

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin D (Homo sapiens (Human)) | BDBM50084626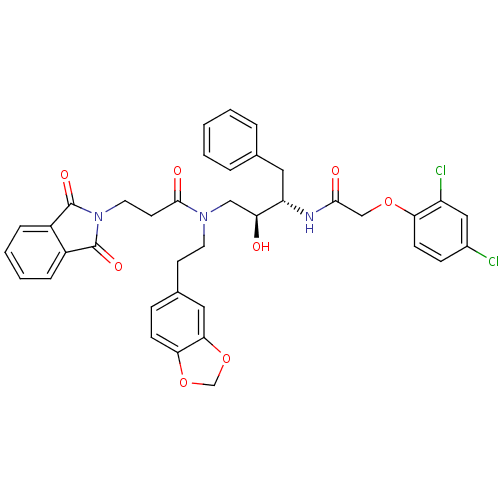 (CHEMBL284440 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of compound against human Cathepsin D | J Med Chem 45: 1412-9 (2002) BindingDB Entry DOI: 10.7270/Q25T3JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50110934 (CHEMBL30483 | N-((1S,3S)-1-Benzyl-3-{[2-(2,4-dichl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of compound against human Cathepsin D | J Med Chem 45: 1412-9 (2002) BindingDB Entry DOI: 10.7270/Q25T3JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50110932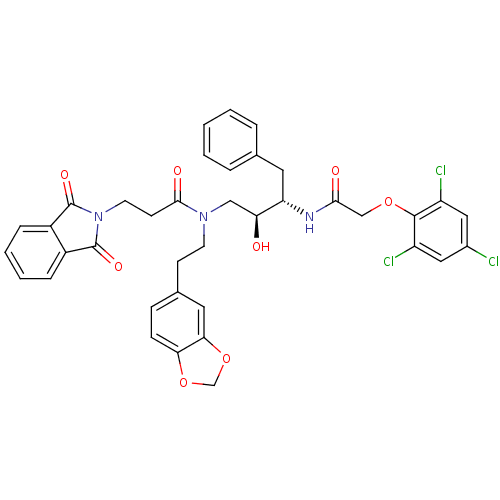 (CHEMBL283863 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of compound against human Cathepsin D | J Med Chem 45: 1412-9 (2002) BindingDB Entry DOI: 10.7270/Q25T3JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50110931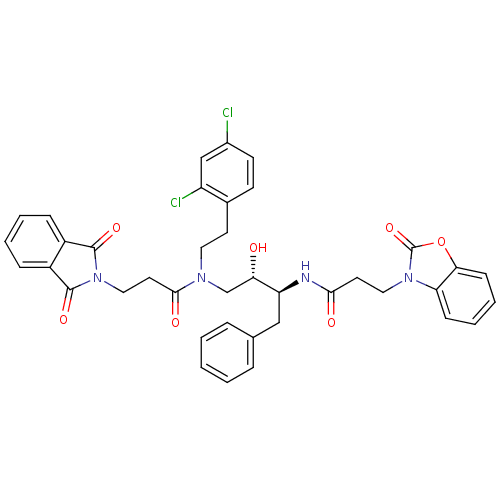 (CHEMBL284441 | N-((1S,3S)-1-Benzyl-3-{[2-(2,4-dich...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of compound against human Cathepsin D | J Med Chem 45: 1412-9 (2002) BindingDB Entry DOI: 10.7270/Q25T3JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50110933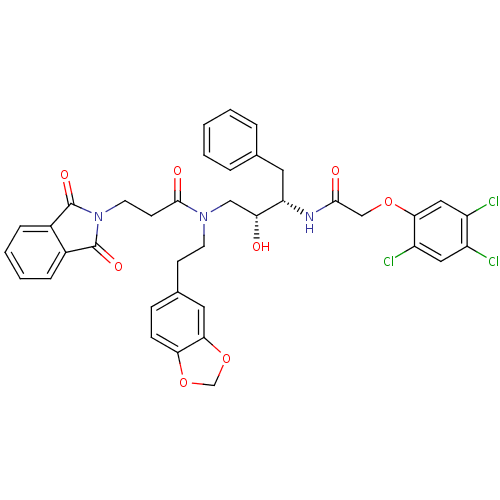 (CHEMBL30571 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of compound against human Cathepsin D | J Med Chem 45: 1412-9 (2002) BindingDB Entry DOI: 10.7270/Q25T3JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50110935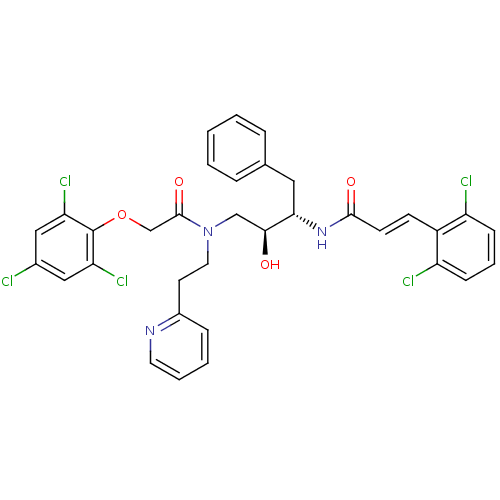 ((E)-N-((1S,3S)-1-Benzyl-2-hydroxy-3-{(2-pyridin-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of compound against human Cathepsin D | J Med Chem 45: 1412-9 (2002) BindingDB Entry DOI: 10.7270/Q25T3JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50279762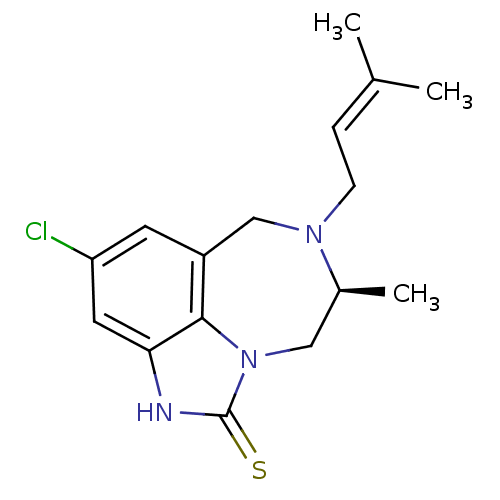 ((5S)-9-chloro-5-methyl-6-(3-methylbut-2-enyl)-4,5,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
