

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50280116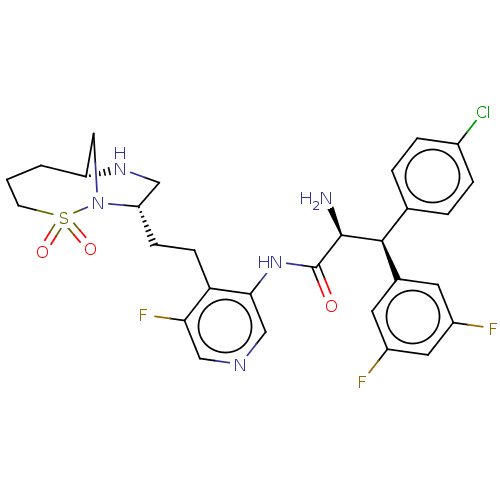 (CHEMBL4177355) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using Val-Ser-Gln-Asn-(beta-naphtyl)Ala-Pro-Ile-Val as substrate preincubated for 30 mins f... | ACS Med Chem Lett 8: 1292-1297 (2017) Article DOI: 10.1021/acsmedchemlett.7b00386 BindingDB Entry DOI: 10.7270/Q2K35X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from Lactobacillus casei | ACS Med Chem Lett 8: 1292-1297 (2017) Article DOI: 10.1021/acsmedchemlett.7b00386 BindingDB Entry DOI: 10.7270/Q2K35X57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using Val-Ser-Gln-Asn-(beta-naphtyl)Ala-Pro-Ile-Val as substrate preincubated for 30 mins f... | ACS Med Chem Lett 8: 1292-1297 (2017) Article DOI: 10.1021/acsmedchemlett.7b00386 BindingDB Entry DOI: 10.7270/Q2K35X57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190623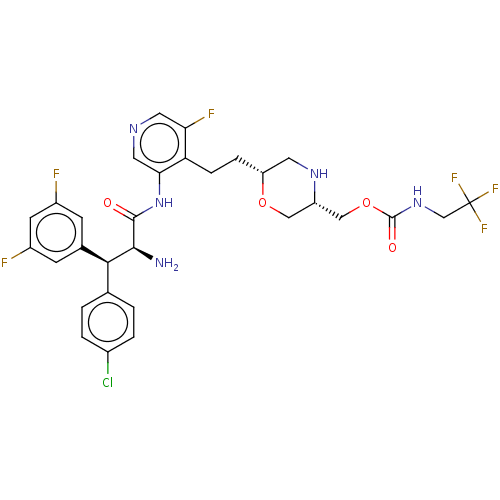 (CHEMBL3828743) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using