

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50365986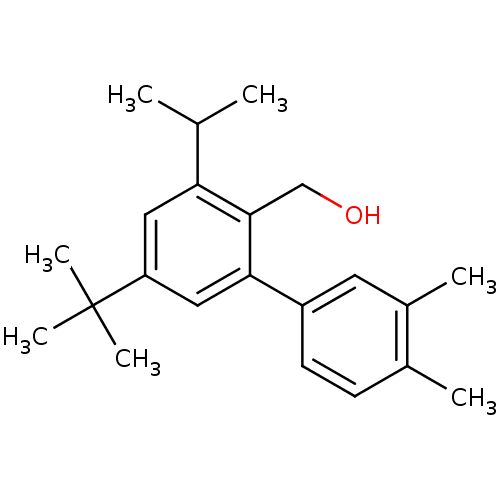 (CHEMBL1956427) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50365985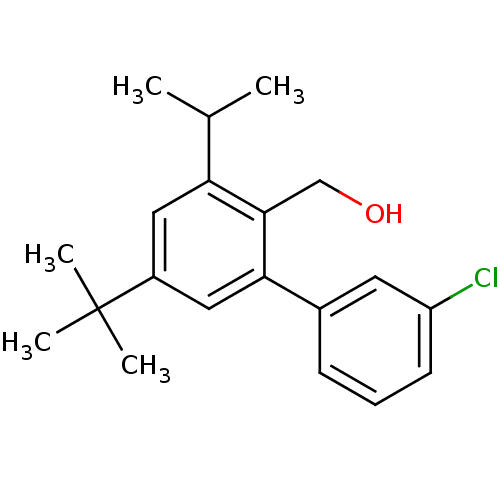 (CHEMBL1956426) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366026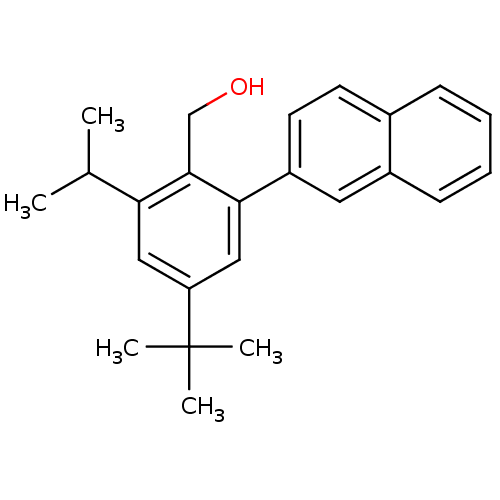 (CHEMBL1956425) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366024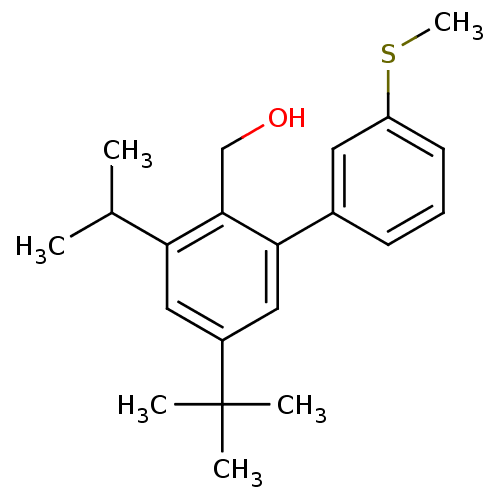 (CHEMBL1956413) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366025 (CHEMBL1956424) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366023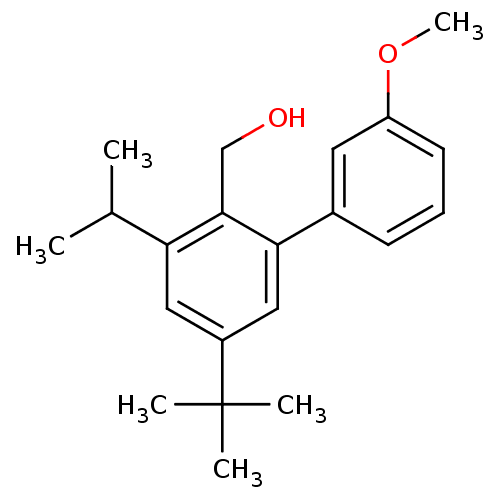 (CHEMBL1956412) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366022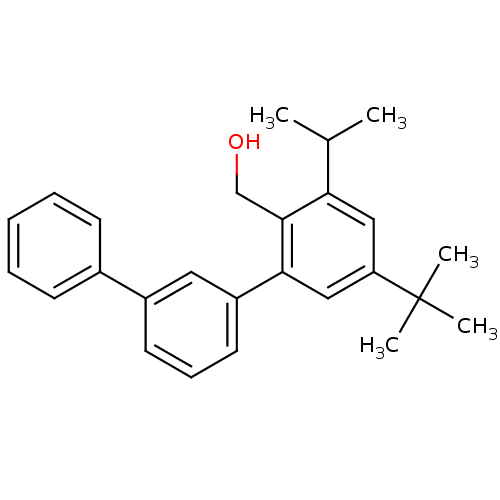 (CHEMBL1956423) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366021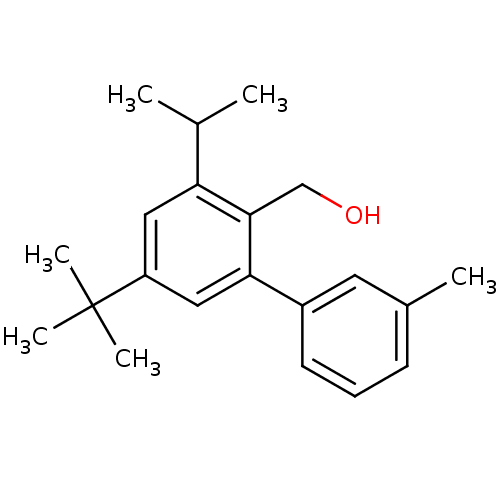 (CHEMBL1956422) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366020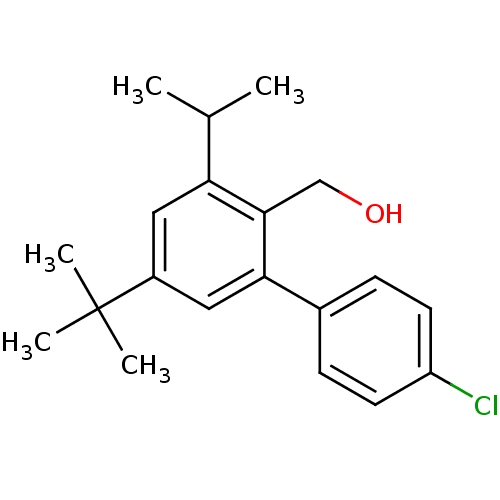 (CHEMBL1956421) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50365996 (CHEMBL1956433) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366019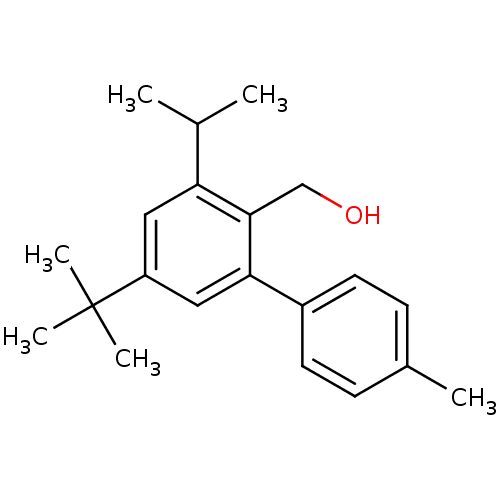 (CHEMBL1956420) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366018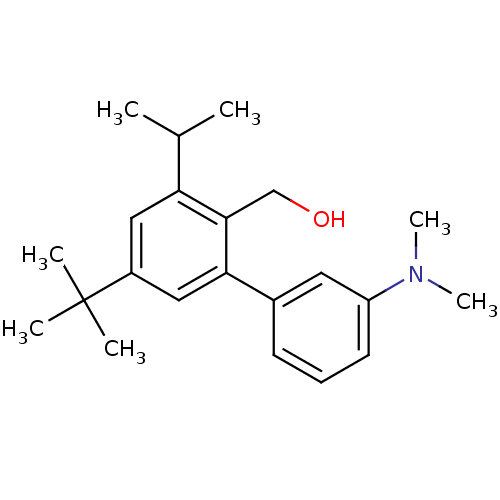 (CHEMBL1956419) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366017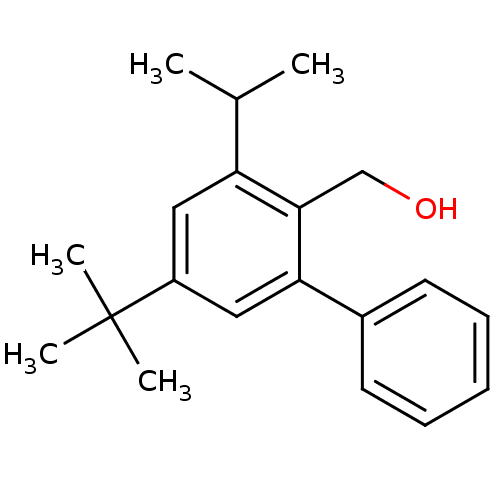 (CHEMBL1956418) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366001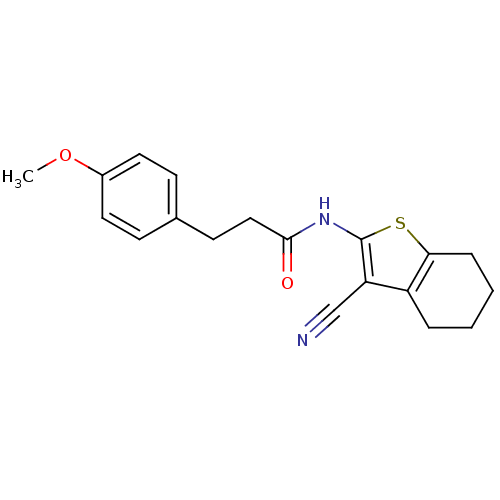 (CHEMBL1956438) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50365994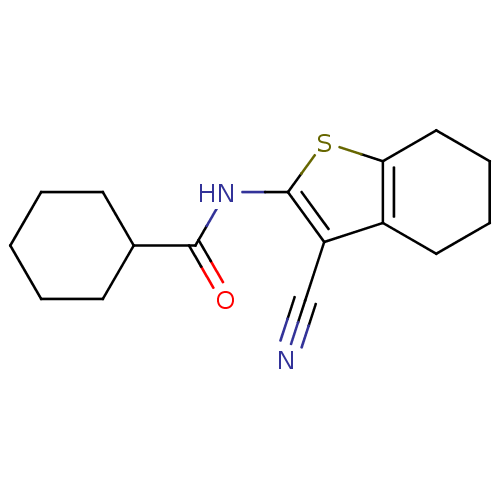 (CHEMBL396627) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50365993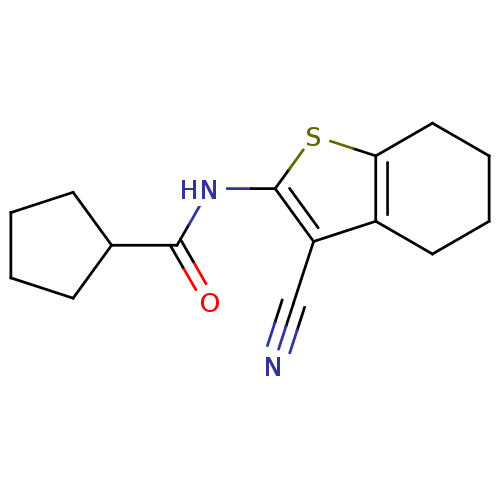 (CHEMBL1956432) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50365999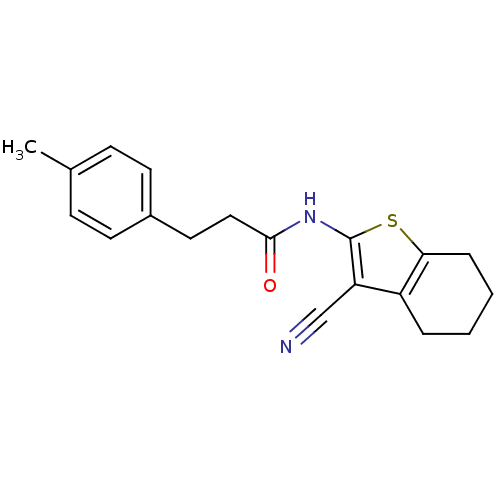 (CHEMBL1956436) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366000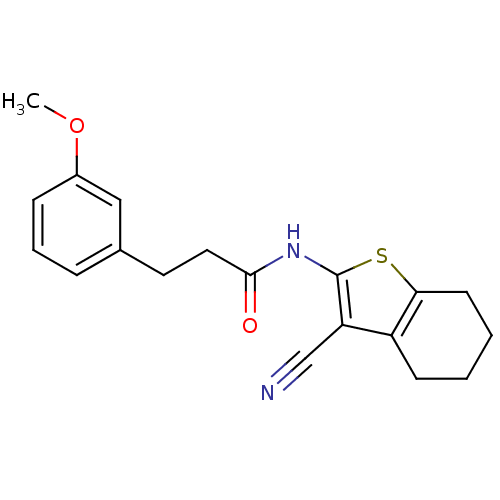 (CHEMBL1956437) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366016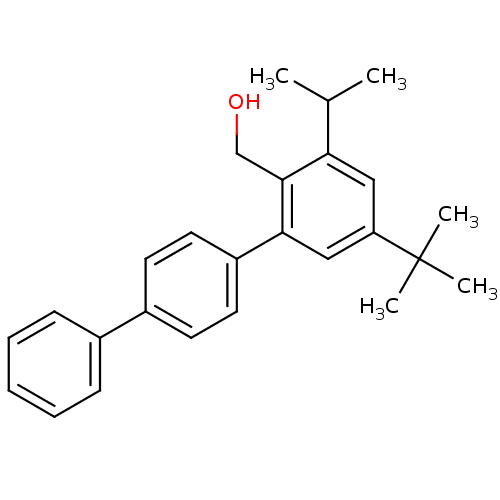 (CHEMBL1956417) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366015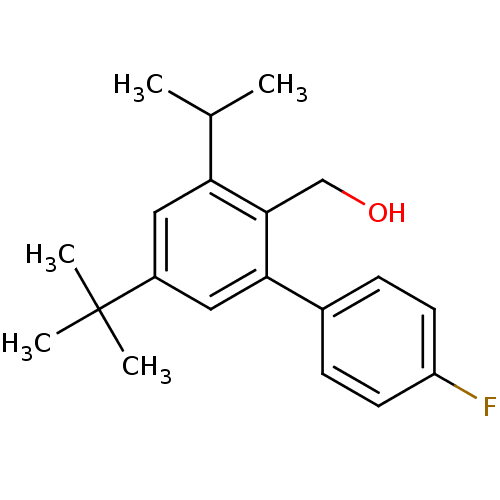 (CHEMBL1956416) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50365998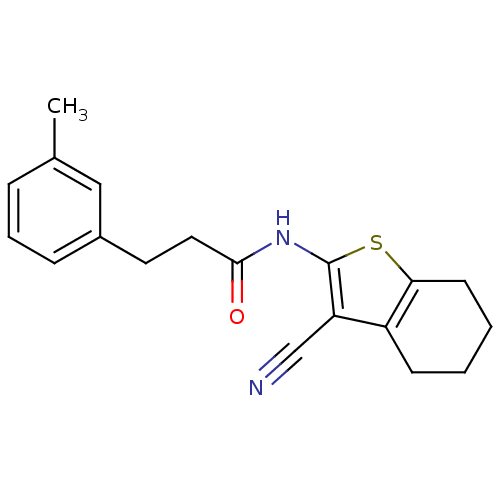 (CHEMBL1956435) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366014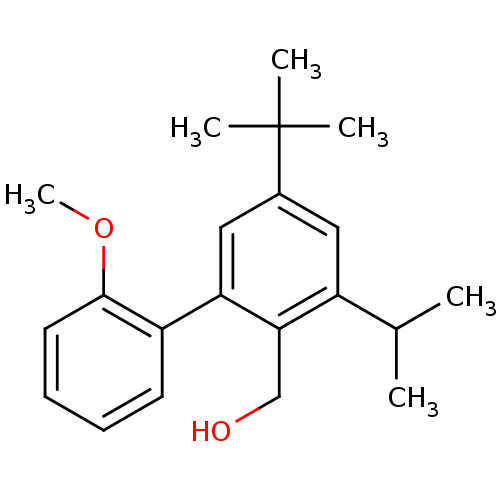 (CHEMBL1956415) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366002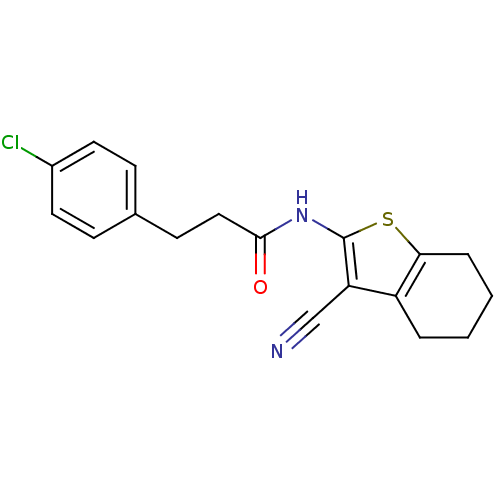 (CHEMBL1956439) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366013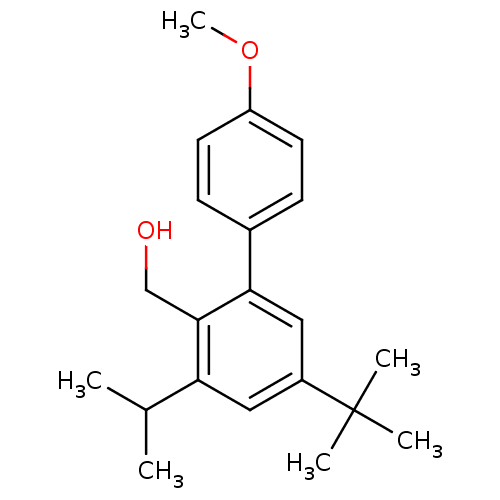 (CHEMBL1956414) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50365995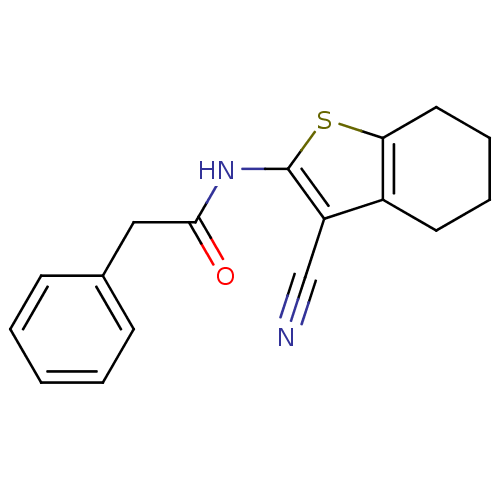 (CHEMBL397167) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50365992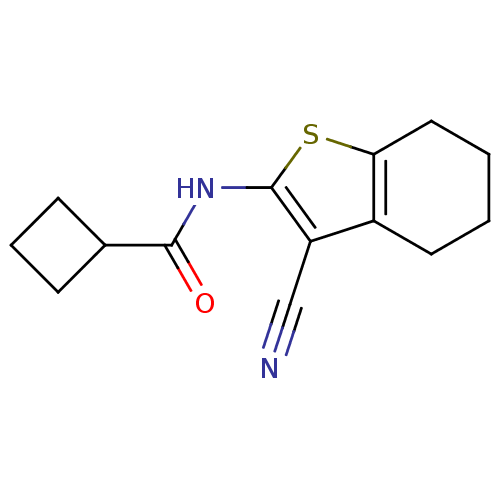 (CHEMBL1956431) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50365990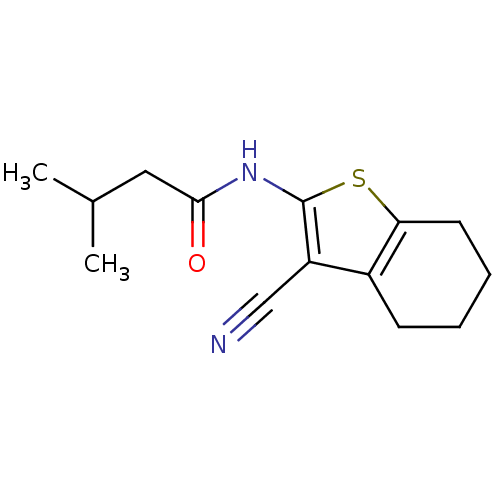 (CHEMBL1956429) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366003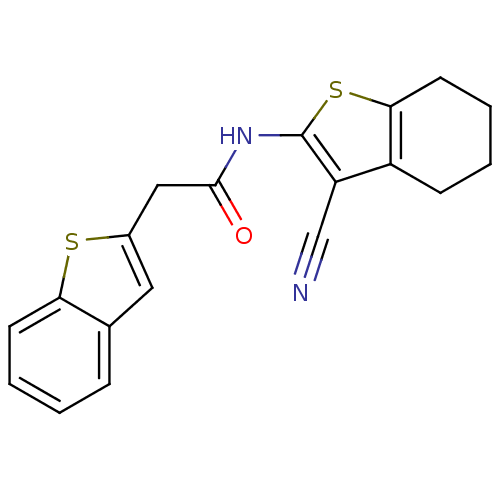 (CHEMBL1956440) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50365991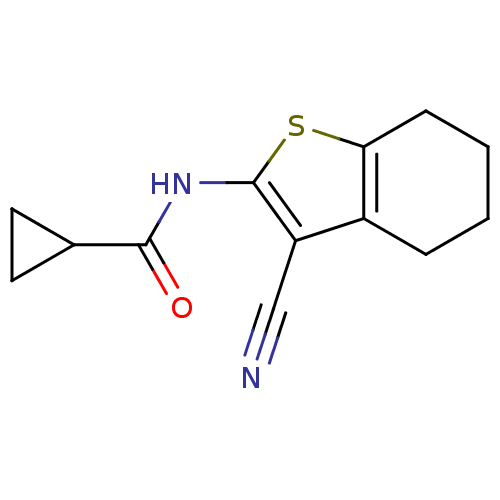 (CHEMBL1956430) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366012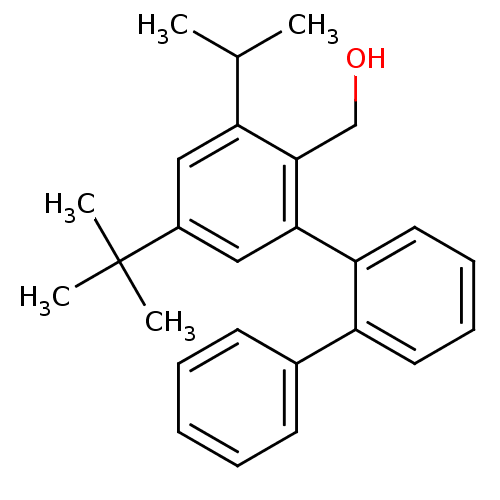 (CHEMBL1956259) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50365989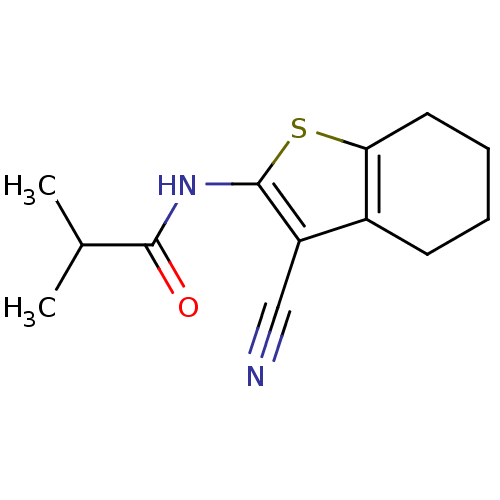 (CHEMBL1632048) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366004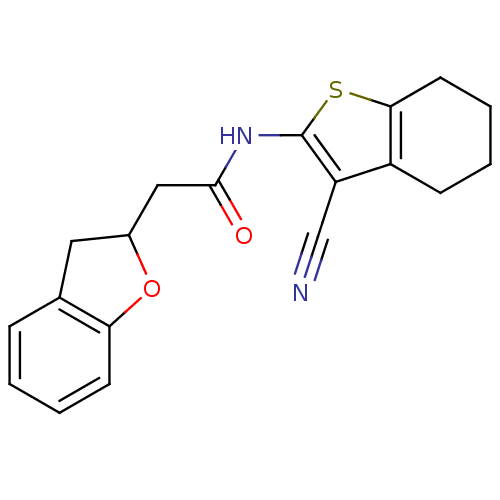 (CHEMBL1956441) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasoactive intestinal polypeptide receptor 1 (Rattus norvegicus) | BDBM50366008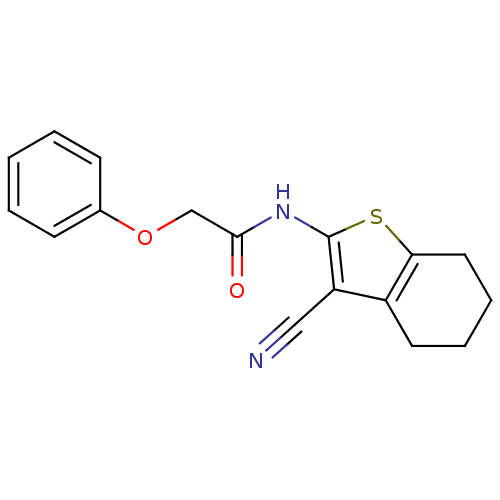 (CHEMBL1956445) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... | Bioorg Med Chem Lett 22: 2287-90 (2012) Article DOI: 10.1016/j.bmcl.2012.01.082 BindingDB Entry DOI: 10.7270/Q2Q240QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-beta (Homo sapiens (Human)) | BDBM50509327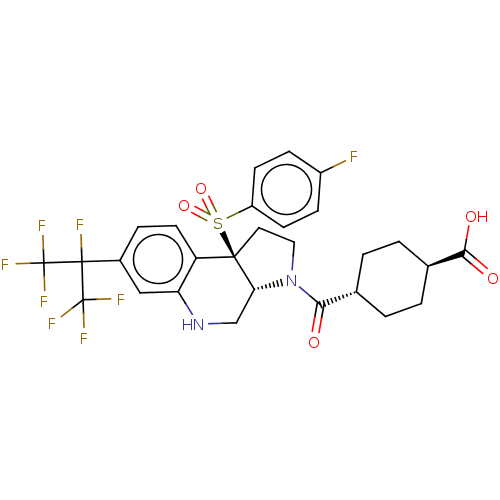 (CHEMBL4464711) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at RORbeta (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM382173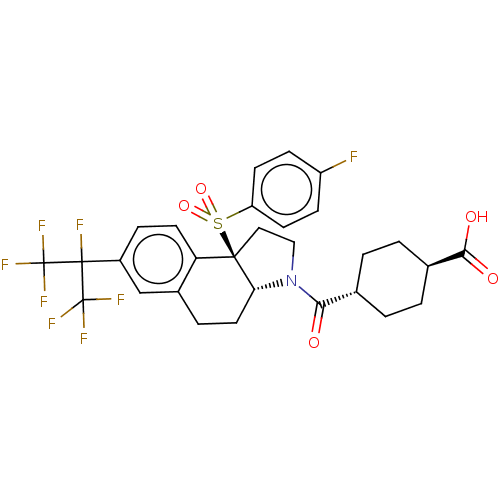 ((1R,4r)-4-((3aR,9bR)-9b-((4-fluorophenyl)sulfonyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at LXRalpha (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM382198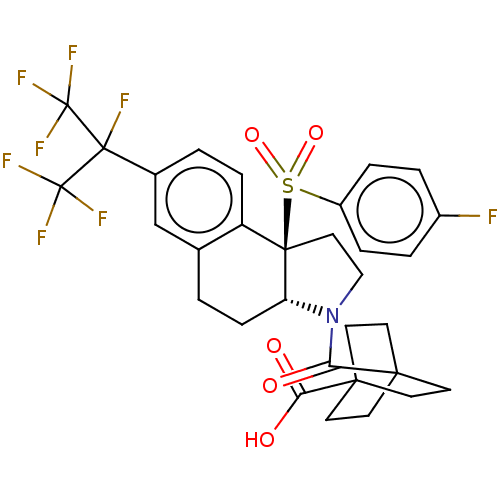 (US10273259, Example 12 | US10711020, Example 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at LXRalpha (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-beta (Homo sapiens (Human)) | BDBM50509330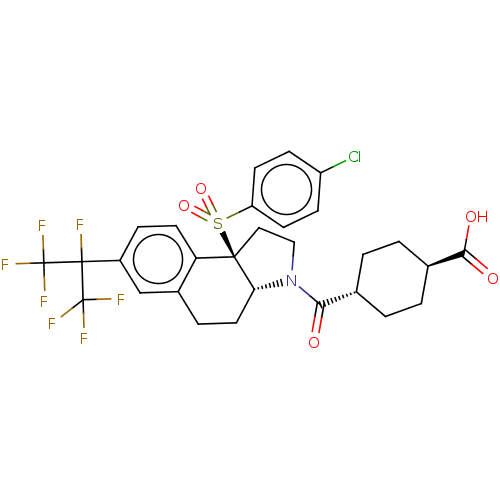 (CHEMBL4525527) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at RORbeta (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-beta (Homo sapiens (Human)) | BDBM382198 (US10273259, Example 12 | US10711020, Example 12) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at RORbeta (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50509331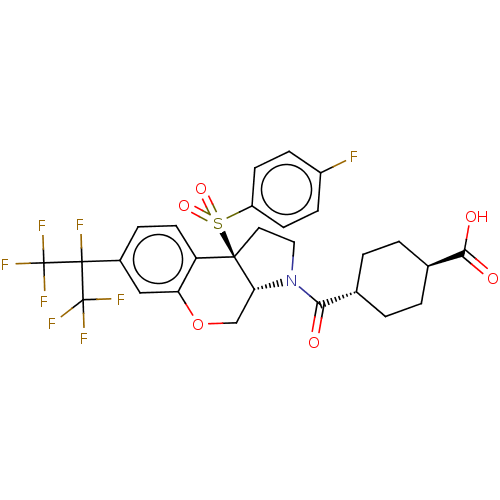 (CHEMBL4527625) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at LXRbeta (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50509327 (CHEMBL4464711) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at LXRalpha (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50509332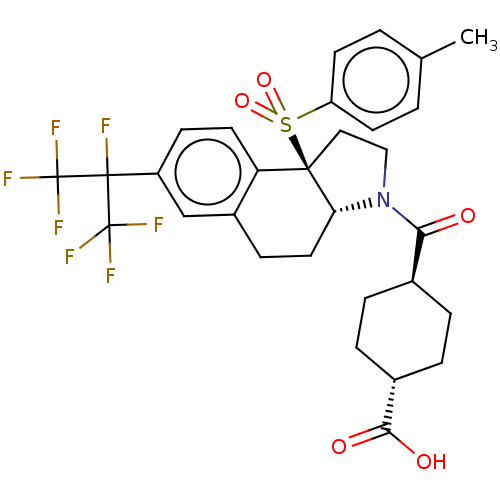 (CHEMBL4462790) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at LXRbeta (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM382181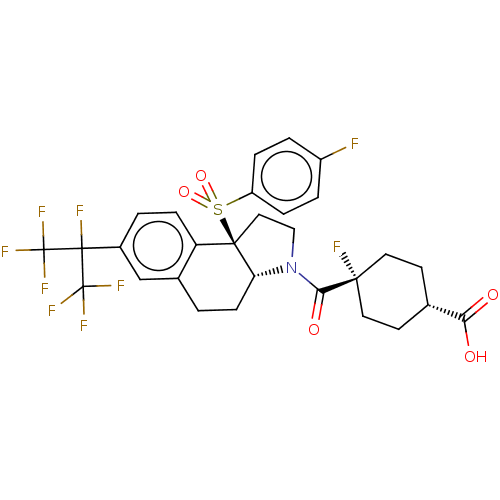 ((1S,4s)-4-fluoro-4-((3aR,9bR)-9b-((4-fluorophenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at LXRbeta (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM382356 (US10273259, Example 69 | US10711020, Example 69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2C9 (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-alpha (Homo sapiens (Human)) | BDBM382356 (US10273259, Example 69 | US10711020, Example 69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at RORalpha (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-alpha (Homo sapiens (Human)) | BDBM50509328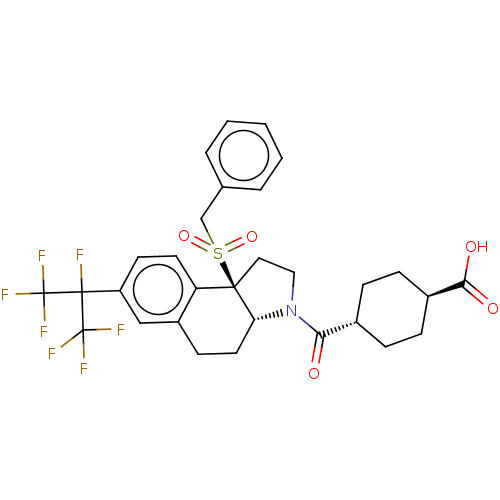 (CHEMBL4452650) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at RORalpha (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-alpha (Homo sapiens (Human)) | BDBM50509331 (CHEMBL4527625) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at RORalpha (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-alpha (Homo