University of California at San Francisco Curated by ChEMBL | Assay Description Effective concentration against HIV-1 reverse transcriptase | J Med Chem 42: 868-81 (1999) Article DOI: 10.1021/jm980277y BindingDB Entry DOI: 10.7270/Q2TB17KD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50074598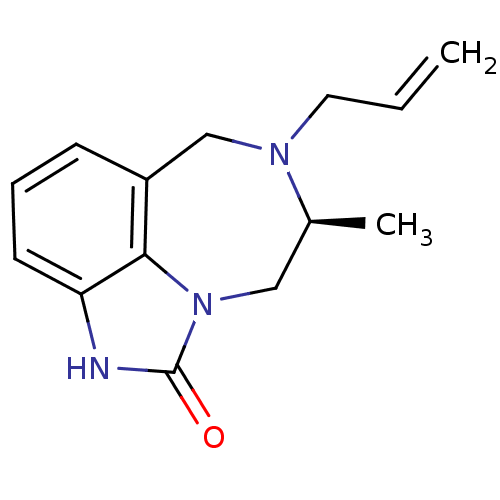 ((S)-7-Allyl-8-methyl-6,7,8,9-tetrahydro-2H-2,7,9a-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a |
University of California at San Francisco Curated by ChEMBL | Assay Description Effective concentration against HIV-1 reverse transcriptase | J Med Chem 42: 868-81 (1999) Article DOI: 10.1021/jm980277y BindingDB Entry DOI: 10.7270/Q2TB17KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50091080 ((+/-)2-(2,3-Dimethoxy-phenyl)-1-methyl-ethylamine ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 2.88E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50024201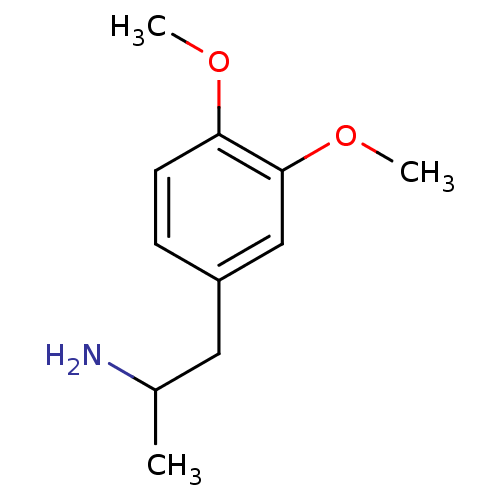 ((+/-)2-(3,4-Dimethoxy-phenyl)-1-methyl-ethylamine ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 3.55E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50005257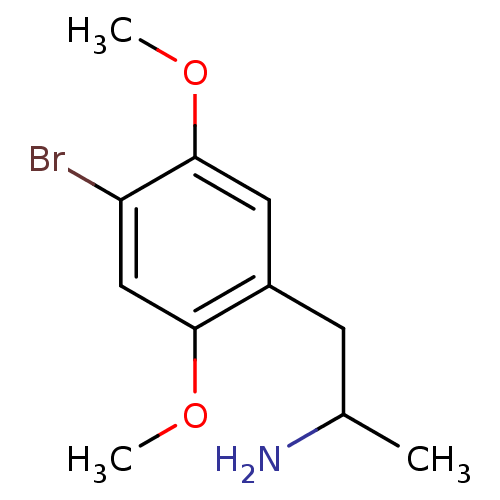 ((+)-2-(4-Bromo-2,5-dimethoxy-phenyl)-1-methyl-ethy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 44.7 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50404653 (CHEMBL30777) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 8.51E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50024211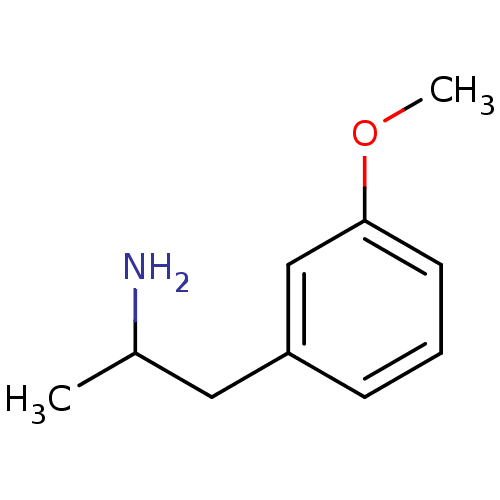 ((+/-)2-(3-Methoxy-phenyl)-1-methyl-ethylamine | 2-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50404654 (CHEMBL284589) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50005251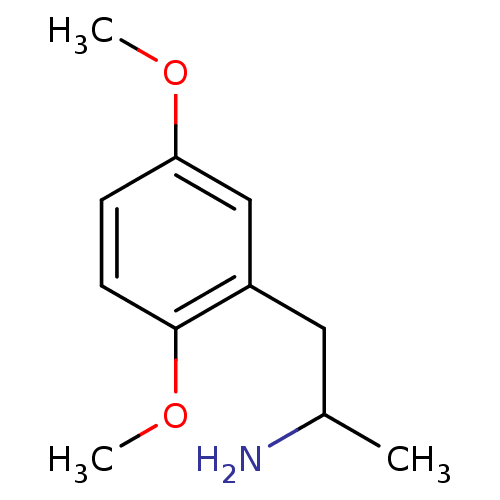 ((+/-)2-(2,5-Dimethoxy-phenyl)-1-methyl-ethylamine ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50005253 ((+/-)1-Methyl-2-(2,4,5-trimethoxy-phenyl)-ethylami...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50024208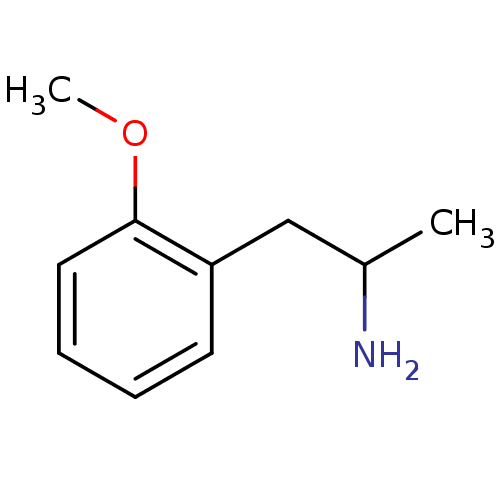 ((+/-)2-(2-Methoxy-phenyl)-1-methyl-ethylamine | 2-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 2.88E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50164323 (2-(2-Bromo-4,5-dimethoxy-phenyl)-1-methyl-ethylami...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50005247 ((+/-)2-Benzo[1,3]dioxol-5-yl-1-methyl-ethylamine |...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50164328 ((+/-)1-Methyl-2-(2,4,6-trimethoxy-phenyl)-ethylami...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 525 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50024209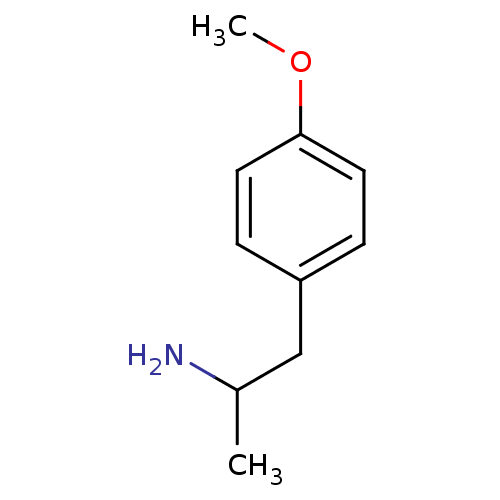 ((+/-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine | (-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 6.92E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50005246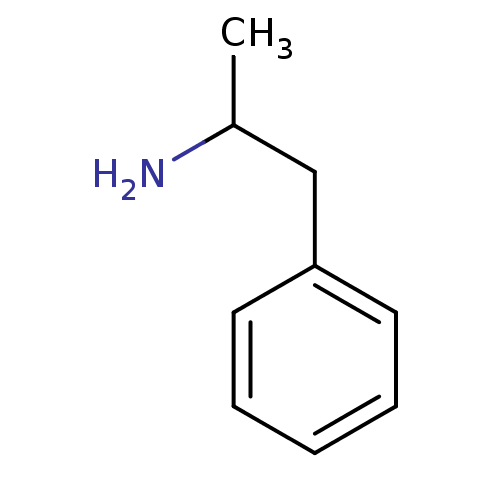 (1-Phenylpropan-2-amin | 1-phenyl-2-aminopropane | ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | 5.37E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against serotonergic receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50404655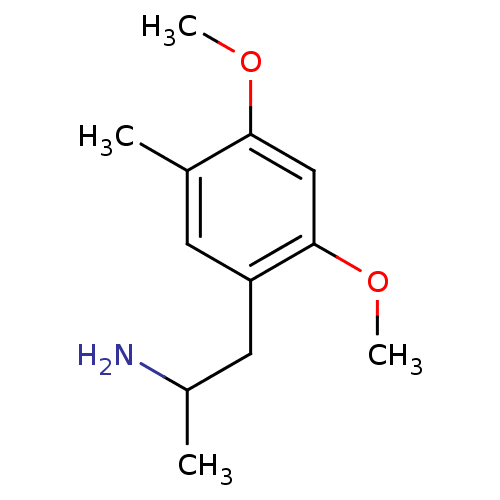 (CHEMBL282183) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50404656 (CHEMBL31787) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50005265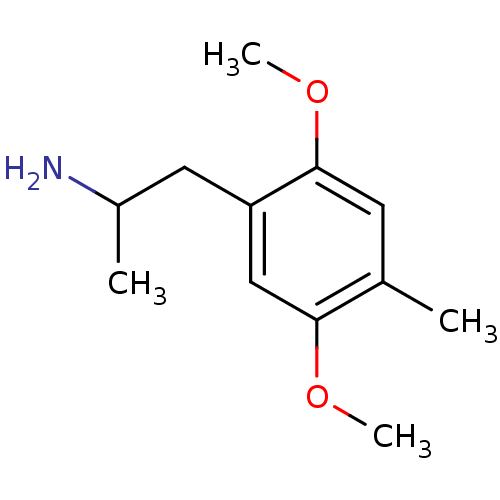 ((+/-)2-(2,5-Dimethoxy-4-methyl-phenyl)-1-methyl-et...