 Found 32 hits with Last Name = 'kauer' and Initial = 'jc'
Found 32 hits with Last Name = 'kauer' and Initial = 'jc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-dependent protein kinase
(Oryctolagus cuniculus (Rabbit)) | BDBM6760

((+)-K-252a | CHEMBL281948 | K-252a | methyl (15S,1...)Show SMILES COC(=O)[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18?,26-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of cAMP dependent Protein kinase A of rabbit Skeletal Muscle. |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
Myosin light chain kinase, smooth muscle
(Gallus gallus (chicken)) | BDBM6760

((+)-K-252a | CHEMBL281948 | K-252a | methyl (15S,1...)Show SMILES COC(=O)[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18?,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Smooth muscle myosin light chain kinase of chicken gizzard |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50031442
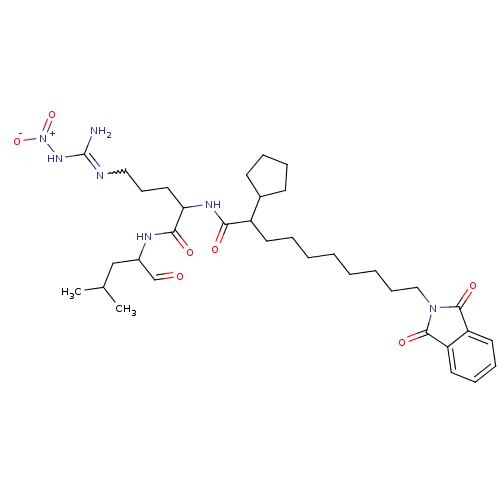
(1N-[4-amino(nitroimino)methylamino-1-(1-formyl-3-m...)Show SMILES CC(C)CC(NC(=O)C(CCCN=C(N)N[N+]([O-])=O)NC(=O)C(CCCCCCCCN1C(=O)c2ccccc2C1=O)C1CCCC1)C=O |w:12.11| Show InChI InChI=1S/C35H53N7O7/c1-24(2)22-26(23-43)38-32(45)30(19-13-20-37-35(36)40-42(48)49)39-31(44)27(25-14-8-9-15-25)16-7-5-3-4-6-12-21-41-33(46)28-17-10-11-18-29(28)34(41)47/h10-11,17-18,23-27,30H,3-9,12-16,19-22H2,1-2H3,(H,38,45)(H,39,44)(H3,36,37,40) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against 20S proteasome from human liver and brain |
J Med Chem 38: 2276-7 (1995)
Article DOI: 10.1021/jm00013a002
BindingDB Entry DOI: 10.7270/Q2V127JJ |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like protease CTRL-1
(Homo sapiens (Human)) | BDBM50031442

(1N-[4-amino(nitroimino)methylamino-1-(1-formyl-3-m...)Show SMILES CC(C)CC(NC(=O)C(CCCN=C(N)N[N+]([O-])=O)NC(=O)C(CCCCCCCCN1C(=O)c2ccccc2C1=O)C1CCCC1)C=O |w:12.11| Show InChI InChI=1S/C35H53N7O7/c1-24(2)22-26(23-43)38-32(45)30(19-13-20-37-35(36)40-42(48)49)39-31(44)27(25-14-8-9-15-25)16-7-5-3-4-6-12-21-41-33(46)28-17-10-11-18-29(28)34(41)47/h10-11,17-18,23-27,30H,3-9,12-16,19-22H2,1-2H3,(H,38,45)(H,39,44)(H3,36,37,40) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of chymotrypsin-like protease of proteasome from postmortem human liver and brain |
Bioorg Med Chem Lett 6: 287-290 (1996)
Article DOI: 10.1016/0960-894X(96)00014-5
BindingDB Entry DOI: 10.7270/Q2JQ1112 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM6760

((+)-K-252a | CHEMBL281948 | K-252a | methyl (15S,1...)Show SMILES COC(=O)[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18?,26-,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit human trk A tyrosine kinase expressed in baculovirus using ELISA based enzyme assay was determined |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50031440
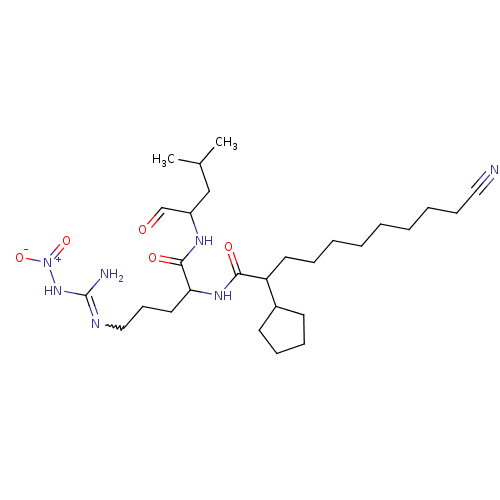
(1N-[4-amino(nitroimino)methylamino-1-(1-formyl-3-m...)Show SMILES CC(C)CC(NC(=O)C(CCCN=C(N)N[N+]([O-])=O)NC(=O)C(CCCCCCCCC#N)C1CCCC1)C=O |w:12.11| Show InChI InChI=1S/C28H49N7O5/c1-21(2)19-23(20-36)32-27(38)25(16-12-18-31-28(30)34-35(39)40)33-26(37)24(22-13-9-10-14-22)15-8-6-4-3-5-7-11-17-29/h20-25H,3-16,18-19H2,1-2H3,(H,32,38)(H,33,37)(H3,30,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against 20S proteasome from human liver and brain |
J Med Chem 38: 2276-7 (1995)
Article DOI: 10.1021/jm00013a002
BindingDB Entry DOI: 10.7270/Q2V127JJ |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like protease CTRL-1
(Homo sapiens (Human)) | BDBM50031440

