

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50507816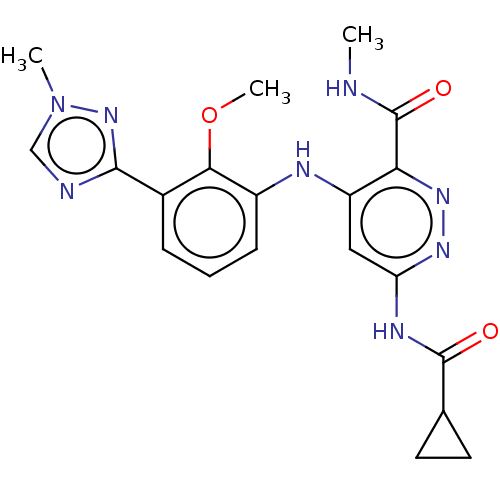 (Bms-986165 | Deucravacitinib) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of fluorescein labeled probe binding to His-tagged human TYK2 pseudokinase domain (575-869 residues) by Morrison titration based HTRF assa... | J Med Chem 62: 8973-8995 (2019) Article DOI: 10.1021/acs.jmedchem.9b00444 BindingDB Entry DOI: 10.7270/Q2930XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198694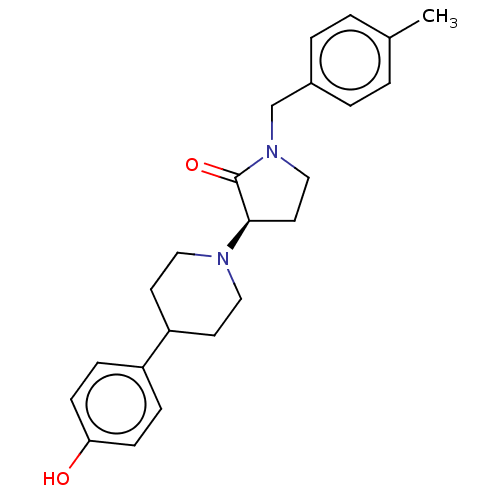 (US9221796, 23b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198665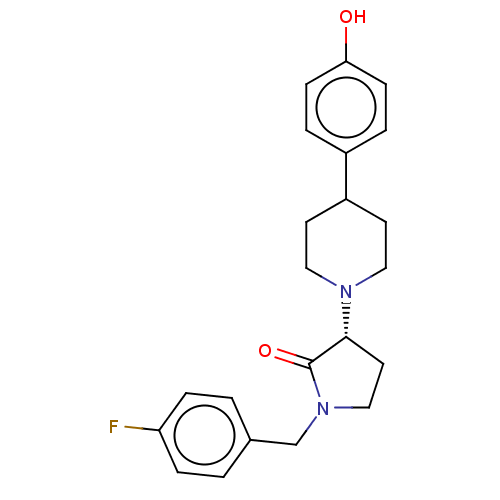 (US9221796, 2b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198728 (US9221796, 46, P-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198726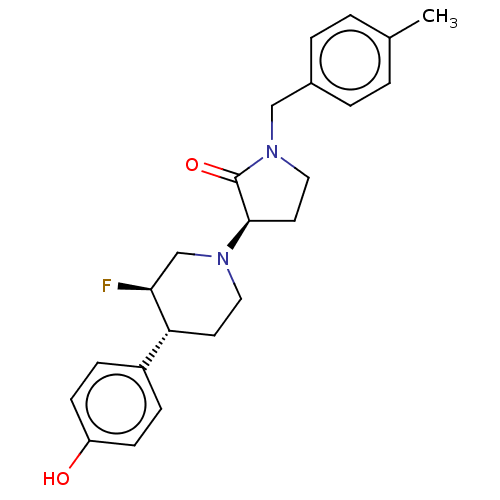 (US9221796, 46, P-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50330324 (CHEMBL4170867) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM198728 (US9221796, 46, P-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to