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 75.9 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50005264 ((+/-)2-(2,4-Dimethoxy-phenyl)-1-methyl-ethylamine ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50091071 ((+/-)2-(2,6-Dimethoxy-phenyl)-1-methyl-ethylamine ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 8.13E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50369418 (CHEMBL58711 | R-87027) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a |
University of California at San Francisco Curated by ChEMBL | Assay Description Effective concentration against HIV-1 reverse transcriptase | J Med Chem 42: 868-81 (1999) Article DOI: 10.1021/jm980277y BindingDB Entry DOI: 10.7270/Q2TB17KD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50074597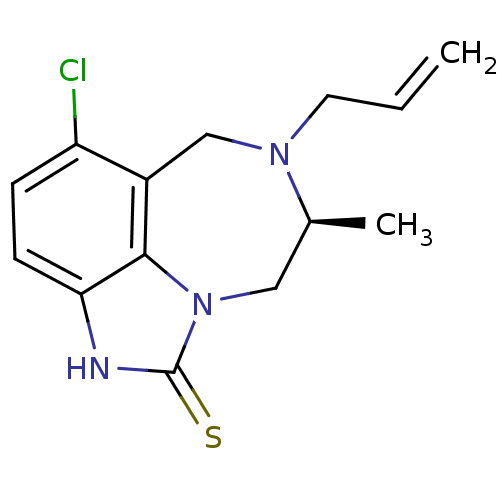 ((S)-7-Allyl-5-chloro-8-methyl-6,7,8,9-tetrahydro-2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a |
University of California at San Francisco Curated by ChEMBL | Assay Description Effective concentration against HIV-1 reverse transcriptase | J Med Chem 42: 868-81 (1999) Article DOI: 10.1021/jm980277y BindingDB Entry DOI: 10.7270/Q2TB17KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50074596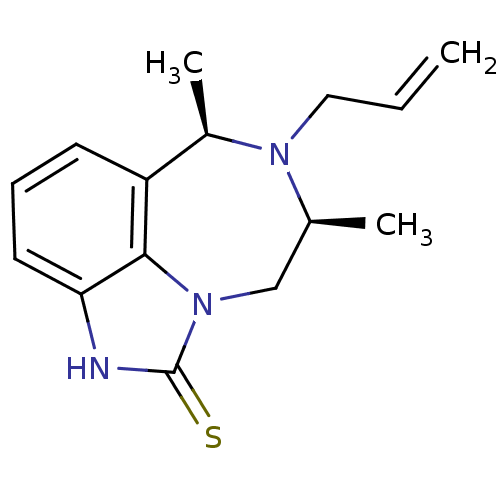 ((6R,8S)-7-Allyl-6,8-dimethyl-6,7,8,9-tetrahydro-2H...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 790 | n/a | n/a | n/a | n/a |
