 Found 69 hits with Last Name = 'knobelsdorf' and Initial = 'ja'
Found 69 hits with Last Name = 'knobelsdorf' and Initial = 'ja' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50071235
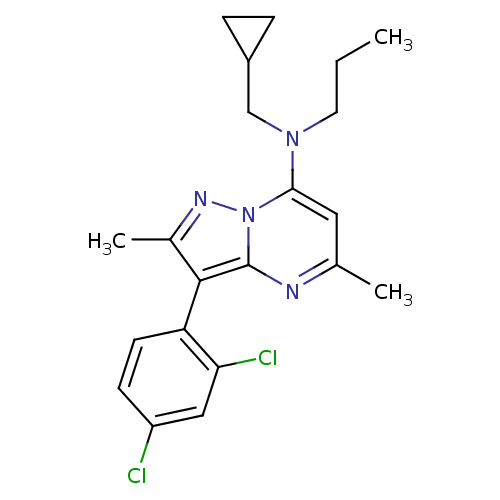
(CHEMBL65078 | Cyclopropylmethyl-[3-(2,4-dichloro-p...)Show SMILES CCCN(CC1CC1)c1cc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl |(17.18,-6.55,;17.15,-8.09,;15.79,-8.82,;15.75,-10.36,;17.06,-11.17,;17.01,-12.71,;18.31,-13.5,;15.65,-13.44,;14.4,-11.1,;14.35,-12.63,;12.98,-13.38,;12.93,-14.91,;11.67,-12.57,;11.72,-11.01,;10.61,-9.94,;11.28,-8.56,;10.55,-7.21,;12.81,-8.78,;13.09,-10.29,;9.11,-10.22,;8.1,-9.04,;6.59,-9.31,;6.08,-10.76,;4.57,-11.03,;7.07,-11.93,;8.59,-11.67,;9.57,-12.83,)| Show InChI InChI=1S/C21H24Cl2N4/c1-4-9-26(12-15-5-6-15)19-10-13(2)24-21-20(14(3)25-27(19)21)17-8-7-16(22)11-18(17)23/h7-8,10-11,15H,4-6,9,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-0-CRF from human Corticotropin releasing factor receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 8: 2067-70 (1999)
BindingDB Entry DOI: 10.7270/Q2125RTC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50071234
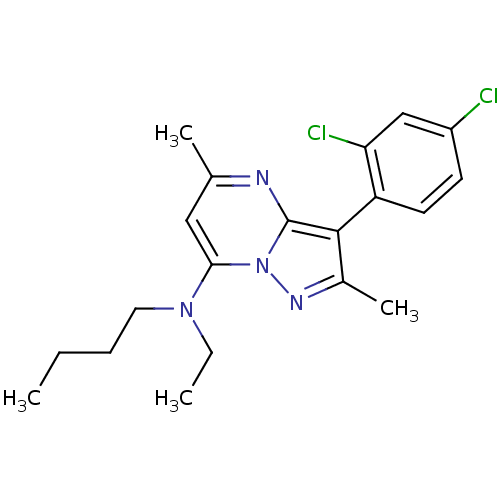
(Butyl-[3-(2,4-dichloro-phenyl)-2,5-dimethyl-pyrazo...)Show SMILES CCCCN(CC)c1cc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl |(3.7,2.11,;2.35,2.88,;1.03,2.11,;-.31,2.86,;-1.64,2.07,;-2.97,2.84,;-4.32,2.07,;-1.64,.53,;-2.97,-.24,;-2.97,-1.78,;-4.29,-2.55,;-1.63,-2.53,;-.29,-1.76,;1.19,-2.22,;2.06,-.96,;3.6,-.94,;1.15,.28,;-.31,-.22,;1.68,-3.67,;.65,-4.82,;1.13,-6.29,;2.64,-6.59,;3.15,-8.05,;3.67,-5.42,;3.18,-3.96,;4.18,-2.8,)| Show InChI InChI=1S/C20H24Cl2N4/c1-5-7-10-25(6-2)18-11-13(3)23-20-19(14(4)24-26(18)20)16-9-8-15(21)12-17(16)22/h8-9,11-12H,5-7,10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-0-CRF from human Corticotropin releasing factor receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 8: 2067-70 (1999)
BindingDB Entry DOI: 10.7270/Q2125RTC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50071238

(3-(2,4-Dichloro-phenyl)-2,5-dimethyl-7-(2-propyl-p...)Show SMILES CCCC1CCCCN1c1cc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl |(11.28,.47,;9.94,1.24,;8.62,.47,;7.28,1.24,;7.28,2.78,;5.94,3.56,;4.61,2.78,;4.61,1.24,;5.94,.45,;5.94,-1.09,;4.61,-1.86,;4.61,-3.41,;3.28,-4.18,;5.94,-4.18,;7.27,-3.41,;8.75,-3.89,;9.68,-2.63,;11.21,-2.63,;8.75,-1.36,;7.27,-1.86,;9.23,-5.35,;10.73,-5.65,;11.21,-7.11,;10.19,-8.27,;10.65,-9.72,;8.68,-7.95,;8.2,-6.48,;6.7,-6.15,)| Show InChI InChI=1S/C22H26Cl2N4/c1-4-7-17-8-5-6-11-27(17)20-12-14(2)25-22-21(15(3)26-28(20)22)18-10-9-16(23)13-19(18)24/h9-10,12-13,17H,4-8,11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-0-CRF from human Corticotropin releasing factor receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 8: 2067-70 (1999)
BindingDB Entry DOI: 10.7270/Q2125RTC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50071229