Val-Ser-Gln-Asn-(beta-naphtyl)Ala-Pro-Ile-Val as substrate preincubated for 30 mins f... | ACS Med Chem Lett 8: 1292-1297 (2017) Article DOI: 10.1021/acsmedchemlett.7b00386 BindingDB Entry DOI: 10.7270/Q2K35X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442673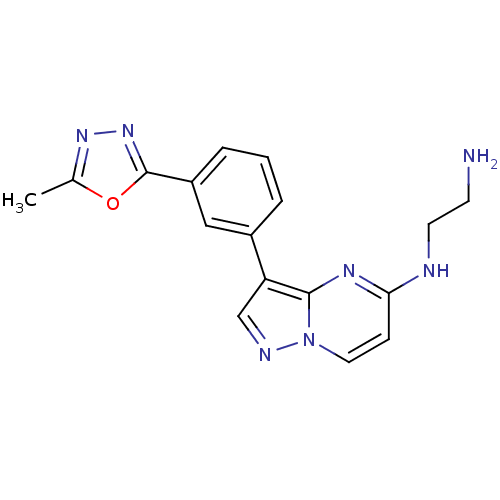 (CHEMBL2442291) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442690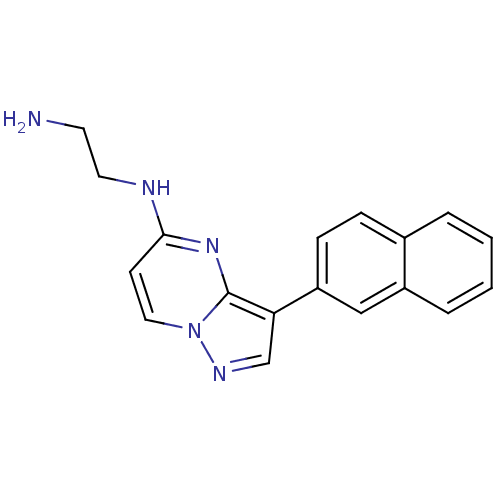 (CHEMBL2442296) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442685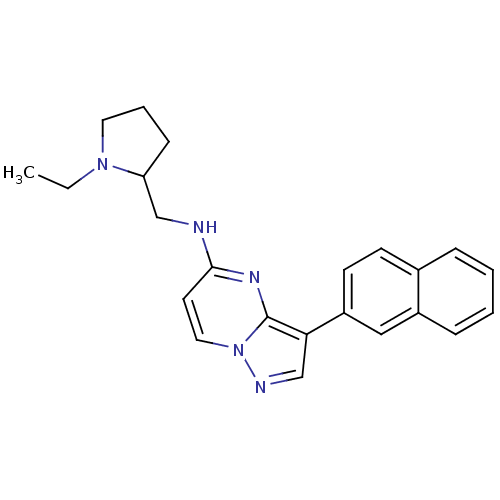 (CHEMBL2442301) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50442673 (CHEMBL2442291) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim3 (unknown origin) using STK1 as substrate preincubated for 30 mins followed by substrate and ATP addition after 60 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442679 (CHEMBL2442302) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442692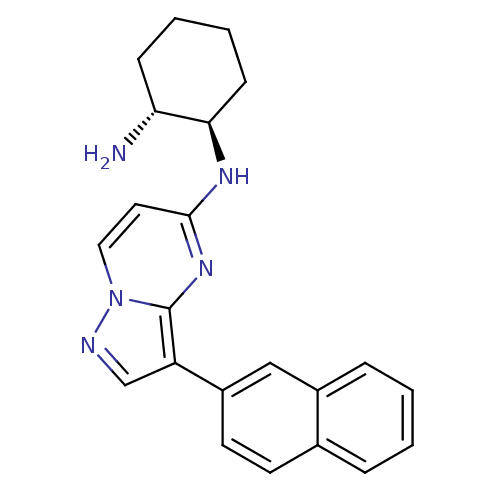 (CHEMBL2442317) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334853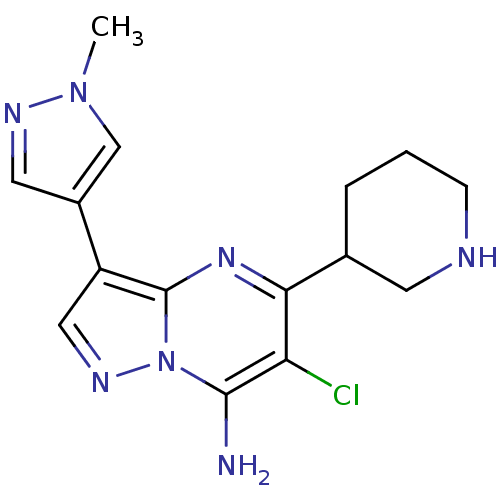 (6-chloro-3-(1-methyl-1H-pyrazol-4-yl)-5-(piperidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334855 (6-iodo-3-(1-methyl-1H-pyrazol-4-yl)-5-(piperidin-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50223567 (3-bromo-5-(2-chlorophenyl)-N-(pyridin-3-ylmethyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334854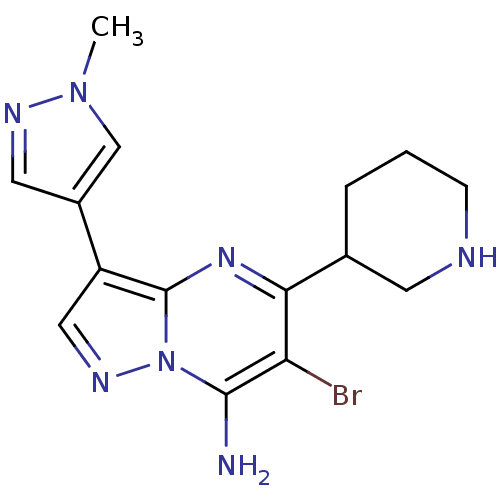 (6-bromo-3-(1-methyl-1H-pyrazol-4-yl)-5-(piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442693 (CHEMBL2442316) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442681 (CHEMBL2442290) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442686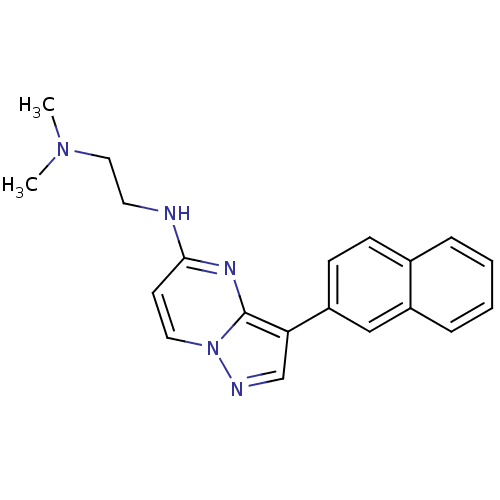 (CHEMBL2442300) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334849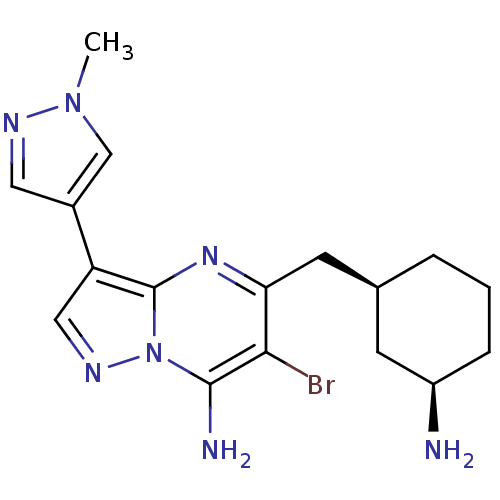 (CHEMBL1643236 | Syn-5-((3-aminocyclohexyl)methyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442687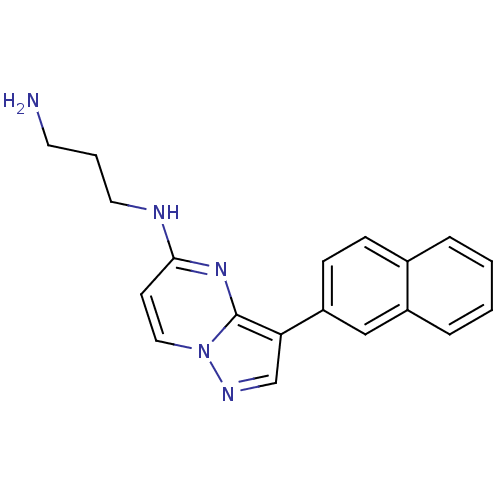 (CHEMBL2442299) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM105239 (US8580782, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334876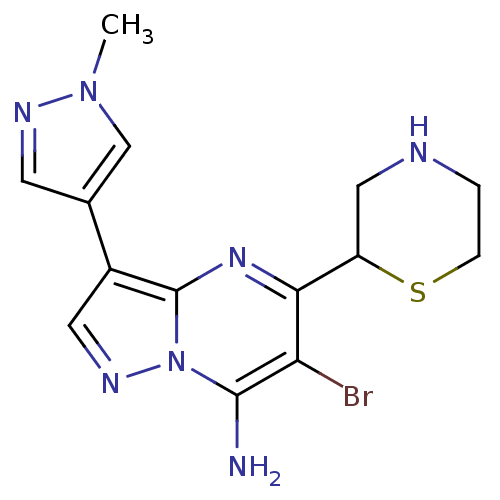 (6-bromo-3-(1-methyl-1H-pyrazol-4-yl)-5-(thiomorpho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442691 (CHEMBL2442295) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442698 (CHEMBL2442287) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334848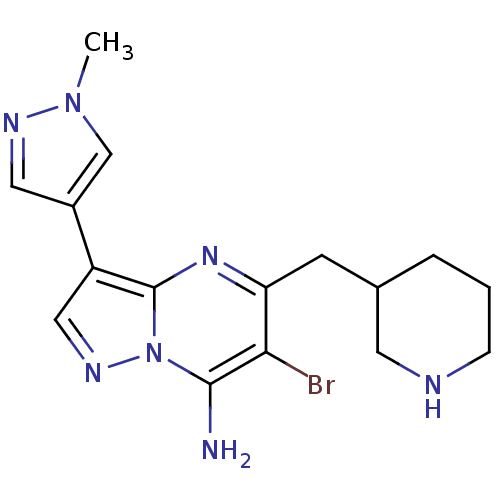 (6-bromo-3-(1-methyl-1H-pyrazol-4-yl)-5-(piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442678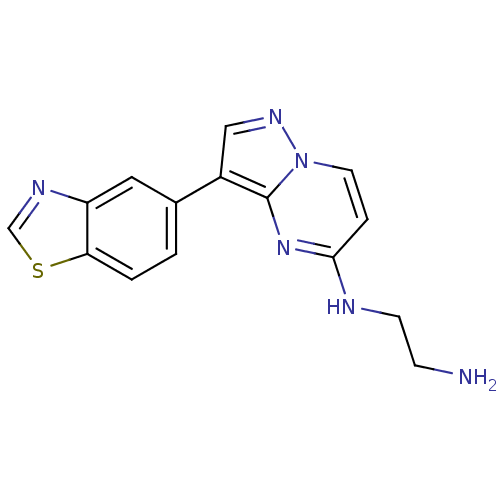 (CHEMBL2442303) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334847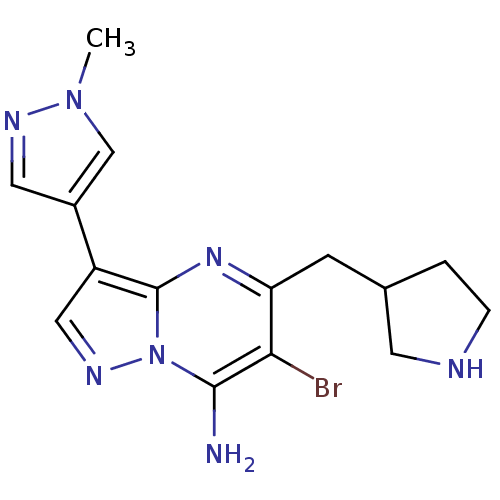 (6-bromo-3-(1-methyl-1H-pyrazol-4-yl)-5-(pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442677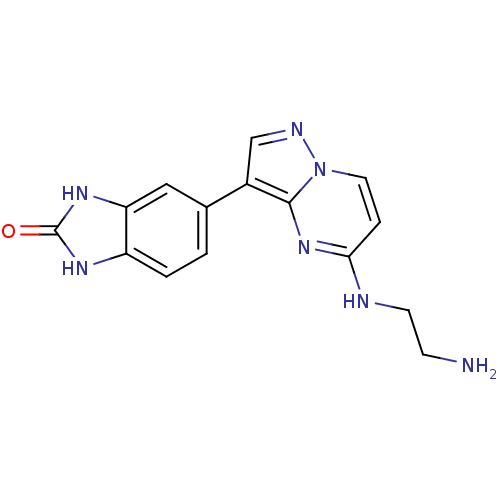 (CHEMBL2442304) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50442673 (CHEMBL2442291) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim2 (unknown origin) using STK1 as substrate preincubated for 30 mins followed by substrate and ATP addition