(1N-[4-amino(nitroimino)methylamino-1-(1-formyl-3-m...)Show SMILES CC(C)CC(NC(=O)C(CCCN=C(N)N[N+]([O-])=O)NC(=O)C(CCCCCCCCC#N)C1CCCC1)C=O |w:12.11| Show InChI InChI=1S/C28H49N7O5/c1-21(2)19-23(20-36)32-27(38)25(16-12-18-31-28(30)34-35(39)40)33-26(37)24(22-13-9-10-14-22)15-8-6-4-3-5-7-11-17-29/h20-25H,3-16,18-19H2,1-2H3,(H,32,38)(H,33,37)(H3,30,31,34) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of chymotrypsin-like protease of proteasome from postmortem human liver and brain |
Bioorg Med Chem Lett 6: 287-290 (1996)
Article DOI: 10.1016/0960-894X(96)00014-5
BindingDB Entry DOI: 10.7270/Q2JQ1112 |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like protease CTRL-1
(Homo sapiens (Human)) | BDBM50288611
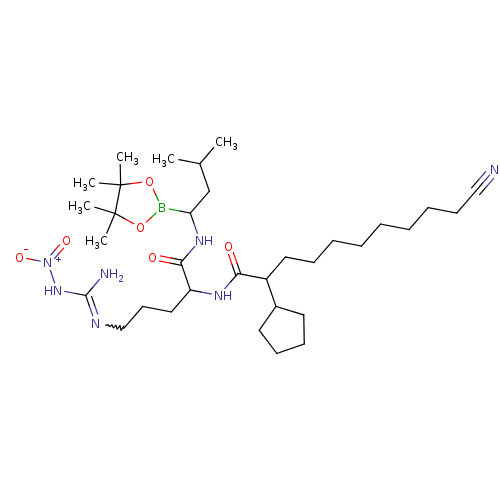
(Alpha-ketocarbonyl boronic ester derivative | CHEM...)Show SMILES CC(C)CC(NC(=O)C(CCCN=C(N)N[N+]([O-])=O)NC(=O)C(CCCCCCCCC#N)C1CCCC1)B1OC(C)(C)C(C)(C)O1 |w:12.11| Show InChI InChI=1S/C33H60BN7O6/c1-24(2)23-28(34-46-32(3,4)33(5,6)47-34)39-30(43)27(20-16-22-37-31(36)40-41(44)45)38-29(42)26(25-17-13-14-18-25)19-12-10-8-7-9-11-15-21-35/h24-28H,7-20,22-23H2,1-6H3,(H,38,42)(H,39,43)(H3,36,37,40) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of chymotrypsin-like protease of proteasome from postmortem human liver and brain |
Bioorg Med Chem Lett 6: 287-290 (1996)
Article DOI: 10.1016/0960-894X(96)00014-5
BindingDB Entry DOI: 10.7270/Q2JQ1112 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase
(Oryctolagus cuniculus (Rabbit)) | BDBM50058332
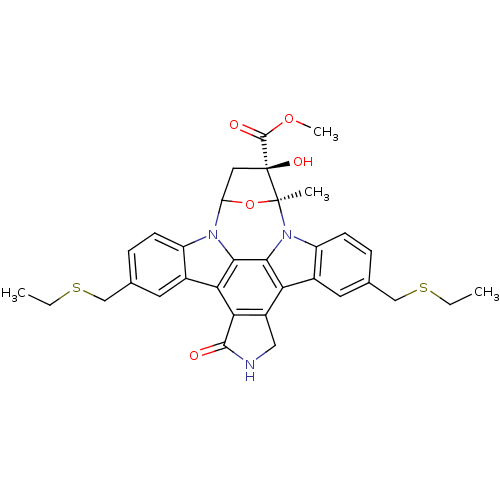
(CHEMBL299496 | methyl 10,23-di(ethylsulfanylmethyl...)Show SMILES CCSCc1ccc2n3C4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(CSCC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C33H33N3O5S2/c1-5-42-15-17-7-9-22-19(11-17)26-27-21(14-34-30(27)37)25-20-12-18(16-43-6-2)8-10-23(20)36-29(25)28(26)35(22)24-13-33(39,31(38)40-4)32(36,3)41-24/h7-12,24,39H,5-6,13-16H2,1-4H3,(H,34,37)/t24?,32-,33-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of cAMP dependent Protein kinase A of rabbit Skeletal Muscle. |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
Myosin light chain kinase, smooth muscle
(Gallus gallus (chicken)) | BDBM50058332