(CHEMBL302645 | [3-(2,4-Dichloro-phenyl)-2,5-dimeth...)Show SMILES CCN(CCOC)c1cc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl |(7.35,2.01,;7.38,.47,;6.03,-.32,;4.69,.45,;4.69,1.99,;3.34,2.76,;3.34,4.31,;6.03,-1.86,;4.69,-2.63,;4.69,-4.18,;3.37,-4.94,;6.03,-4.94,;7.35,-4.18,;8.82,-4.66,;9.75,-3.4,;11.29,-3.4,;8.82,-2.13,;7.35,-2.63,;9.3,-6.12,;10.81,-6.42,;11.29,-7.88,;10.26,-9.04,;10.74,-10.49,;8.76,-8.71,;8.28,-7.25,;6.77,-6.92,)| Show InChI InChI=1S/C19H22Cl2N4O/c1-5-24(8-9-26-4)17-10-12(2)22-19-18(13(3)23-25(17)19)15-7-6-14(20)11-16(15)21/h6-7,10-11H,5,8-9H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-0-CRF from human Corticotropin releasing factor receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 8: 2067-70 (1999)
BindingDB Entry DOI: 10.7270/Q2125RTC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50071233
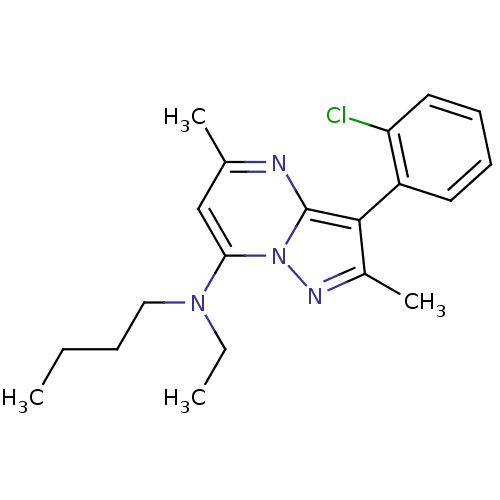
(Butyl-[3-(2-chloro-phenyl)-2,5-dimethyl-pyrazolo[1...)Show SMILES CCCCN(CC)c1cc(C)nc2c(c(C)nn12)-c1ccccc1Cl |(8.21,3.42,;8.21,1.88,;6.89,1.11,;6.89,-.43,;5.55,-1.22,;4.23,-.45,;4.23,1.09,;5.55,-2.76,;4.23,-3.52,;4.23,-5.07,;2.89,-5.84,;5.55,-5.84,;6.89,-5.07,;8.36,-5.55,;9.29,-4.29,;10.81,-4.29,;8.36,-3.02,;6.89,-3.52,;8.84,-7.02,;7.82,-8.15,;8.3,-9.61,;9.81,-9.93,;10.83,-8.78,;10.35,-7.32,;11.38,-6.16,)| Show InChI InChI=1S/C20H25ClN4/c1-5-7-12-24(6-2)18-13-14(3)22-20-19(15(4)23-25(18)20)16-10-8-9-11-17(16)21/h8-11,13H,5-7,12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-0-CRF from human Corticotropin releasing factor receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 8: 2067-70 (1999)
BindingDB Entry DOI: 10.7270/Q2125RTC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50071226

(Butyl-[3-(2,4-dichloro-phenyl)-5-methyl-pyrazolo[1...)Show InChI InChI=1S/C19H22Cl2N4/c1-4-6-9-24(5-2)18-10-13(3)23-19-16(12-22-25(18)19)15-8-7-14(20)11-17(15)21/h7-8,10-12H,4-6,9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-0-CRF from human Corticotropin releasing factor receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 8: 2067-70 (1999)
BindingDB Entry DOI: 10.7270/Q2125RTC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50071232

