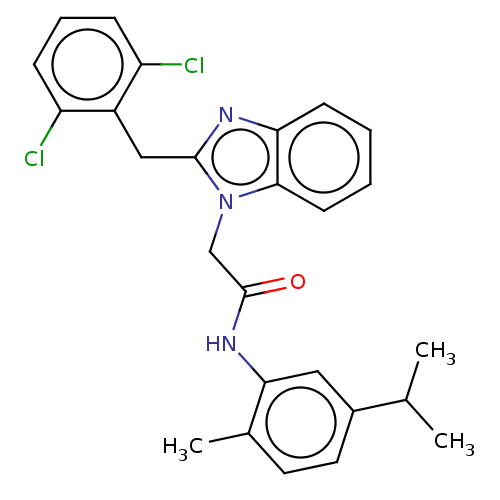 (CHEMBL3948914) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from TSPO in Sprague-Dawley rat cerebral cortex membranes after 60 mins by microbeta liquid scintillation counting method | Eur J Med Chem 125: 1172-1192 (2017) Article DOI: 10.1016/j.ejmech.2016.11.017 BindingDB Entry DOI: 10.7270/Q25Q4Z83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Rattus norvegicus) | BDBM50241203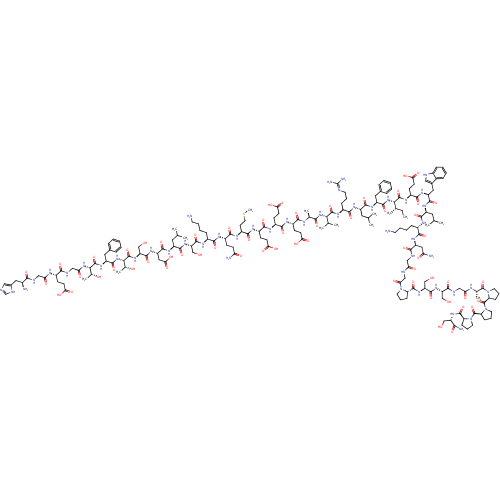 (CHEMBL414357 | HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
SungKyunKwan University Curated by ChEMBL | Assay Description Displacement of [125I]exendin-4 from GLP1 receptor in rat RINm5F cells after 2 hrs by gamma counting | J Med Chem 52: 6889-96 (2009) Article DOI: 10.1021/jm901153x BindingDB Entry DOI: 10.7270/Q2RJ4JNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Rattus norvegicus) | BDBM50314037 (CHEMBL1092704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
SungKyunKwan University Curated by ChEMBL | Assay Description Displacement of [125I]exendin-4 from GLP1 receptor in rat RINm5F cells after 2 hrs by gamma counting | J Med Chem 52: 6889-96 (2009) Article DOI: 10.1021/jm901153x BindingDB Entry DOI: 10.7270/Q2RJ4JNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Rattus norvegicus) | BDBM50314036 (CHEMBL1092373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
SungKyunKwan University Curated by ChEMBL | Assay Description Displacement of [125I]exendin-4 from GLP1 receptor in rat RINm5F cells after 2 hrs by gamma counting | J Med Chem 52: 6889-96 (2009) Article DOI: 10.1021/jm901153x BindingDB Entry DOI: 10.7270/Q2RJ4JNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Rattus norvegicus) | BDBM50314039 (CHEMBL1092706) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
SungKyunKwan University Curated by ChEMBL | Assay Description Displacement of [125I]exendin-4 from GLP1 receptor in rat RINm5F cells after 2 hrs by gamma counting | J Med Chem 52: 6889-96 (2009) Article DOI: 10.1021/jm901153x BindingDB Entry DOI: 10.7270/Q2RJ4JNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Rattus norvegicus) | BDBM50314041 (CHEMBL1092708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
SungKyunKwan University Curated by ChEMBL | Assay Description Displacement of [125I]exendin-4 from GLP1 receptor in rat RINm5F cells after 2 hrs by gamma counting | J Med Chem 52: 6889-96 (2009) Article DOI: 10.1021/jm901153x BindingDB Entry DOI: 10.7270/Q2RJ4JNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Homo sapiens (Human)) | BDBM50185957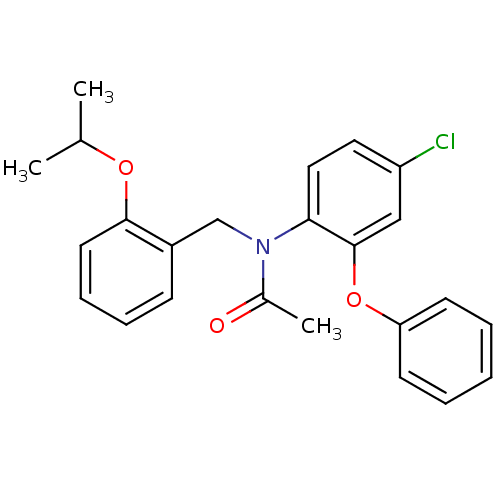 (CHEMBL205767 | N-(2-isopropoxybenzyl)-N-(4-chloro-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of TSPO (unknown origin) | Eur J Med Chem 125: 1172-1192 (2017) Article DOI: 10.1016/j.ejmech.2016.11.017 BindingDB Entry DOI: 10.7270/Q25Q4Z83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Homo sapiens (Human)) | BDBM22032 (1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoq...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of TSPO (unknown origin) | Eur J Med Chem 125: 1172-1192 (2017) Article DOI: 10.1016/j.ejmech.2016.11.017 BindingDB Entry DOI: 10.7270/Q25Q4Z83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM22032 (1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoq...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from TSPO in Sprague-Dawley rat cerebral cortex membranes after 60 mins by microbeta liquid scintillation counting method | Eur J Med Chem 125: 1172-1192 (2017) Article DOI: 10.1016/j.ejmech.2016.11.017 BindingDB Entry DOI: 10.7270/Q25Q4Z83 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Translocator protein (Homo sapiens (Human)) | BDBM50210943 (CHEMBL3970955) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of TSPO (unknown origin) | Eur J Med Chem 125: 1172-1192 (2017) Article DOI: 10.1016/j.ejmech.2016.11.017 BindingDB Entry DOI: 10.7270/Q25Q4Z83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Rattus norvegicus) | BDBM50314038 (CHEMBL1092705) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.18 | n/a | n/a | n/a | n/a | n/a | n/a |
SungKyunKwan University Curated by ChEMBL | Assay Description Displacement of [125I]exendin-4 from GLP1 receptor in rat RINm5F cells after 2 hrs by gamma counting | J Med Chem 52: 6889-96 (2009) Article DOI: 10.1021/jm901153x BindingDB Entry DOI: 10.7270/Q2RJ4JNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM22032 (1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoq...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST) Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from TSPO in Sprague-Dawley rat cerebral cortex membranes after 60 mins by liquid scintillation counting analysis | Eur J Med Chem 141: 240-256 (2017) Article DOI: 10.1016/j.ejmech.2017.09.033 BindingDB Entry DOI: 10.7270/Q2ZG6VRM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucagon-like peptide 1 receptor (Rattus norvegicus) | BDBM50314040 (CHEMBL1092707) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.1 | n/a | n/a | n/a | n/a | n/a | n/a |
SungKyunKwan University Curated by ChEMBL | Assay Description Displacement of [125I]exendin-4 from GLP1 receptor in rat RINm5F cells after 2 hrs by gamma counting | J Med Chem 52: 6889-96 (2009) Article DOI: 10.1021/jm901153x BindingDB Entry DOI: 10.7270/Q2RJ4JNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM22040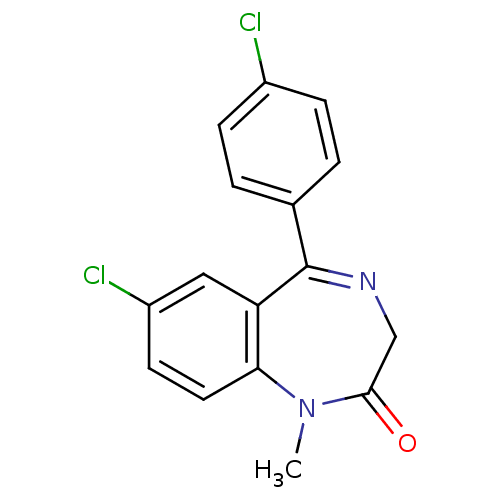 (4 -chlorodiazepam | 4' Cl-diazepam | 7-chloro-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST) Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from TSPO in Sprague-Dawley rat cerebral cortex membranes after 60 mins by liquid scintillation counting analysis | Eur J Med Chem 141: 240-256 (2017) Article DOI: 10.1016/j.ejmech.2017.09.033 BindingDB Entry DOI: 10.7270/Q2ZG6VRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584729 (US11524968, Example 478) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584734 (US11524968, Example 483) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584739 (US11524968, Example 488) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584774 (US11524968, Example 523) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584775 (US11524968, Example 524) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50210941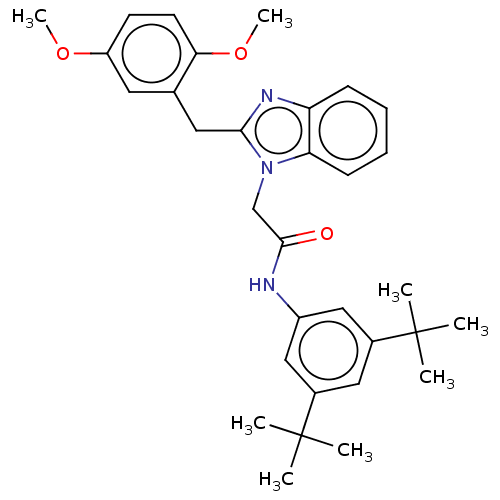 (CHEMBL3899686) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHOK1 cells by automated patch clamp method | Eur J Med Chem 125: 1172-1192 (2017) Article DOI: 10.1016/j.ejmech.2016.11.017 BindingDB Entry DOI: 10.7270/Q25Q4Z83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584318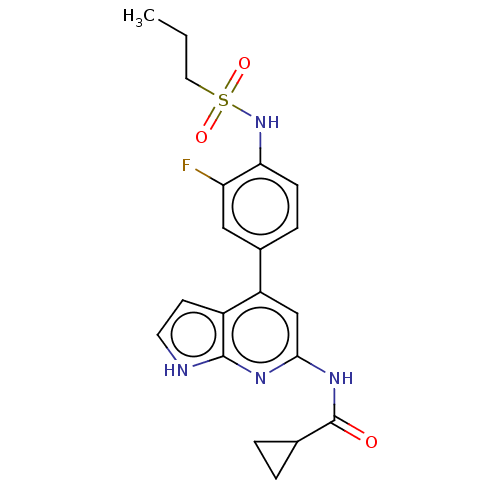 (US11524968, Example 66) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584319 (US11524968, Example 67) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584320 (US11524968, Example 68) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584321 (US11524968, Example 69) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584323 (US11524968, Example 71) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584324 (US11524968, Example 72) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584325 (US11524968, Example 73) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584327 (US11524968, Example 75) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584328 (US11524968, Example 76) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584329 (US11524968, Example 77) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584330 (US11524968, Example 78) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584331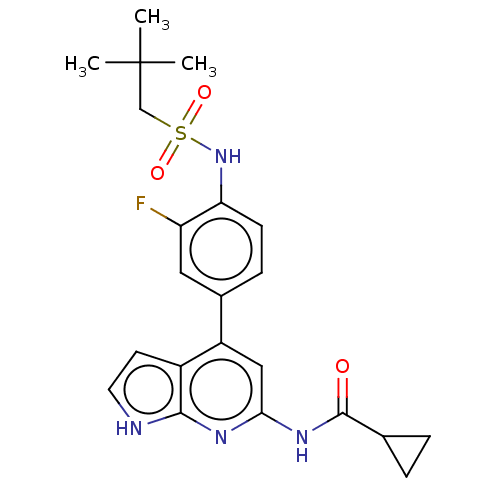 (US11524968, Example 79) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584332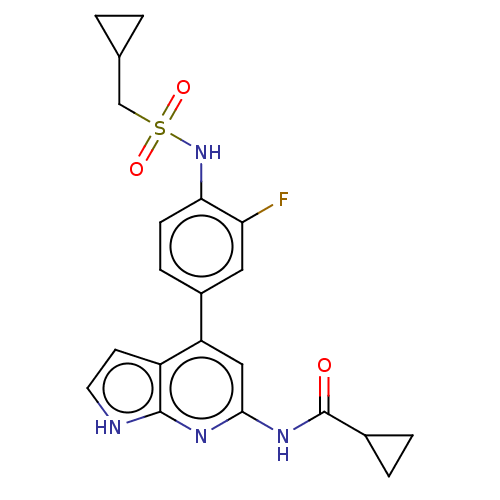 (US11524968, Example 80) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584335 (US11524968, Example 83) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584336 (US11524968, Example 84) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584337 (US11524968, Example 85) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584338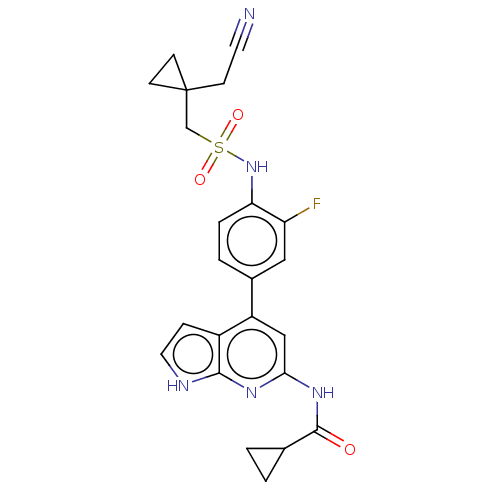 (US11524968, Example 86) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584340 (US11524968, Example 88) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584341 (US11524968, Example 89) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584877 (US11524968, Example 626) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584878 (US11524968, Example 627) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584879 (US11524968, Example 628) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584887 (US11524968, Example 636) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584918 (US11524968, Example 667) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584928 (US11524968, Example 677 | US11524968, Example 688) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584937 (US11524968, Example 686) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM584942 (US11524968, Example 691) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A control material and a test material were prepared for each concentration, in such a way that they were diluted by means of DMSO. At the same time,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HD80H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1412 total ) | Next | Last >> |