(CHEMBL299496 | methyl 10,23-di(ethylsulfanylmethyl...)Show SMILES CCSCc1ccc2n3C4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(CSCC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C33H33N3O5S2/c1-5-42-15-17-7-9-22-19(11-17)26-27-21(14-34-30(27)37)25-20-12-18(16-43-6-2)8-10-23(20)36-29(25)28(26)35(22)24-13-33(39,31(38)40-4)32(36,3)41-24/h7-12,24,39H,5-6,13-16H2,1-4H3,(H,34,37)/t24?,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Smooth muscle myosin light chain kinase of chicken gizzard |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50073850
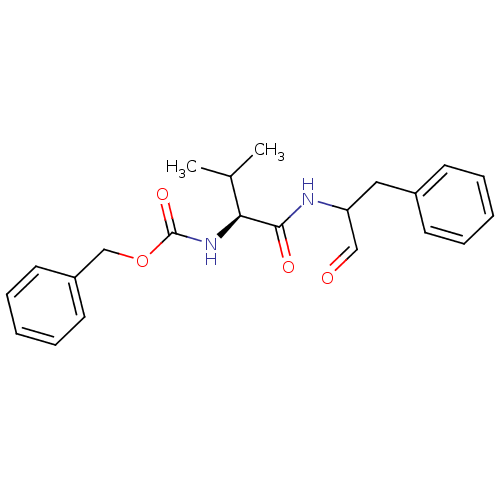
((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C=O |r| Show InChI InChI=1S/C22H26N2O4/c1-16(2)20(24-22(27)28-15-18-11-7-4-8-12-18)21(26)23-19(14-25)13-17-9-5-3-6-10-17/h3-12,14,16,19-20H,13,15H2,1-2H3,(H,23,26)(H,24,27)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against recombinant human Calpain 1 |
Bioorg Med Chem Lett 6: 1619-1622 (1996)
Article DOI: 10.1016/S0960-894X(96)00286-7
BindingDB Entry DOI: 10.7270/Q2RN37V7 |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50470197
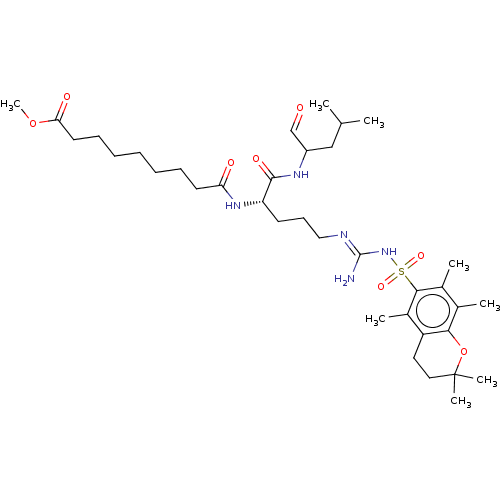
(CHEMBL2114122)Show SMILES COC(=O)CCCCCCCC(=O)N[C@@H](CCC\N=C(/N)NS(=O)(=O)c1c(C)c(C)c2OC(C)(C)CCc2c1C)C(=O)NC(CC(C)C)C=O Show InChI InChI=1S/C36H59N5O8S/c1-23(2)21-27(22-42)39-34(45)29(40-30(43)16-12-10-9-11-13-17-31(44)48-8)15-14-20-38-35(37)41-50(46,47)33-25(4)24(3)32-28(26(33)5)18-19-36(6,7)49-32/h22-23,27,29H,9-21H2,1-8H3,(H,39,45)(H,40,43)(H3,37,38,41)/t27?,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against 20S proteasome from human liver and brain |
J Med Chem 38: 2276-7 (1995)
Article DOI: 10.1021/jm00013a002
BindingDB Entry DOI: 10.7270/Q2V127JJ |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like protease CTRL-1
(Homo sapiens (Human)) | BDBM50288609

(Alpha-ketocarbonyl derivative | CHEMBL317337)Show SMILES CCNC(=O)C(=O)C(CC(C)C)NC(=O)C(CCCN=C(N)N[N+]([O-])=O)NC(=O)C(CCCCCCCCC#N)C1CCCC1 |w:19.18| Show InChI InChI=1S/C31H54N8O6/c1-4-34-30(43)27(40)26(21-22(2)3)37-29(42)25(18-14-20-35-31(33)38-39(44)45)36-28(41)24(23-15-11-12-16-23)17-10-8-6-5-7-9-13-19-32/h22-26H,4-18,20-21H2,1-3H3,(H,34,43)(H,36,41)(H,37,42)(H3,33,35,38) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of chymotrypsin-like protease of proteasome from postmortem human liver and brain |
Bioorg Med Chem Lett 6: 287-290 (1996)
Article DOI: 10.1016/0960-894X(96)00014-5
BindingDB Entry DOI: 10.7270/Q2JQ1112 |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like protease CTRL-1
(Homo sapiens (Human)) | BDBM50288609