(3-(2,4-Dichloro-phenyl)-2,5-dimethyl-7-piperidin-1...)Show SMILES Cc1nn2c(cc(C)nc2c1-c1ccc(Cl)cc1Cl)N1CCCCC1 |(11.25,-2.63,;9.71,-2.63,;8.78,-1.36,;7.31,-1.86,;5.97,-1.09,;4.65,-1.86,;4.65,-3.41,;3.31,-4.18,;5.97,-4.18,;7.31,-3.41,;8.78,-3.89,;9.26,-5.35,;10.77,-5.65,;11.25,-7.11,;10.22,-8.27,;10.68,-9.72,;8.71,-7.95,;8.23,-6.48,;6.73,-6.15,;5.97,.45,;7.32,1.24,;7.32,2.78,;5.99,3.56,;4.65,2.78,;4.65,1.24,)| Show InChI InChI=1S/C19H20Cl2N4/c1-12-10-17(24-8-4-3-5-9-24)25-19(22-12)18(13(2)23-25)15-7-6-14(20)11-16(15)21/h6-7,10-11H,3-5,8-9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-0-CRF from human Corticotropin releasing factor receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 8: 2067-70 (1999)
BindingDB Entry DOI: 10.7270/Q2125RTC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50058163
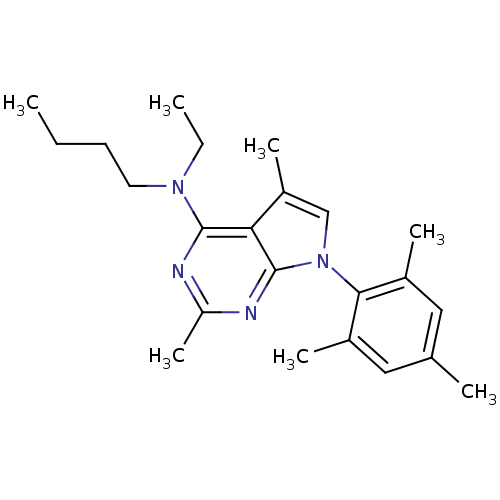
(Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...)Show SMILES CCCCN(CC)c1nc(C)nc2n(cc(C)c12)-c1c(C)cc(C)cc1C |(-10.62,2.69,;-9.29,3.46,;-7.95,2.7,;-6.62,3.48,;-5.28,2.71,;-3.95,3.49,;-3.96,5.03,;-5.27,1.17,;-6.6,.4,;-6.6,-1.14,;-7.94,-1.91,;-5.27,-1.91,;-3.92,-1.14,;-2.44,-1.61,;-1.54,-.35,;-2.46,.9,;-1.99,2.37,;-3.93,.41,;-1.96,-3.07,;-2.99,-4.22,;-4.49,-3.91,;-2.5,-5.68,;-.99,-5.99,;-.51,-7.46,;.03,-4.83,;-.46,-3.38,;.56,-2.22,)| Show InChI InChI=1S/C23H32N4/c1-8-10-11-26(9-2)22-20-18(6)14-27(23(20)25-19(7)24-22)21-16(4)12-15(3)13-17(21)5/h12-14H,8-11H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-0-CRF from human Corticotropin releasing factor receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 8: 2067-70 (1999)
BindingDB Entry DOI: 10.7270/Q2125RTC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50071228

(Butyl-[3-(2,4-dichloro-phenyl)-2,5-dimethyl-pyrazo...)Show SMILES CCCCNc1cc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl |(8.63,4.31,;8.65,2.78,;7.31,2.01,;7.32,.47,;5.97,-.32,;5.97,-1.86,;4.65,-2.63,;4.65,-4.18,;3.31,-4.94,;5.97,-4.94,;7.31,-4.18,;8.78,-4.66,;9.71,-3.4,;11.25,-3.4,;8.78,-2.13,;7.31,-2.63,;9.26,-6.12,;10.77,-6.42,;11.25,-7.88,;10.22,-9.04,;10.68,-10.49,;8.71,-8.71,;8.23,-7.25,;6.73,-6.92,)| Show InChI InChI=1S/C18H20Cl2N4/c1-4-5-8-21-16-9-11(2)22-18-17(12(3)23-24(16)18)14-7-6-13(19)10-15(14)20/h6-7,9-10,21H,4-5,8H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-0-CRF from human Corticotropin releasing factor receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 8: 2067-70 (1999)
BindingDB Entry DOI: 10.7270/Q2125RTC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50071237
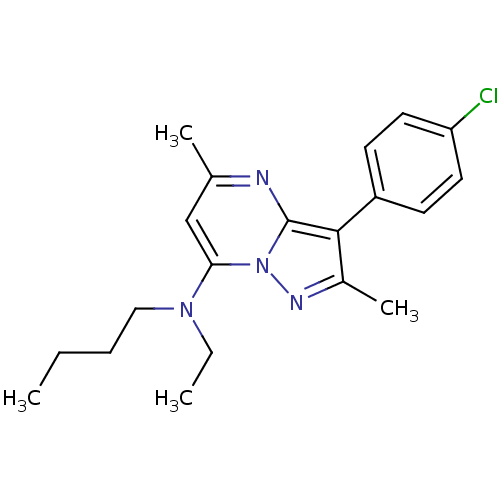
(Butyl-[3-(4-chloro-phenyl)-2,5-dimethyl-pyrazolo[1...)Show SMILES CCCCN(CC)c1cc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C20H25ClN4/c1-5-7-12-24(6-2)18-13-14(3)22-20-19(15(4)23-25(18)20)16-8-10-17(21)11-9-16/h8-11,13H,5-7,12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-0-CRF from human Corticotropin releasing factor receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 8: 2067-70 (1999)
BindingDB Entry DOI: 10.7270/Q2125RTC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50071230