sapiens (Human)) | BDBM50509329 (CHEMBL4578712) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at RORalpha (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-alpha (Homo sapiens (Human)) | BDBM50509334 (CHEMBL4436417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at RORalpha (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-beta (Homo sapiens (Human)) | BDBM382173 ((1R,4r)-4-((3aR,9bR)-9b-((4-fluorophenyl)sulfonyl)...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at RORbeta (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-beta (Homo sapiens (Human)) | BDBM50509337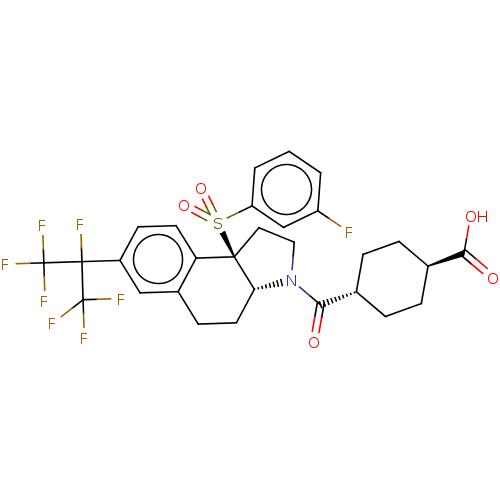 (CHEMBL4555822) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Activity at RORbeta (unknown origin) | J Med Chem 62: 9931-9946 (2019) Article DOI: 10.1021/acs.jmedchem.9b01369 BindingDB Entry DOI: 10.7270/Q2G44TKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 236 total ) | Next | Last >> |