(Alpha-ketocarbonyl derivative | CHEMBL317337)Show SMILES CCNC(=O)C(=O)C(CC(C)C)NC(=O)C(CCCN=C(N)N[N+]([O-])=O)NC(=O)C(CCCCCCCCC#N)C1CCCC1 |w:19.18| Show InChI InChI=1S/C31H54N8O6/c1-4-34-30(43)27(40)26(21-22(2)3)37-29(42)25(18-14-20-35-31(33)38-39(44)45)36-28(41)24(23-15-11-12-16-23)17-10-8-6-5-7-9-13-19-32/h22-26H,4-18,20-21H2,1-3H3,(H,34,43)(H,36,41)(H,37,42)(H3,33,35,38) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of chymotrypsin-like protease of proteasome from postmortem human liver and brain |
Bioorg Med Chem Lett 6: 287-290 (1996)
Article DOI: 10.1016/0960-894X(96)00014-5
BindingDB Entry DOI: 10.7270/Q2JQ1112 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50287391
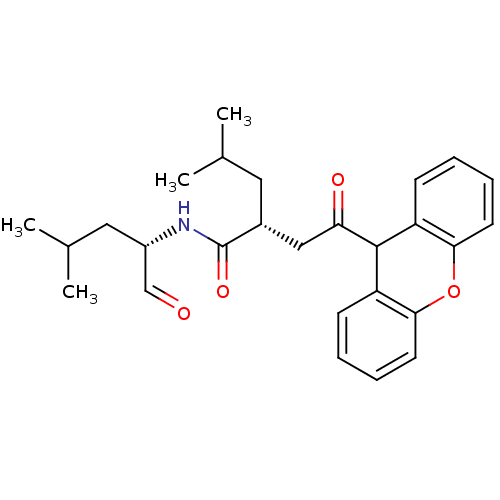
((R)-4-Methyl-2-[2-oxo-2-(9H-xanthen-9-yl)-ethyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)CC(=O)C1c2ccccc2Oc2ccccc12)C=O Show InChI InChI=1S/C27H33NO4/c1-17(2)13-19(27(31)28-20(16-29)14-18(3)4)15-23(30)26-21-9-5-7-11-24(21)32-25-12-8-6-10-22(25)26/h5-12,16-20,26H,13-15H2,1-4H3,(H,28,31)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against recombinant human Calpain 1 |
Bioorg Med Chem Lett 6: 1619-1622 (1996)
Article DOI: 10.1016/S0960-894X(96)00286-7
BindingDB Entry DOI: 10.7270/Q2RN37V7 |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50470199