(Butyl-[2,5-dimethyl-3-(2,4,6-trichloro-phenyl)-pyr...)Show SMILES CCCCN(CC)c1cc(C)nc2c(c(C)nn12)-c1c(Cl)cc(Cl)cc1Cl |(8.21,4.32,;8.21,2.78,;6.89,2.01,;6.89,.47,;5.55,-.32,;4.23,.45,;4.23,1.99,;5.55,-1.86,;4.23,-2.63,;4.23,-4.18,;2.89,-4.95,;5.55,-4.95,;6.89,-4.18,;8.36,-4.66,;9.29,-3.4,;10.81,-3.4,;8.36,-2.13,;6.89,-2.63,;8.84,-6.12,;10.35,-6.42,;11.38,-5.27,;10.83,-7.88,;9.81,-9.04,;10.26,-10.49,;8.3,-8.72,;7.82,-7.25,;6.31,-6.92,)| Show InChI InChI=1S/C20H23Cl3N4/c1-5-7-8-26(6-2)17-9-12(3)24-20-18(13(4)25-27(17)20)19-15(22)10-14(21)11-16(19)23/h9-11H,5-8H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-0-CRF from human Corticotropin releasing factor receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 8: 2067-70 (1999)
BindingDB Entry DOI: 10.7270/Q2125RTC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50071227
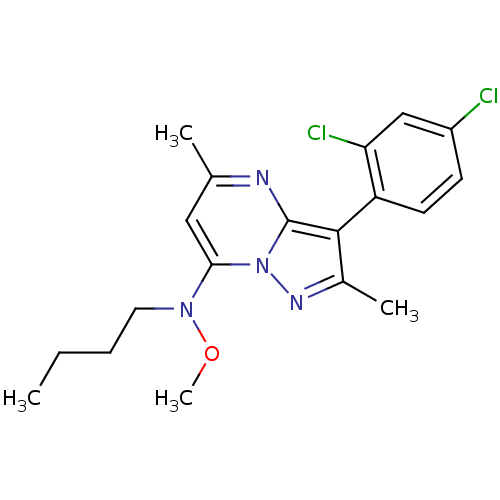
(CHEMBL65574 | N-Butyl-N-[3-(2,4-dichloro-phenyl)-2...)Show SMILES CCCCN(OC)c1cc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl |(8.63,4.31,;8.65,2.78,;7.31,2.01,;7.32,.47,;5.97,-.32,;4.65,.47,;4.65,2.01,;5.97,-1.86,;4.65,-2.63,;4.65,-4.18,;3.31,-4.94,;5.97,-4.94,;7.31,-4.18,;8.78,-4.66,;9.71,-3.4,;11.25,-3.4,;8.78,-2.13,;7.31,-2.63,;9.26,-6.12,;10.77,-6.42,;11.25,-7.88,;10.22,-9.04,;10.68,-10.49,;8.71,-8.71,;8.23,-7.25,;6.73,-6.92,)| Show InChI InChI=1S/C19H22Cl2N4O/c1-5-6-9-24(26-4)17-10-12(2)22-19-18(13(3)23-25(17)19)15-8-7-14(20)11-16(15)21/h7-8,10-11H,5-6,9H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-0-CRF from human Corticotropin releasing factor receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 8: 2067-70 (1999)
BindingDB Entry DOI: 10.7270/Q2125RTC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50071236