GluN2B receptor in human cortex | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50330409 (CHEMBL4168402) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50330410 (CHEMBL4161899) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198735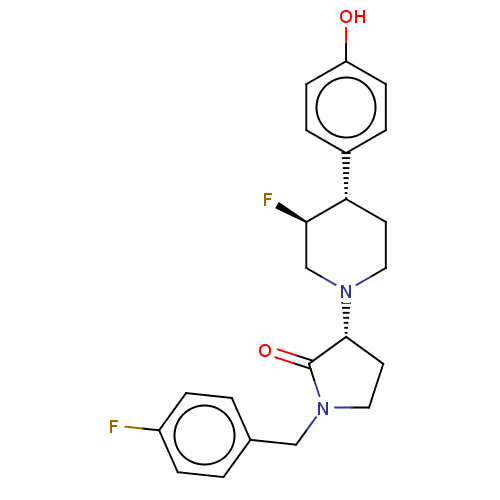 (US9221796, 48, P-3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM141485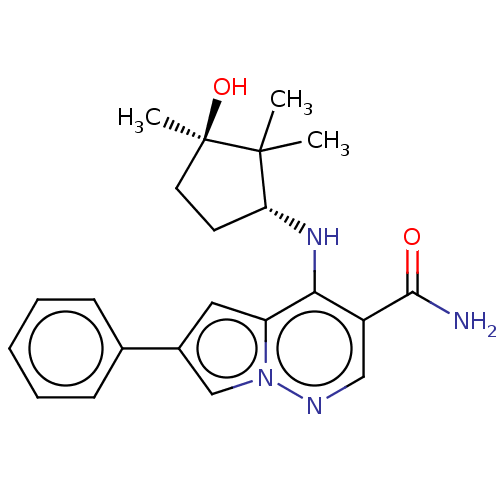 (US8921368, 293) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substr... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM151040 (US8987268, 59) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50507816 (Bms-986165 | Deucravacitinib) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Allosteric inhibition of fluorescein labeled probe binding to His-tagged recombinant human TYK2 pseudokinase JH2 domain (575-869 residues) incubated ... | J Med Chem 62: 8973-8995 (2019) Article DOI: 10.1021/acs.jmedchem.9b00444 BindingDB Entry DOI: 10.7270/Q2930XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM151063 (US8987268, 184) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141485 (US8921368, 293) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM151051 (US8987268, 105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141459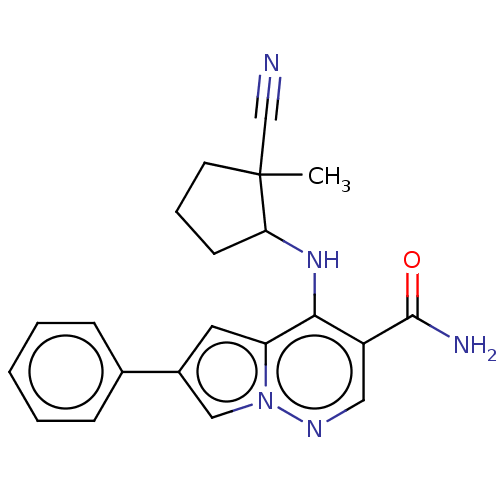 (US8921368, 123) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM151084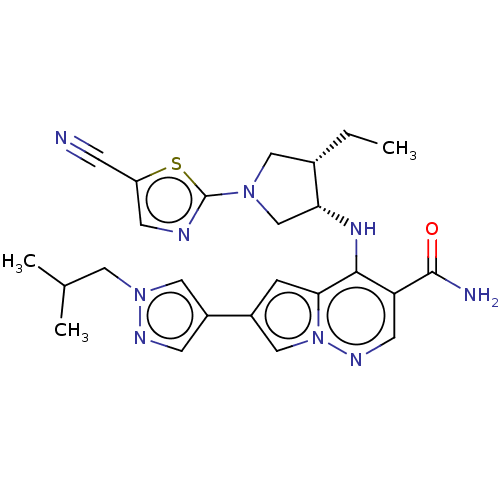 (US8987268, 183) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM230106 (US10106559, Example 31 | US10435415, Example 31 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141480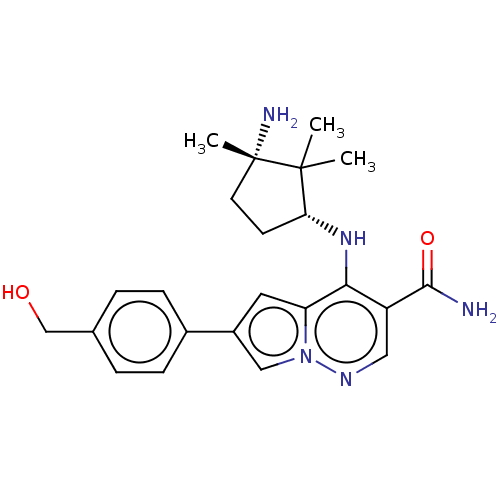 (US8921368, 271) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM151083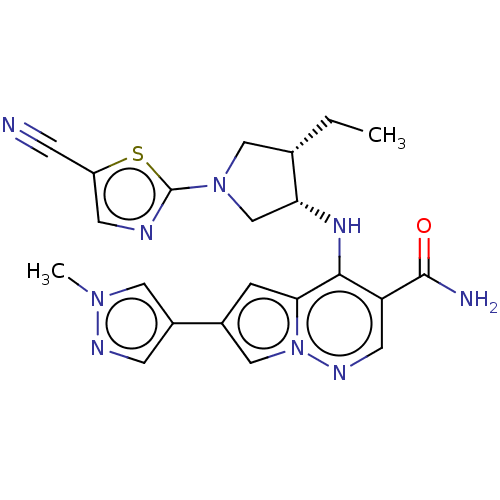 (US8987268, 182) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM151064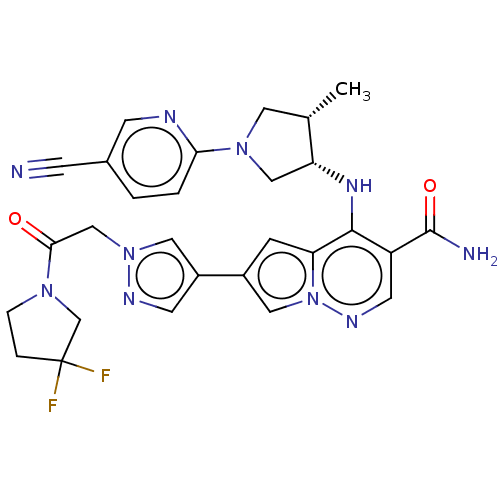 (US8987268, 185) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM151042 (US8987268, 61) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141456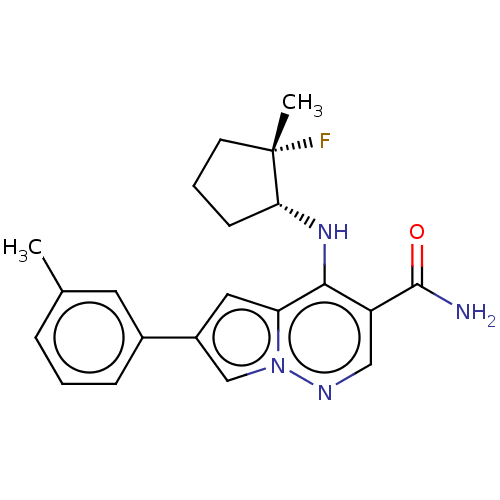 (US8921368, 72) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM151072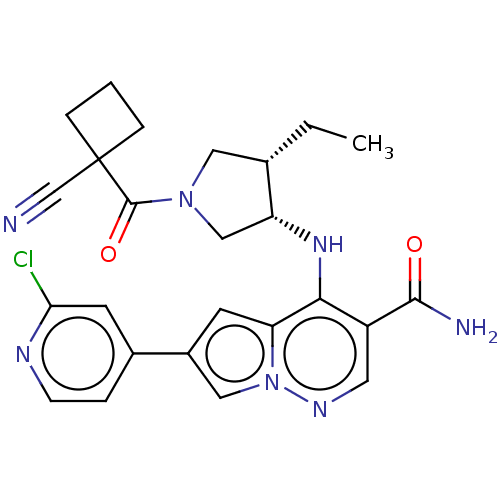 (US8987268, 257) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM151047 (US8987268, 70) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141498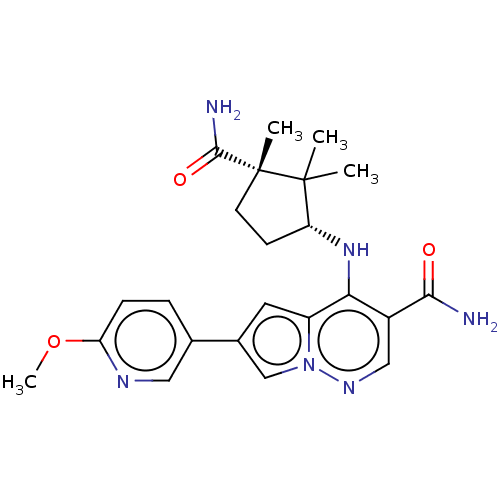 (US8921368, 338) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM151044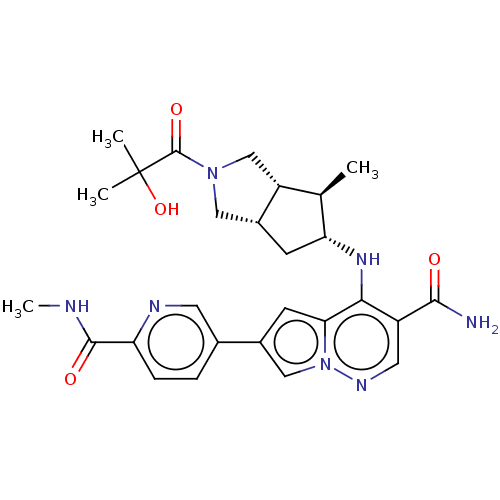 (US8987268, 63) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM151063 (US8987268, 184) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM151061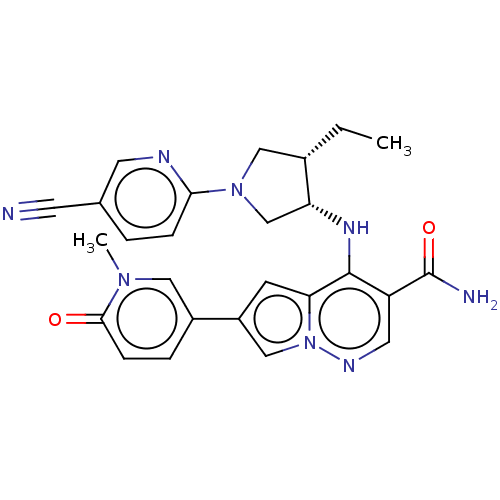 (US8987268, 173) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM151049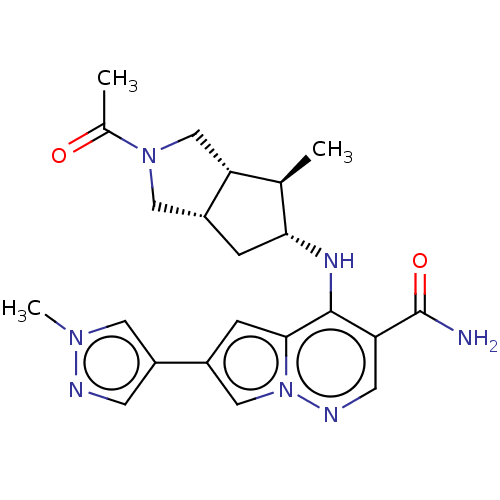 (US8987268, 93) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM151047 (US8987268, 70) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM151045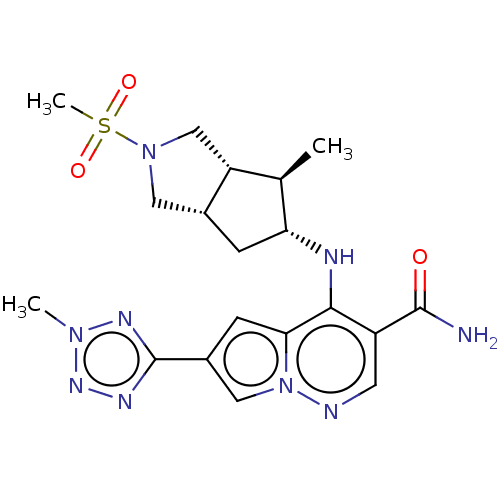 (US8987268, 67) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM151042 (US8987268, 61) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141497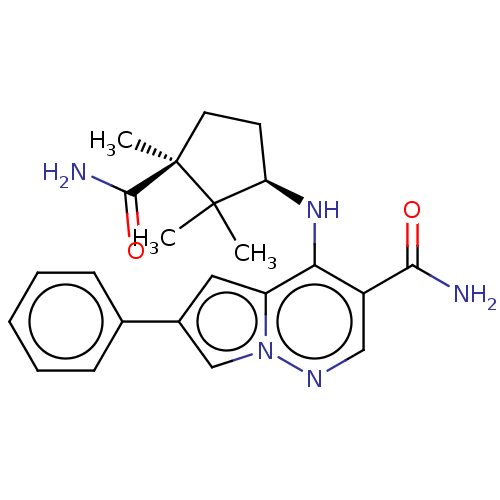 (US8921368, 337) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM151085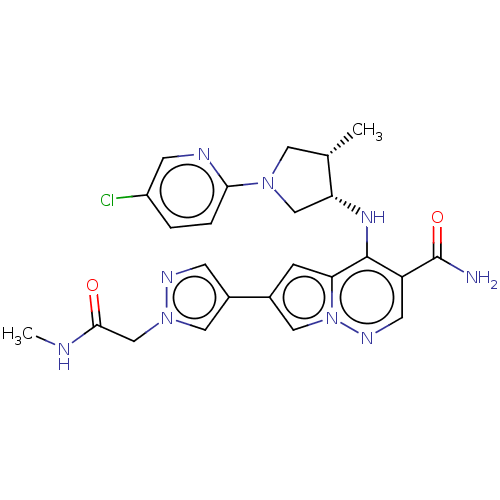 (US8987268, 188) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267827 (CHEMBL4076794) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267796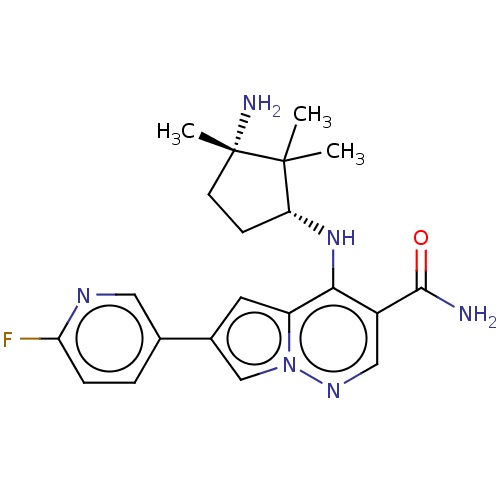 (CHEMBL4098840) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50267826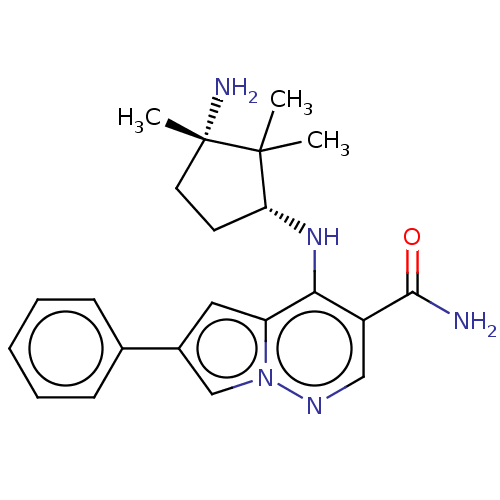 (CHEMBL4096145) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of GST-tagged JAK1 (unknown origin) after 3 hrs by Caliper assay | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50547850 (CHEMBL4741099) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00335 BindingDB Entry DOI: 10.7270/Q2PN9966 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267804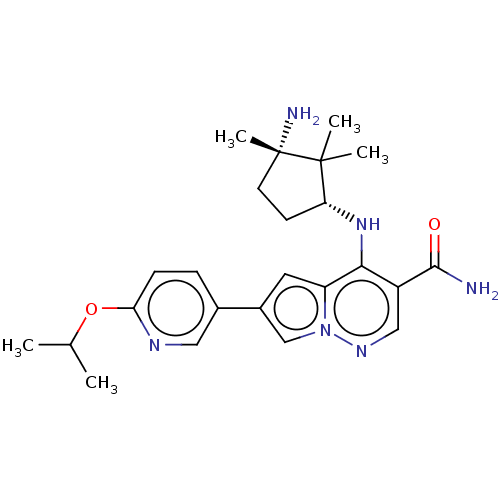 (CHEMBL4070136) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM151075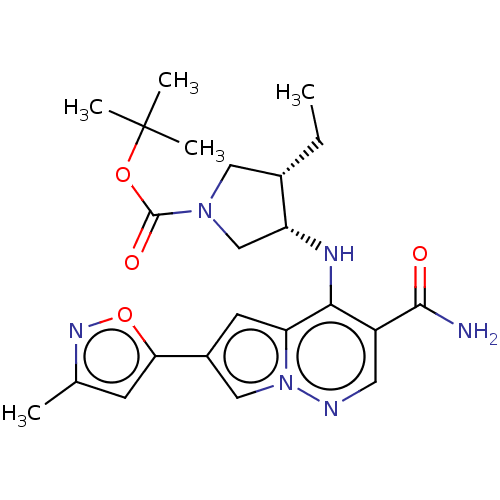 (US8987268, 292) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141457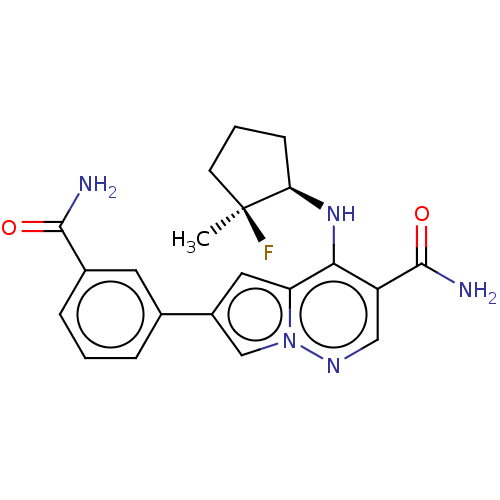 (US8921368, 94) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50267806 (CHEMBL4071255) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of GST-tagged JAK1 (unknown origin) after 3 hrs by Caliper assay | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM151085 (US8987268, 188) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141463 (US8921368, 159) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM151076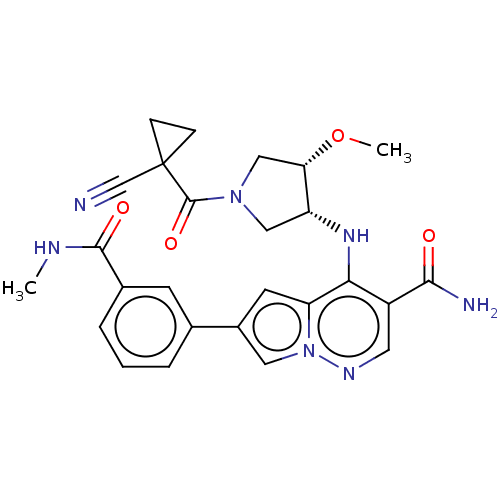 (US8987268, 303) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM151070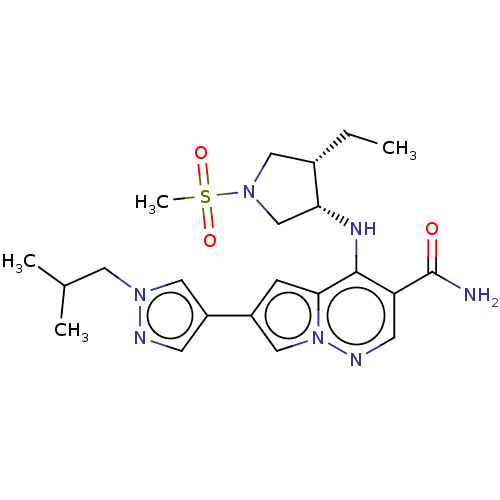 (US8987268, 239) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM151066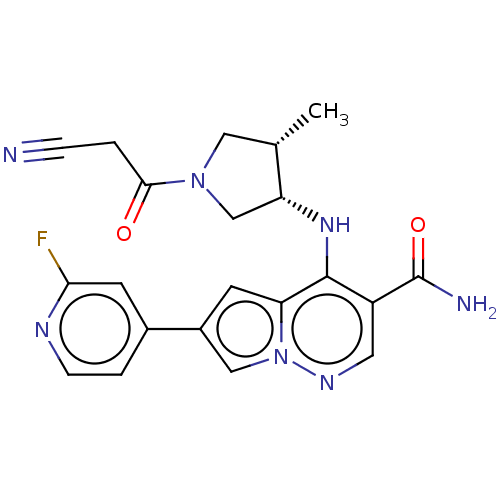 (US8987268, 201) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substrat... | US Patent US8987268 (2015) BindingDB Entry DOI: 10.7270/Q2NG4PC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141488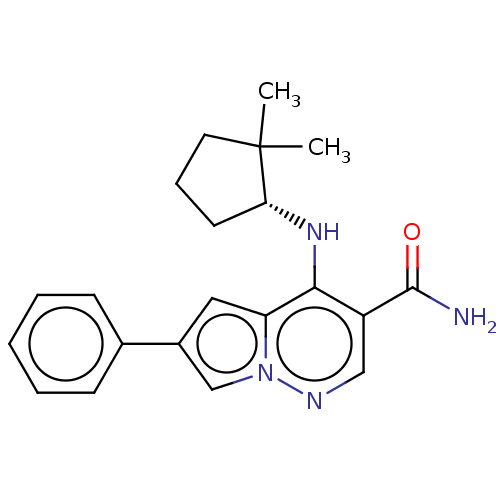 (US8921368, 304) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1272 total ) | Next | Last >> |