(CHEMBL76284)Show SMILES COC(=O)CCCCCCCC(=O)NC(CCC\N=C(/N)N[N+]([O-])=O)C(=O)NC(CC(C)C)C=O Show InChI InChI=1S/C22H40N6O7/c1-16(2)14-17(15-29)25-21(32)18(10-9-13-24-22(23)27-28(33)34)26-19(30)11-7-5-4-6-8-12-20(31)35-3/h15-18H,4-14H2,1-3H3,(H,25,32)(H,26,30)(H3,23,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against 20S proteasome from human liver and brain |
J Med Chem 38: 2276-7 (1995)
Article DOI: 10.1021/jm00013a002
BindingDB Entry DOI: 10.7270/Q2V127JJ |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50287394
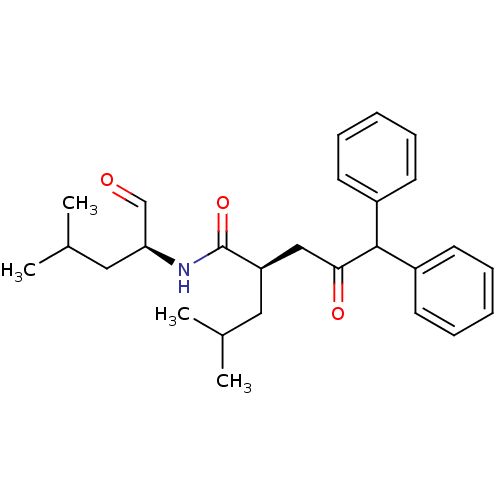
((R)-2-Isobutyl-4-oxo-5,5-diphenyl-pentanoic acid (...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)CC(=O)C(c1ccccc1)c1ccccc1)C=O Show InChI InChI=1S/C27H35NO3/c1-19(2)15-23(27(31)28-24(18-29)16-20(3)4)17-25(30)26(21-11-7-5-8-12-21)22-13-9-6-10-14-22/h5-14,18-20,23-24,26H,15-17H2,1-4H3,(H,28,31)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against recombinant human Calpain 1 |
Bioorg Med Chem Lett 6: 1619-1622 (1996)
Article DOI: 10.1016/S0960-894X(96)00286-7
BindingDB Entry DOI: 10.7270/Q2RN37V7 |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50470198
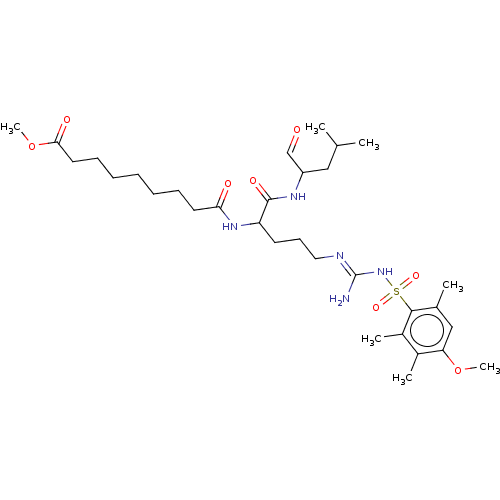
(CHEMBL73602)Show SMILES COC(=O)CCCCCCCC(=O)NC(CCC\N=C(/N)NS(=O)(=O)c1c(C)cc(OC)c(C)c1C)C(=O)NC(CC(C)C)C=O Show InChI InChI=1S/C32H53N5O8S/c1-21(2)18-25(20-38)35-31(41)26(36-28(39)15-11-9-8-10-12-16-29(40)45-7)14-13-17-34-32(33)37-46(42,43)30-22(3)19-27(44-6)23(4)24(30)5/h19-21,25-26H,8-18H2,1-7H3,(H,35,41)(H,36,39)(H3,33,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against 20S proteasome from human liver and brain |
J Med Chem 38: 2276-7 (1995)
Article DOI: 10.1021/jm00013a002
BindingDB Entry DOI: 10.7270/Q2V127JJ |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50287392
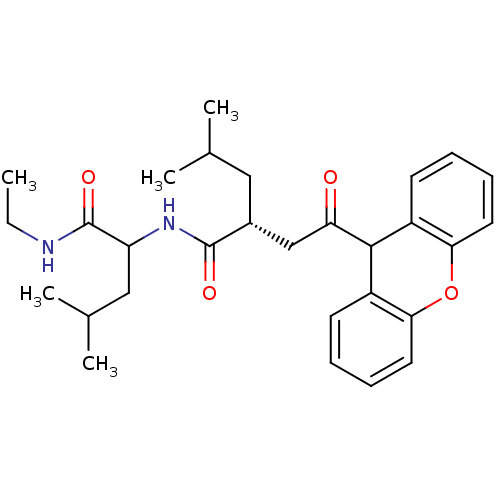
((R)-4-Methyl-2-[2-oxo-2-(9H-xanthen-9-yl)-ethyl]-p...)Show SMILES CCNC(=O)C(CC(C)C)NC(=O)[C@H](CC(C)C)CC(=O)C1c2ccccc2Oc2ccccc12 Show InChI InChI=1S/C29H38N2O4/c1-6-30-29(34)23(16-19(4)5)31-28(33)20(15-18(2)3)17-24(32)27-21-11-7-9-13-25(21)35-26-14-10-8-12-22(26)27/h7-14,18-20,23,27H,6,15-17H2,1-5H3,(H,30,34)(H,31,33)/t20-,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against recombinant human Calpain 1 |
Bioorg Med Chem Lett 6: 1619-1622 (1996)
Article DOI: 10.1016/S0960-894X(96)00286-7
BindingDB Entry DOI: 10.7270/Q2RN37V7 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50287390
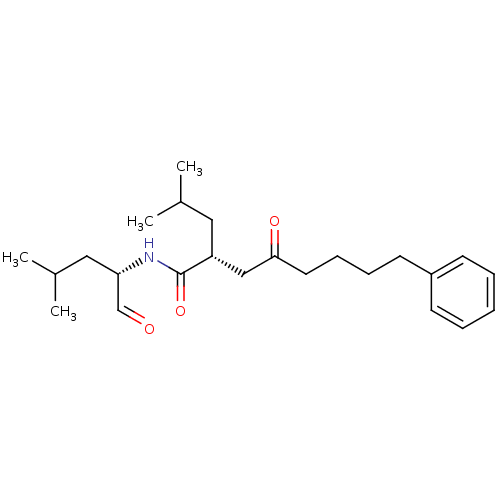
((R)-2-Isobutyl-4-oxo-8-phenyl-octanoic acid ((S)-1...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)CC(=O)CCCCc1ccccc1)C=O Show InChI InChI=1S/C24H37NO3/c1-18(2)14-21(24(28)25-22(17-26)15-19(3)4)16-23(27)13-9-8-12-20-10-6-5-7-11-20/h5-7,10-11,17-19,21-22H,8-9,12-16H2,1-4H3,(H,25,28)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against recombinant human Calpain 1 |
Bioorg Med Chem Lett 6: 1619-1622 (1996)
Article DOI: 10.1016/S0960-894X(96)00286-7
BindingDB Entry DOI: 10.7270/Q2RN37V7 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50058336