(3-(2,4-Dichloro-phenyl)-2,5-dimethyl-7-morpholin-4...)Show SMILES Cc1nn2c(cc(C)nc2c1-c1ccc(Cl)cc1Cl)N1CCOCC1 |(11.25,-2.67,;9.71,-2.67,;8.78,-1.39,;7.31,-1.9,;5.97,-1.13,;4.65,-1.9,;4.65,-3.44,;3.31,-4.21,;5.97,-4.21,;7.31,-3.44,;8.78,-3.92,;9.26,-5.39,;10.77,-5.68,;11.25,-7.16,;10.22,-8.31,;10.68,-9.75,;8.72,-7.99,;8.24,-6.52,;6.73,-6.2,;5.97,.41,;7.32,1.21,;7.32,2.75,;5.99,3.51,;4.65,2.75,;4.65,1.21,)| Show InChI InChI=1S/C18H18Cl2N4O/c1-11-9-16(23-5-7-25-8-6-23)24-18(21-11)17(12(2)22-24)14-4-3-13(19)10-15(14)20/h3-4,9-10H,5-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-0-CRF from human Corticotropin releasing factor receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 8: 2067-70 (1999)
BindingDB Entry DOI: 10.7270/Q2125RTC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50071231
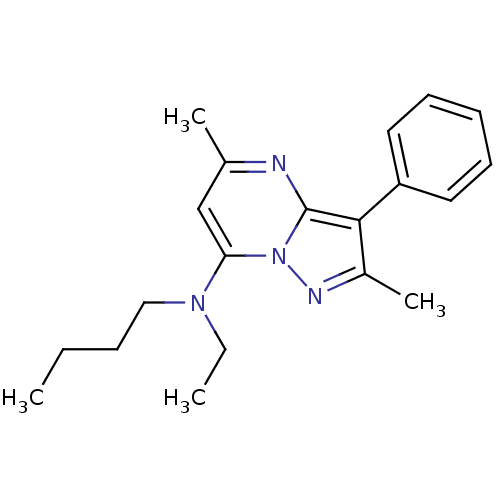
(Butyl-(2,5-dimethyl-3-phenyl-pyrazolo[1,5-a]pyrimi...)Show InChI InChI=1S/C20H26N4/c1-5-7-13-23(6-2)18-14-15(3)21-20-19(16(4)22-24(18)20)17-11-9-8-10-12-17/h8-12,14H,5-7,13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 511 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-0-CRF from human Corticotropin releasing factor receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 8: 2067-70 (1999)
BindingDB Entry DOI: 10.7270/Q2125RTC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50207400
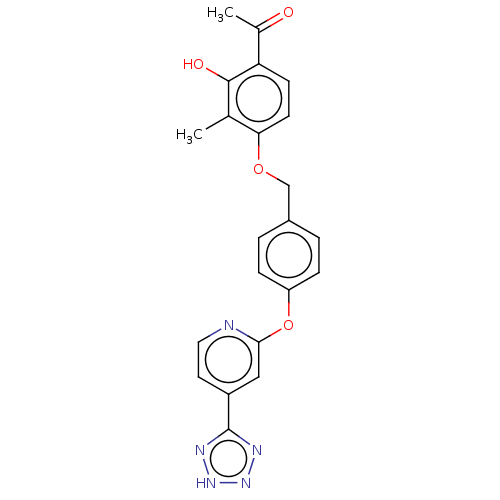
(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.79E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in CHO cells after 120 mins by scintillation counting method |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Activity at human 5HT1D receptor |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.97E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Activity at human 5HT1F receptor |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.02E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Activity at human 5HT1A receptor |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Activity at human 5T2B receptor |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.58E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Activity at human 5HT1B receptor |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.98E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT7 receptor expressed in CHO cells after 120 mins by scintillation counting method |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1E
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.37E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Activity at human 5HT1E receptor |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >6.31E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of human recombinant 5HT4E receptor expressed in CHO cells after 60 mins by scintillation counting method |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 5A
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >6.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of human 5HT5A receptor expressed in HEK293 cells after 120 mins by scintillation counting method |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >7.47E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Activity at human 5HT2A receptor |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >7.87E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Activity at human 5T2C receptor |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50207410
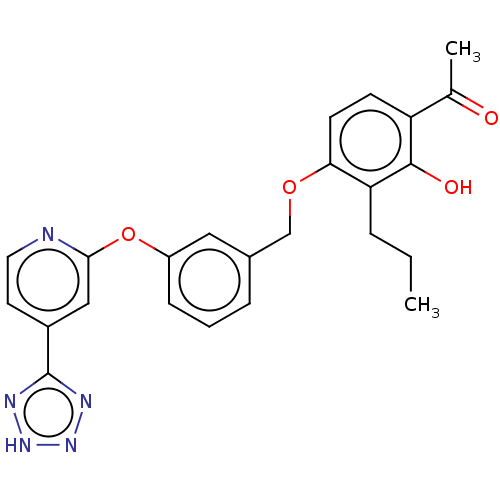
(CHEMBL3909995)Show SMILES CCCc1c(OCc2cccc(Oc3cc(ccn3)-c3nn[nH]n3)c2)ccc(C(C)=O)c1O Show InChI InChI=1S/C24H23N5O4/c1-3-5-20-21(9-8-19(15(2)30)23(20)31)32-14-16-6-4-7-18(12-16)33-22-13-17(10-11-25-22)24-26-28-29-27-24/h4,6-13,31H,3,5,14H2,1-2H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50207406
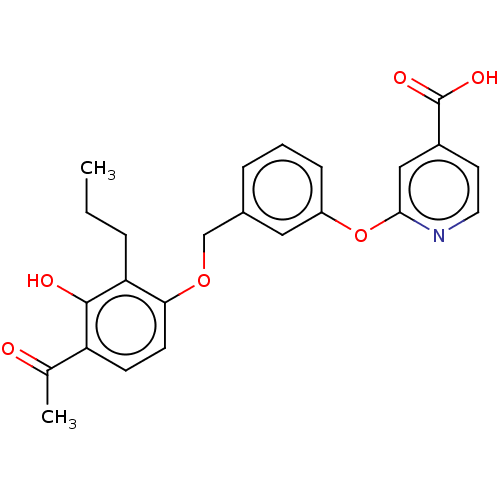
(CHEMBL3890848)Show SMILES CCCc1c(OCc2cccc(Oc3cc(ccn3)C(O)=O)c2)ccc(C(C)=O)c1O Show InChI InChI=1S/C24H23NO6/c1-3-5-20-21(9-8-19(15(2)26)23(20)27)30-14-16-6-4-7-18(12-16)31-22-13-17(24(28)29)10-11-25-22/h4,6-13,27H,3,5,14H2,1-2H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50207398
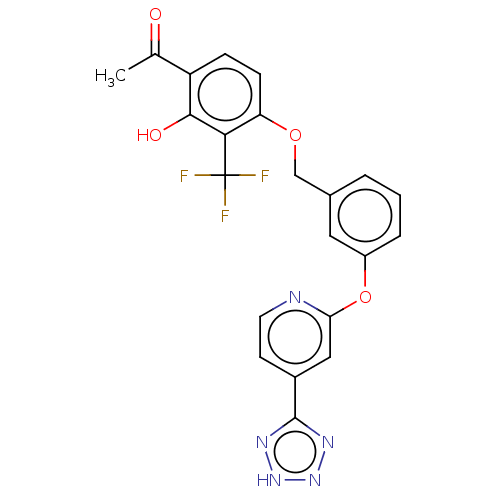
(CHEMBL3965244)Show SMILES CC(=O)c1ccc(OCc2cccc(Oc3cc(ccn3)-c3nn[nH]n3)c2)c(c1O)C(F)(F)F Show InChI InChI=1S/C22H16F3N5O4/c1-12(31)16-5-6-17(19(20(16)32)22(23,24)25)33-11-13-3-2-4-15(9-13)34-18-10-14(7-8-26-18)21-27-29-30-28-21/h2-10,32H,11H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50207404