after 60 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334871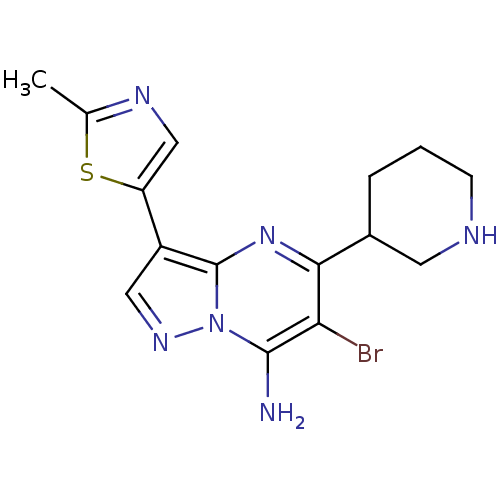 (6-bromo-3-(2-methylthiazol-5-yl)-5-(piperidin-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334852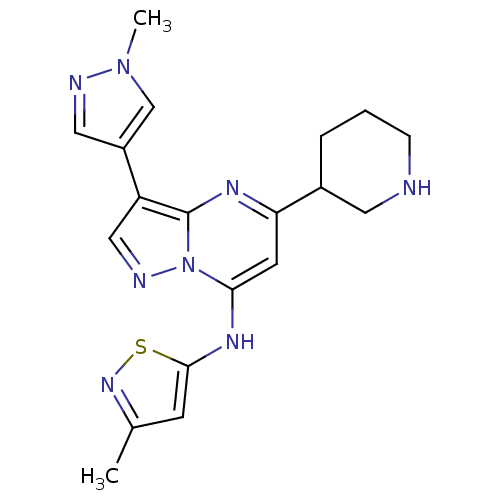 (3-methyl-N-(3-(1-methyl-1H-pyrazol-4-yl)-5-(piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334852 (3-methyl-N-(3-(1-methyl-1H-pyrazol-4-yl)-5-(piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant His-CHK1 expressed in baculovirus after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 467-70 (2010) Article DOI: 10.1016/j.bmcl.2010.10.113 BindingDB Entry DOI: 10.7270/Q2J966MH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442676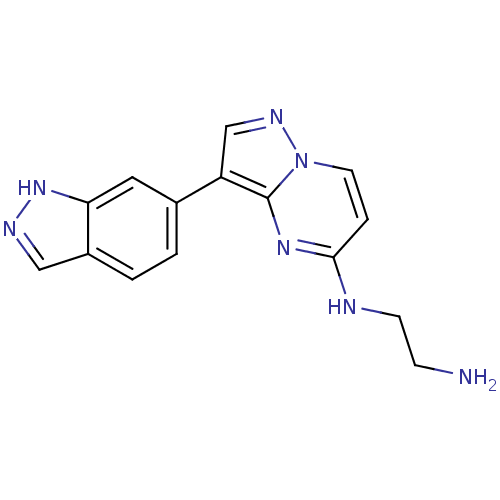 (CHEMBL2442305) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442675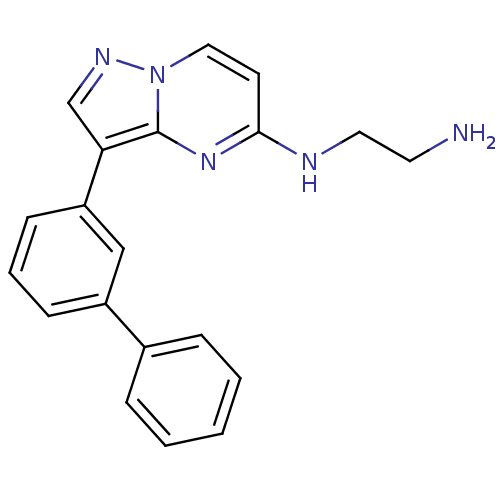 (CHEMBL2442307) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM105239 (US8580782, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM105236 (US8580782, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50334914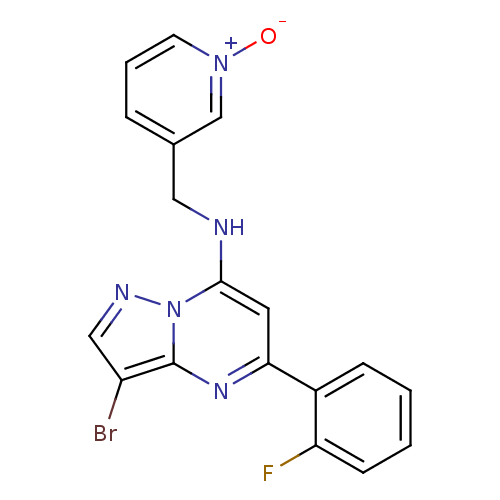 (3-((3-bromo-5-(2-fluorophenyl)pyrazolo[1,5-a]pyrim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A | Bioorg Med Chem Lett 21: 467-70 (2010) Article DOI: 10.1016/j.bmcl.2010.10.113 BindingDB Entry DOI: 10.7270/Q2J966MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334869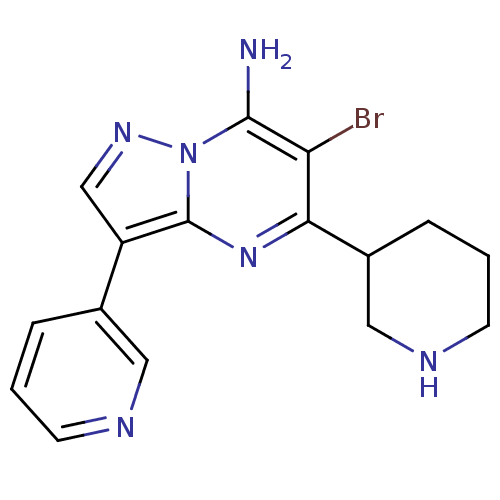 (6-bromo-5-(piperidin-3-yl)-3-(pyridin-3-yl)pyrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334858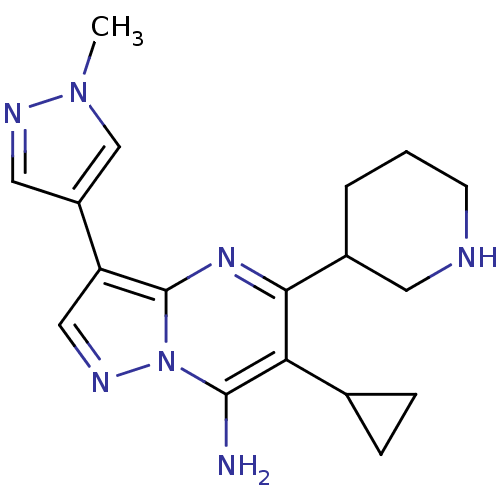 (6-cyclopropyl-3-(1-methyl-1H-pyrazol-4-yl)-5-(pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442694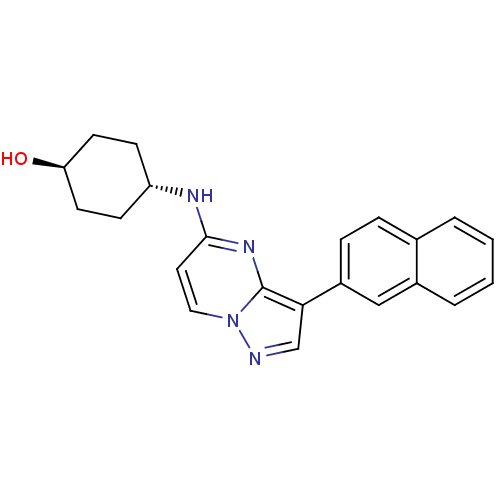 (CHEMBL2442315) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50442677 (CHEMBL2442304) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim2 (unknown origin) using STK1 as substrate preincubated for 30 mins followed by substrate and ATP addition after 60 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM105234 (US8580782, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334912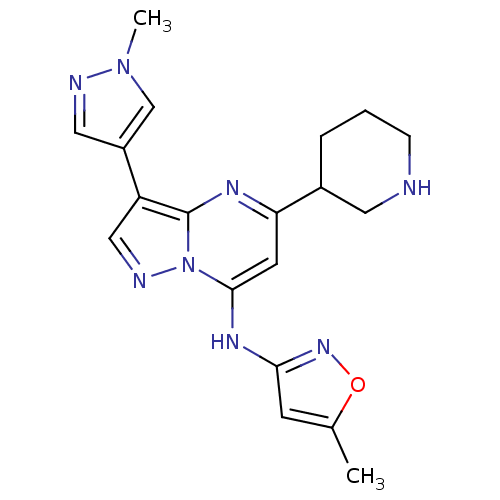 (5-methyl-N-(3-(1-methyl-1H-pyrazol-4-yl)-5-(piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant His-CHK1 expressed in baculovirus after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 467-70 (2010) Article DOI: 10.1016/j.bmcl.2010.10.113 BindingDB Entry DOI: 10.7270/Q2J966MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM105237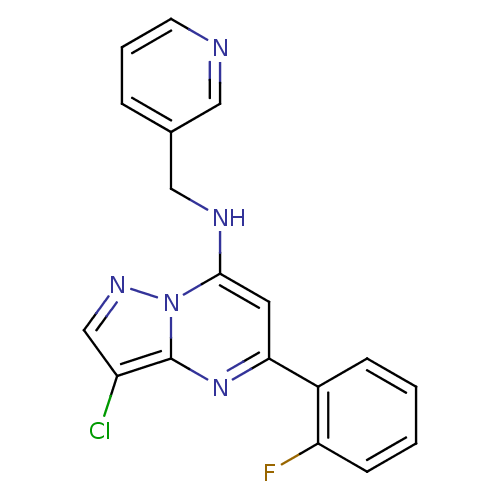 (US8575203, I-4 | US8580782, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50442676 (CHEMBL2442305) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim2 (unknown origin) using STK1 as substrate preincubated for 30 mins followed by substrate and ATP addition after 60 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM105235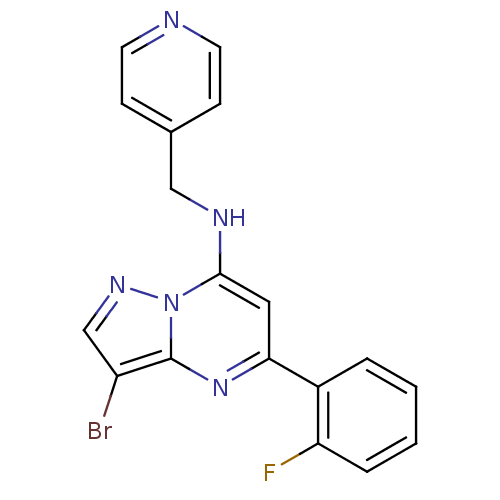 (US8580782, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334870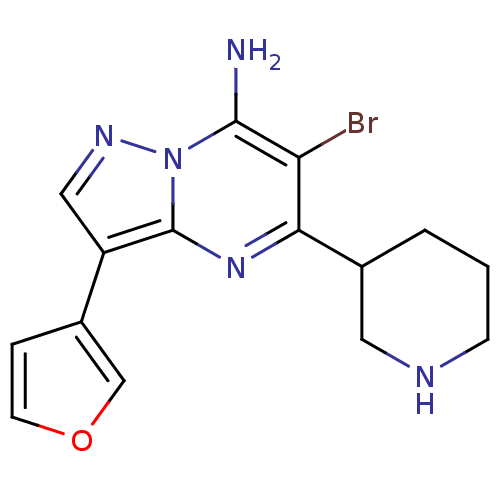 (6-bromo-3-(furan-3-yl)-5-(piperidin-3-yl)pyrazolo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442689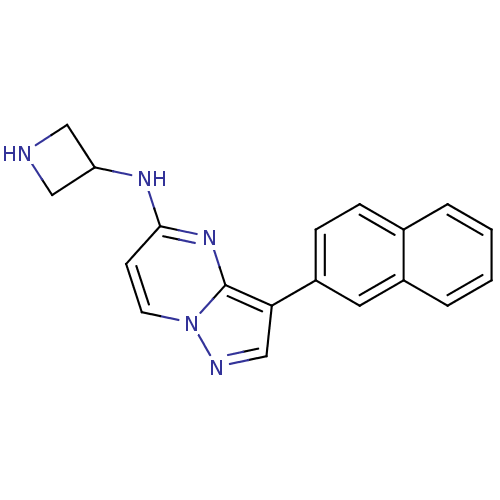 (CHEMBL2442297) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334859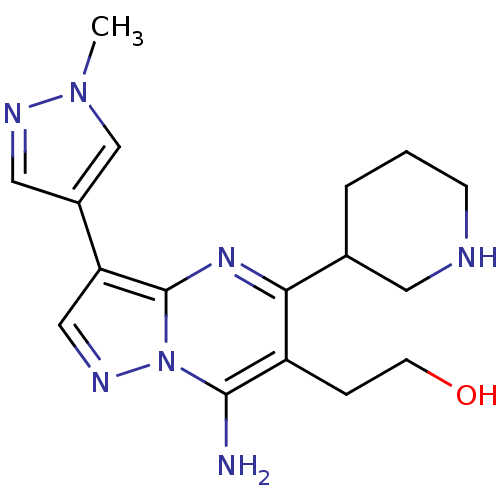 (2-(7-amino-3-(1-methyl-1H-pyrazol-4-yl)-5-(piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50543904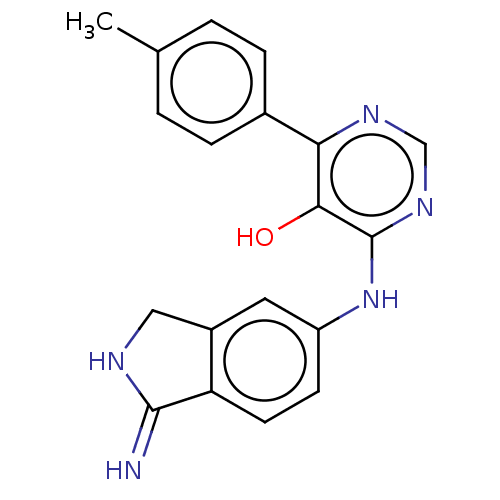 (CHEMBL4648414) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of activated factor IX (unknown origin) using CH3SO2-D-CHG-Gly-Arg-pNA:AcOH as substrate by fluorescence based assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127279 BindingDB Entry DOI: 10.7270/Q2JH3QSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50223615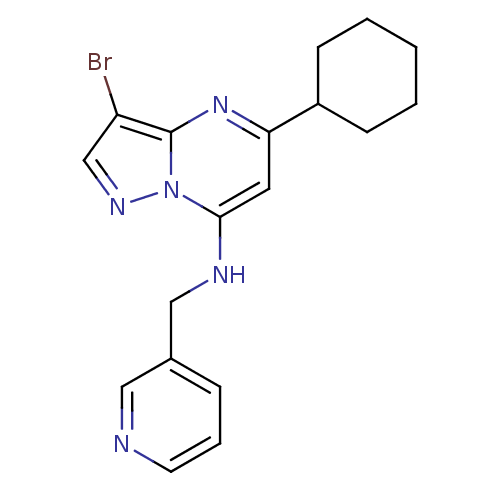 (3-bromo-5-cyclohexyl-N-(pyridin-3-ylmethyl)pyrazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of human GSK3beta | Bioorg Med Chem Lett 17: 6220-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.017 BindingDB Entry DOI: 10.7270/Q27M07ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 347 total ) | Next | Last >> |