(CHEMBL53885 | methyl 10,23-di(ethoxymethyl)-16-hyd...)Show SMILES CCOCc1ccc2n3C4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(COCC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C33H33N3O7/c1-5-41-15-17-7-9-22-19(11-17)26-27-21(14-34-30(27)37)25-20-12-18(16-42-6-2)8-10-23(20)36-29(25)28(26)35(22)24-13-33(39,31(38)40-4)32(36,3)43-24/h7-12,24,39H,5-6,13-16H2,1-4H3,(H,34,37)/t24?,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit human trk A tyrosine kinase expressed in baculovirus using ELISA based enzyme assay was determined |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50287393

(CHEMBL296285 | [(S)-1-(1-Ethylaminooxalyl-propylca...)Show SMILES CCNC(=O)C(=O)C(CC)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C21H31N3O5/c1-5-16(18(25)20(27)22-6-2)23-19(26)17(12-14(3)4)24-21(28)29-13-15-10-8-7-9-11-15/h7-11,14,16-17H,5-6,12-13H2,1-4H3,(H,22,27)(H,23,26)(H,24,28)/t16?,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against recombinant human Calpain 1 |
Bioorg Med Chem Lett 6: 1619-1622 (1996)
Article DOI: 10.1016/S0960-894X(96)00286-7
BindingDB Entry DOI: 10.7270/Q2RN37V7 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50058333
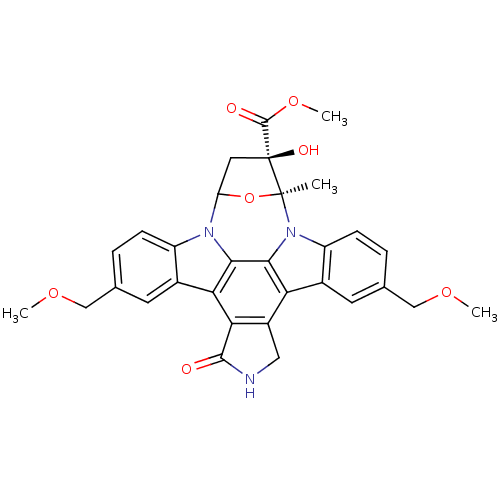
(CHEMBL291985 | methyl 16-hydroxy-10,23-di(methoxym...)Show SMILES COCc1ccc2n3C4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(COC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C31H29N3O7/c1-30-31(37,29(36)40-4)11-22(41-30)33-20-7-5-15(13-38-2)9-17(20)24-25-19(12-32-28(25)35)23-18-10-16(14-39-3)6-8-21(18)34(30)27(23)26(24)33/h5-10,22,37H,11-14H2,1-4H3,(H,32,35)/t22?,30-,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit human trk A tyrosine kinase expressed in baculovirus using ELISA based enzyme assay was determined |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like protease CTRL-1
(Homo sapiens (Human)) | BDBM50288610
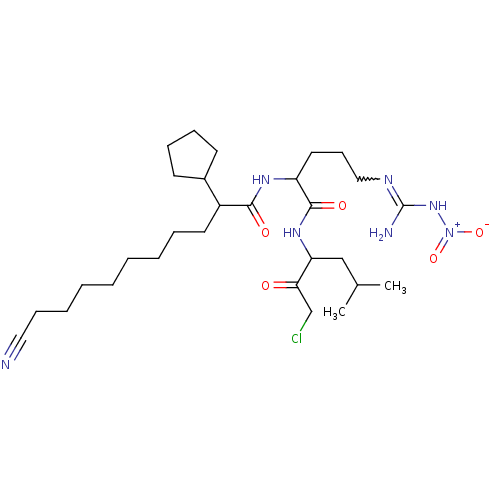
(Alpha-ketocarbonyl derivative | CHEMBL98937)Show SMILES CC(C)CC(NC(=O)C(CCCN=C(N)N[N+]([O-])=O)NC(=O)C(CCCCCCCCC#N)C1CCCC1)C(=O)CCl |w:12.11| Show InChI InChI=1S/C29H50ClN7O5/c1-21(2)19-25(26(38)20-30)35-28(40)24(16-12-18-33-29(32)36-37(41)42)34-27(39)23(22-13-9-10-14-22)15-8-6-4-3-5-7-11-17-31/h21-25H,3-16,18-20H2,1-2H3,(H,34,39)(H,35,40)(H3,32,33,36) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of chymotrypsin-like protease of proteasome from postmortem human liver and brain |
Bioorg Med Chem Lett 6: 287-290 (1996)
Article DOI: 10.1016/0960-894X(96)00014-5
BindingDB Entry DOI: 10.7270/Q2JQ1112 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50058329
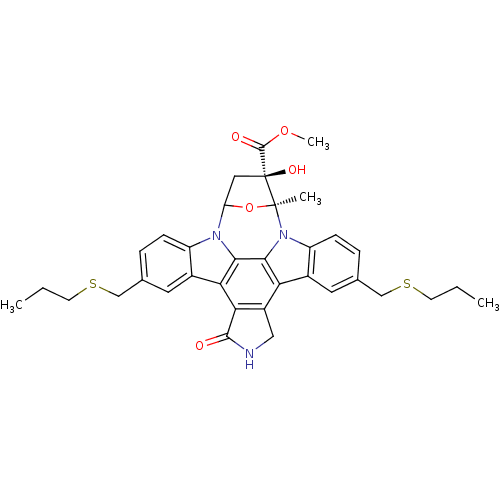
(CHEMBL55567 | methyl 16-hydroxy-15-methyl-3-oxo-10...)Show SMILES CCCSCc1ccc2n3C4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(CSCCC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C35H37N3O5S2/c1-5-11-44-17-19-7-9-24-21(13-19)28-29-23(16-36-32(29)39)27-22-14-20(18-45-12-6-2)8-10-25(22)38-31(27)30(28)37(24)26-15-35(41,33(40)42-4)34(38,3)43-26/h7-10,13-14,26,41H,5-6,11-12,15-18H2,1-4H3,(H,36,39)/t26?,34-,35-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit human trk A tyrosine kinase expressed in baculovirus using ELISA based enzyme assay was determined |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50058331
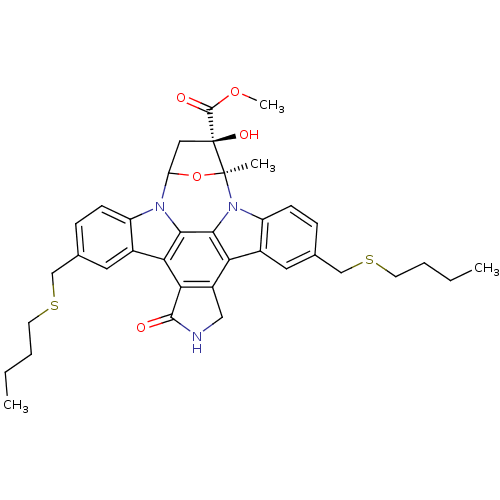
(CHEMBL444039 | methyl 10,23-di(butylsulfanylmethyl...)Show SMILES CCCCSCc1ccc2n3C4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(CSCCCC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C37H41N3O5S2/c1-5-7-13-46-19-21-9-11-26-23(15-21)30-31-25(18-38-34(31)41)29-24-16-22(20-47-14-8-6-2)10-12-27(24)40-33(29)32(30)39(26)28-17-37(43,35(42)44-4)36(40,3)45-28/h9-12,15-16,28,43H,5-8,13-14,17-20H2,1-4H3,(H,38,41)/t28?,36-,37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit human trk A tyrosine kinase expressed in baculovirus using ELISA based enzyme assay was determined |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like protease CTRL-1
(Homo sapiens (Human)) | BDBM50288610