(CHEMBL3935529)Show SMILES CCCc1c(OCc2ccc(Oc3ccc(cn3)C(O)=O)cc2)ccc(C(C)=O)c1O Show InChI InChI=1S/C24H23NO6/c1-3-4-20-21(11-10-19(15(2)26)23(20)27)30-14-16-5-8-18(9-6-16)31-22-12-7-17(13-25-22)24(28)29/h5-13,27H,3-4,14H2,1-2H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50207411
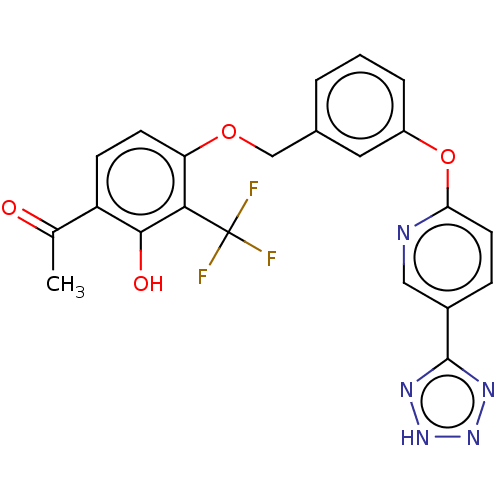
(CHEMBL3973048)Show SMILES CC(=O)c1ccc(OCc2cccc(Oc3ccc(cn3)-c3nn[nH]n3)c2)c(c1O)C(F)(F)F Show InChI InChI=1S/C22H16F3N5O4/c1-12(31)16-6-7-17(19(20(16)32)22(23,24)25)33-11-13-3-2-4-15(9-13)34-18-8-5-14(10-26-18)21-27-29-30-28-21/h2-10,32H,11H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50207399

(CHEMBL3913281)Show SMILES CCCc1c(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)ccc(C(C)=O)c1O Show InChI InChI=1S/C24H23N5O4/c1-3-4-20-21(10-9-19(15(2)30)23(20)31)32-14-16-5-7-18(8-6-16)33-22-13-17(11-12-25-22)24-26-28-29-27-24/h5-13,31H,3-4,14H2,1-2H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50207405
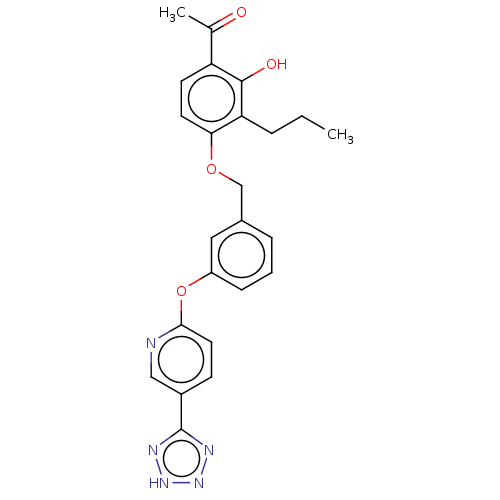
(CHEMBL3918914)Show SMILES CCCc1c(OCc2cccc(Oc3ccc(cn3)-c3nn[nH]n3)c2)ccc(C(C)=O)c1O Show InChI InChI=1S/C24H23N5O4/c1-3-5-20-21(10-9-19(15(2)30)23(20)31)32-14-16-6-4-7-18(12-16)33-22-11-8-17(13-25-22)24-26-28-29-27-24/h4,6-13,31H,3,5,14H2,1-2H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50207413