University of California at San Francisco Curated by ChEMBL | Assay Description Effective concentration against HIV-1 reverse transcriptase | J Med Chem 42: 868-81 (1999) Article DOI: 10.1021/jm980277y BindingDB Entry DOI: 10.7270/Q2TB17KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50074594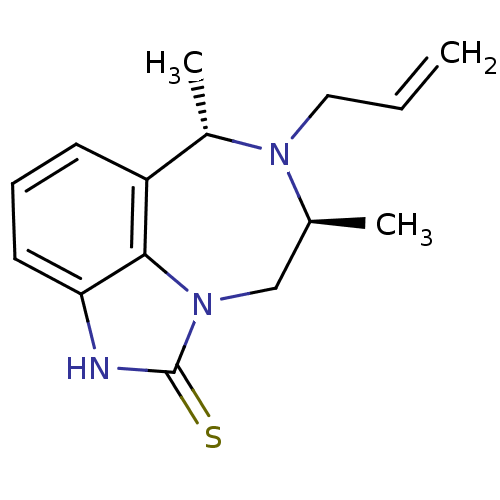 ((6S,8S)-7-Allyl-6,8-dimethyl-6,7,8,9-tetrahydro-2H...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
University of California at San Francisco Curated by ChEMBL | Assay Description Effective concentration against HIV-1 reverse transcriptase | J Med Chem 42: 868-81 (1999) Article DOI: 10.1021/jm980277y BindingDB Entry DOI: 10.7270/Q2TB17KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1433 ((11S)-11-methyl-10-(3-methylbut-2-en-1-yl)-1,3,10-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
University of California at San Francisco Curated by ChEMBL | Assay Description Effective concentration against HIV-1 reverse transcriptase | J Med Chem 42: 868-81 (1999) Article DOI: 10.1021/jm980277y BindingDB Entry DOI: 10.7270/Q2TB17KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50074592 ((S)-7-Allyl-5,8-dimethyl-6,7,8,9-tetrahydro-2H-2,7...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
University of California at San Francisco Curated by ChEMBL | Assay Description Effective concentration against HIV-1 reverse transcriptase | J Med Chem 42: 868-81 (1999) Article DOI: 10.1021/jm980277y BindingDB Entry DOI: 10.7270/Q2TB17KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (RAT) | BDBM50005256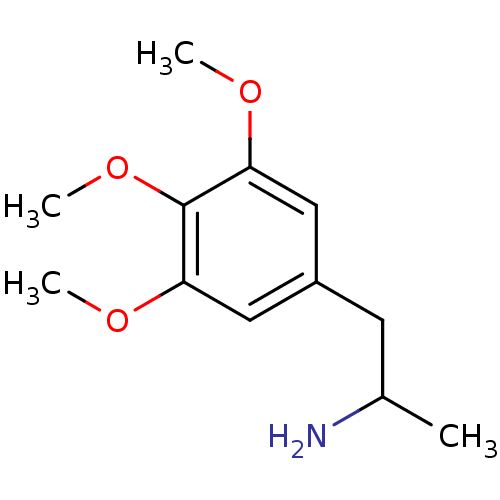 ((+/-)1-Methyl-2-(3,4,5-trimethoxy-phenyl)-ethylami...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundus | J Med Chem 24: 1414-21 (1982) BindingDB Entry DOI: 10.7270/Q2SX6FD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||