(Alpha-ketocarbonyl derivative | CHEMBL98937)Show SMILES CC(C)CC(NC(=O)C(CCCN=C(N)N[N+]([O-])=O)NC(=O)C(CCCCCCCCC#N)C1CCCC1)C(=O)CCl |w:12.11| Show InChI InChI=1S/C29H50ClN7O5/c1-21(2)19-25(26(38)20-30)35-28(40)24(16-12-18-33-29(32)36-37(41)42)34-27(39)23(22-13-9-10-14-22)15-8-6-4-3-5-7-11-17-31/h21-25H,3-16,18-20H2,1-2H3,(H,34,39)(H,35,40)(H3,32,33,36) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for thromboxane TXA2 synthase inhibitory activity using human platelet |
Bioorg Med Chem Lett 6: 287-290 (1996)
Article DOI: 10.1016/0960-894X(96)00014-5
BindingDB Entry DOI: 10.7270/Q2JQ1112 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50058334
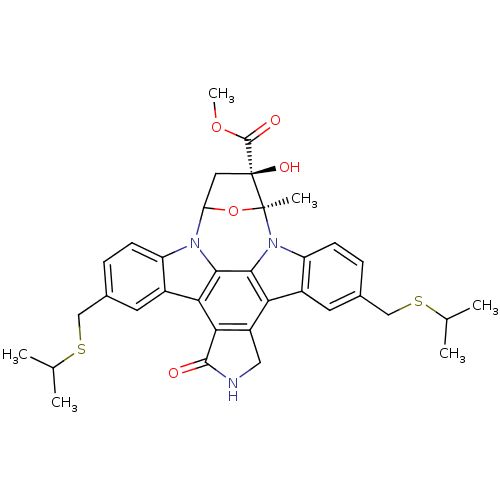
(CHEMBL301144 | methyl 16-hydroxy-10,23-di(isopropy...)Show SMILES COC(=O)[C@@]1(O)CC2O[C@]1(C)n1c3ccc(CSC(C)C)cc3c3c4CNC(=O)c4c4c5cc(CSC(C)C)ccc5n2c4c13 Show InChI InChI=1S/C35H37N3O5S2/c1-17(2)44-15-19-7-9-24-21(11-19)28-29-23(14-36-32(29)39)27-22-12-20(16-45-18(3)4)8-10-25(22)38-31(27)30(28)37(24)26-13-35(41,33(40)42-6)34(38,5)43-26/h7-12,17-18,26,41H,13-16H2,1-6H3,(H,36,39)/t26?,34-,35-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit human trk A tyrosine kinase expressed in baculovirus using ELISA based enzyme assay was determined |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50058335