(CHEMBL3945882)Show SMILES CCCc1c(OCc2ccc(Oc3ncccc3-c3nn[nH]n3)cc2)ccc(C(C)=O)c1O Show InChI InChI=1S/C24H23N5O4/c1-3-5-19-21(12-11-18(15(2)30)22(19)31)32-14-16-7-9-17(10-8-16)33-24-20(6-4-13-25-24)23-26-28-29-27-23/h4,6-13,31H,3,5,14H2,1-2H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50207397
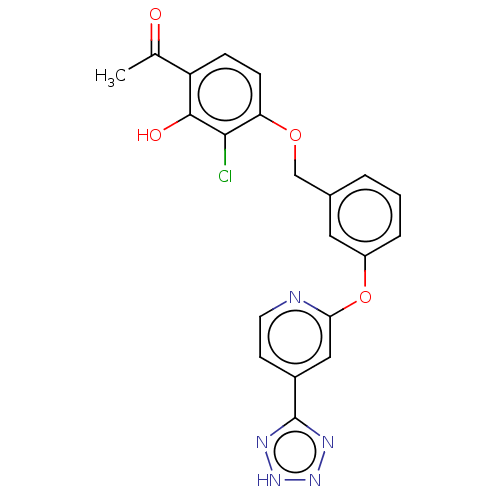
(CHEMBL3927834)Show SMILES CC(=O)c1ccc(OCc2cccc(Oc3cc(ccn3)-c3nn[nH]n3)c2)c(Cl)c1O Show InChI InChI=1S/C21H16ClN5O4/c1-12(28)16-5-6-17(19(22)20(16)29)30-11-13-3-2-4-15(9-13)31-18-10-14(7-8-23-18)21-24-26-27-25-21/h2-10,29H,11H2,1H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 342 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
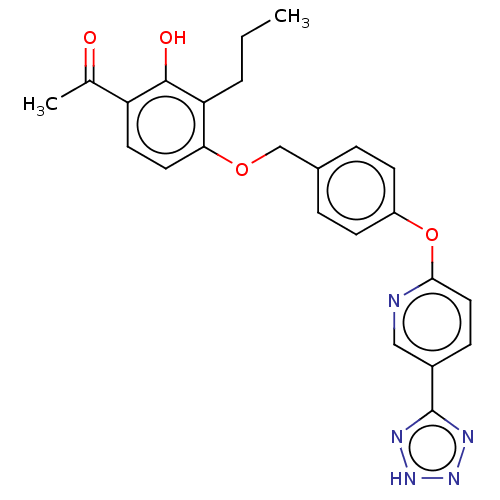
(Homo sapiens (Human)) | BDBM50207403
(CHEMBL3911282)Show SMILES CCCc1c(OCc2ccc(Oc3ccc(cn3)-c3nn[nH]n3)cc2)ccc(C(C)=O)c1O Show InChI InChI=1S/C24H23N5O4/c1-3-4-20-21(11-10-19(15(2)30)23(20)31)32-14-16-5-8-18(9-6-16)33-22-12-7-17(13-25-22)24-26-28-29-27-24/h5-13,31H,3-4,14H2,1-2H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 346 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50207402
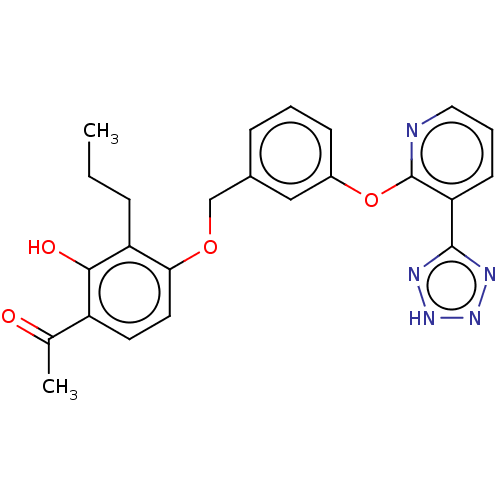
(CHEMBL3980125)Show SMILES CCCc1c(OCc2cccc(Oc3ncccc3-c3nn[nH]n3)c2)ccc(C(C)=O)c1O Show InChI InChI=1S/C24H23N5O4/c1-3-6-19-21(11-10-18(15(2)30)22(19)31)32-14-16-7-4-8-17(13-16)33-24-20(9-5-12-25-24)23-26-28-29-27-23/h4-5,7-13,31H,3,6,14H2,1-2H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50207407
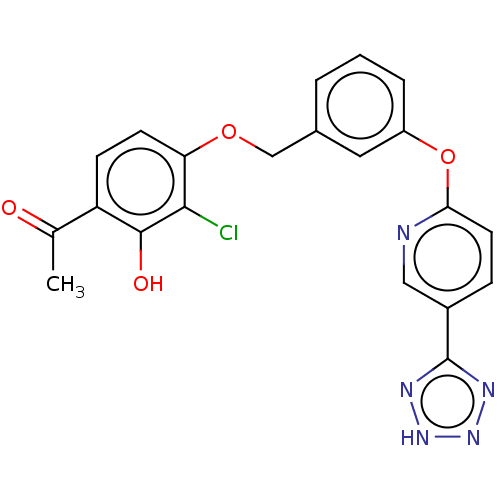
(CHEMBL3936830)Show SMILES CC(=O)c1ccc(OCc2cccc(Oc3ccc(cn3)-c3nn[nH]n3)c2)c(Cl)c1O Show InChI InChI=1S/C21H16ClN5O4/c1-12(28)16-6-7-17(19(22)20(16)29)30-11-13-3-2-4-15(9-13)31-18-8-5-14(10-23-18)21-24-26-27-25-21/h2-10,29H,11H2,1H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 475 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50207412

(CHEMBL3920609)Show SMILES CCCc1c(OCc2ccc(Oc3cccc(c3)-c3nn[nH]n3)nc2)ccc(C(C)=O)c1O Show InChI InChI=1S/C24H23N5O4/c1-3-5-20-21(10-9-19(15(2)30)23(20)31)32-14-16-8-11-22(25-13-16)33-18-7-4-6-17(12-18)24-26-28-29-27-24/h4,6-13,31H,3,5,14H2,1-2H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 484 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50207409
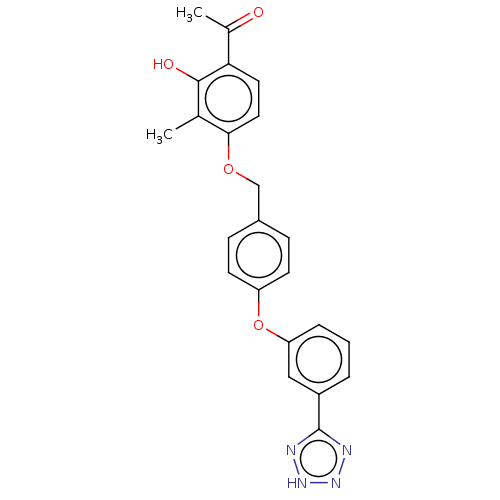
(CHEMBL3892611)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cccc(c3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C23H20N4O4/c1-14-21(11-10-20(15(2)28)22(14)29)30-13-16-6-8-18(9-7-16)31-19-5-3-4-17(12-19)23-24-26-27-25-23/h3-12,29H,13H2,1-2H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50207408

(CHEMBL3943547)Show SMILES CC(=O)c1ccc(OCc2cccc(Oc3ccc(cn3)-c3nn[nH]n3)c2)c(F)c1O Show InChI InChI=1S/C21H16FN5O4/c1-12(28)16-6-7-17(19(22)20(16)29)30-11-13-3-2-4-15(9-13)31-18-8-5-14(10-23-18)21-24-26-27-25-21/h2-10,29H,11H2,1H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CysLT1 receptor expressed in Syrian hamster AV12-664 cells assessed as decrease in LTD4-induced intracellular calcium le... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human mGlu1 receptor expressed in hamster AV12 cells expressing EAAT1 assessed as inhibition of glutamate-induced ... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human mGlu2 receptor expressed in hamster AV12 cells expressing EAAT1 assessed as inhibition of glutamate-induced ... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human mGlu3 receptor expressed in hamster AV12 cells expressing EAAT1 assessed as inhibition of glutamate-induced ... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human mGlu5 receptor expressed in hamster AV12 cells expressing EAAT1 assessed as inhibition of glutamate-induced ... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50207399

(CHEMBL3913281)Show SMILES CCCc1c(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)ccc(C(C)=O)c1O Show InChI InChI=1S/C24H23N5O4/c1-3-4-20-21(10-9-19(15(2)30)23(20)31)32-14-16-5-7-18(8-6-16)33-22-13-17(11-12-25-22)24-26-28-29-27-24/h5-13,31H,3-4,14H2,1-2H3,(H,26,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 470 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of recombinant human mGlu2 receptor expressed in hamster AV12 cells expressing EAAT1 assessed as potentiation of gluta... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50207402

(CHEMBL3980125)Show SMILES CCCc1c(OCc2cccc(Oc3ncccc3-c3nn[nH]n3)c2)ccc(C(C)=O)c1O Show InChI InChI=1S/C24H23N5O4/c1-3-6-19-21(11-10-18(15(2)30)22(19)31)32-14-16-7-4-8-17(13-16)33-24-20(9-5-12-25-24)23-26-28-29-27-23/h4-5,7-13,31H,3,6,14H2,1-2H3,(H,26,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 227 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of recombinant human mGlu2 receptor expressed in hamster AV12 cells expressing EAAT1 assessed as potentiation of gluta... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50207405

(CHEMBL3918914)Show SMILES CCCc1c(OCc2cccc(Oc3ccc(cn3)-c3nn[nH]n3)c2)ccc(C(C)=O)c1O Show InChI InChI=1S/C24H23N5O4/c1-3-5-20-21(10-9-19(15(2)30)23(20)31)32-14-16-6-4-7-18(12-16)33-22-11-8-17(13-25-22)24-26-28-29-27-24/h4,6-13,31H,3,5,14H2,1-2H3,(H,26,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 319 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of recombinant human mGlu2 receptor expressed in hamster AV12 cells expressing EAAT1 assessed as potentiation of gluta... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50207400

(CHEMBL3899832)Show SMILES CC(=O)c1ccc(OCc2ccc(Oc3cc(ccn3)-c3nn[nH]n3)cc2)c(C)c1O Show InChI InChI=1S/C22H19N5O4/c1-13-19(8-7-18(14(2)28)21(13)29)30-12-15-3-5-17(6-4-15)31-20-11-16(9-10-23-20)22-24-26-27-25-22/h3-11,29H,12H2,1-2H3,(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human mGlu5 expressed in hamster AV12 cells expressing EAAT1 assessed as increase in intracellular calcium level meas... |
Bioorg Med Chem Lett 27: 323-328 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.049
BindingDB Entry DOI: 10.7270/Q2DN471R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data