(CHEMBL433281 | methyl 10,23-di(allylsulfanylmethyl...)Show SMILES COC(=O)[C@@]1(O)CC2O[C@]1(C)n1c3ccc(CSCC=C)cc3c3c4CNC(=O)c4c4c5cc(CSCC=C)ccc5n2c4c13 Show InChI InChI=1S/C35H33N3O5S2/c1-5-11-44-17-19-7-9-24-21(13-19)28-29-23(16-36-32(29)39)27-22-14-20(18-45-12-6-2)8-10-25(22)38-31(27)30(28)37(24)26-15-35(41,33(40)42-4)34(38,3)43-26/h5-10,13-14,26,41H,1-2,11-12,15-18H2,3-4H3,(H,36,39)/t26?,34-,35-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit human trk A tyrosine kinase expressed in baculovirus using ELISA based enzyme assay was determined |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50058332

(CHEMBL299496 | methyl 10,23-di(ethylsulfanylmethyl...)Show SMILES CCSCc1ccc2n3C4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(CSCC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C33H33N3O5S2/c1-5-42-15-17-7-9-22-19(11-17)26-27-21(14-34-30(27)37)25-20-12-18(16-43-6-2)8-10-23(20)36-29(25)28(26)35(22)24-13-33(39,31(38)40-4)32(36,3)41-24/h7-12,24,39H,5-6,13-16H2,1-4H3,(H,34,37)/t24?,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit human trk A tyrosine kinase expressed in baculovirus using ELISA based enzyme assay was determined |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50470200

(CHEMBL2369773)Show SMILES COC(=O)CCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C30H45N5O10/c1-18(2)26(35-28(41)21(12-14-24(37)38)32-23(36)13-15-25(39)45-3)29(42)33-20(11-7-8-16-31)27(40)34-22(30(43)44)17-19-9-5-4-6-10-19/h4-6,9-10,18,20-22,26H,7-8,11-17,31H2,1-3H3,(H,32,36)(H,33,42)(H,34,40)(H,35,41)(H,37,38)(H,43,44)/t20-,21-,22-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against 20S proteasome from human liver and brain |
J Med Chem 38: 2276-7 (1995)
Article DOI: 10.1021/jm00013a002
BindingDB Entry DOI: 10.7270/Q2V127JJ |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50470201
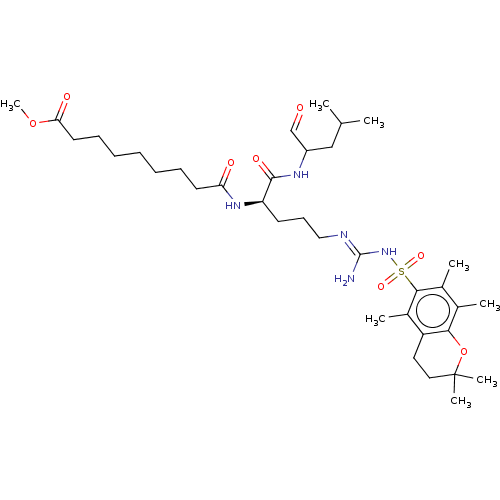
(CHEMBL406736)Show SMILES COC(=O)CCCCCCCC(=O)N[C@H](CCC\N=C(/N)NS(=O)(=O)c1c(C)c(C)c2OC(C)(C)CCc2c1C)C(=O)NC(CC(C)C)C=O Show InChI InChI=1S/C36H59N5O8S/c1-23(2)21-27(22-42)39-34(45)29(40-30(43)16-12-10-9-11-13-17-31(44)48-8)15-14-20-38-35(37)41-50(46,47)33-25(4)24(3)32-28(26(33)5)18-19-36(6,7)49-32/h22-23,27,29H,9-21H2,1-8H3,(H,39,45)(H,40,43)(H3,37,38,41)/t27?,29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against 20S proteasome from human liver and brain |
J Med Chem 38: 2276-7 (1995)
Article DOI: 10.1021/jm00013a002
BindingDB Entry DOI: 10.7270/Q2V127JJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data