

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM194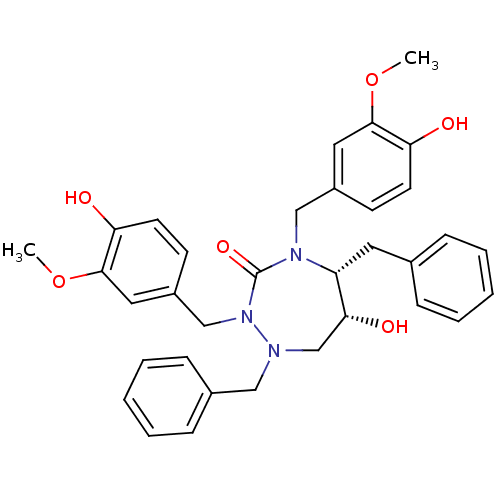 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(4-hydroxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00500 | -65.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50519598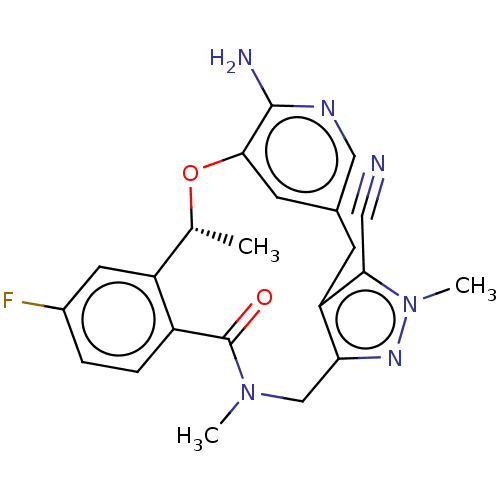 (CHEMBL4436406) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of ALK (unknown origin) | J Med Chem 62: 10927-10954 (2019) Article DOI: 10.1021/acs.jmedchem.9b00446 BindingDB Entry DOI: 10.7270/Q2S185WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM195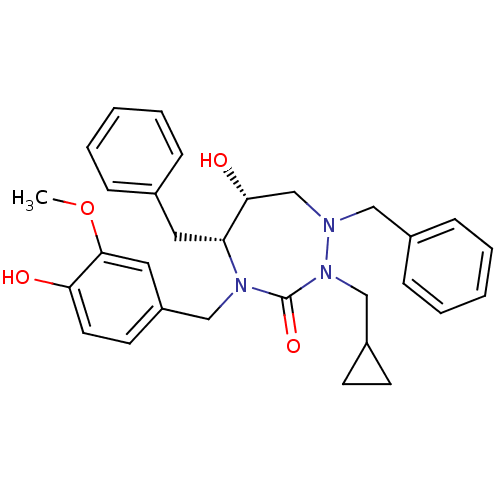 ((5R,6R)-1,5-dibenzyl-2-(cyclopropylmethyl)-6-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | -59.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50519598 (CHEMBL4436406) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of ROS1 (unknown origin) | J Med Chem 62: 10927-10954 (2019) Article DOI: 10.1021/acs.jmedchem.9b00446 BindingDB Entry DOI: 10.7270/Q2S185WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM192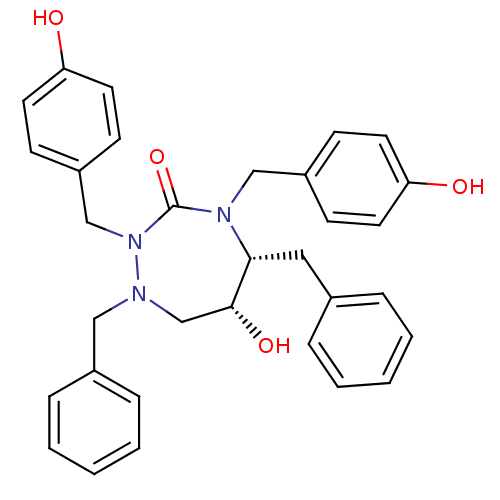 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(4-hydroxyp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | -58.9 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50111887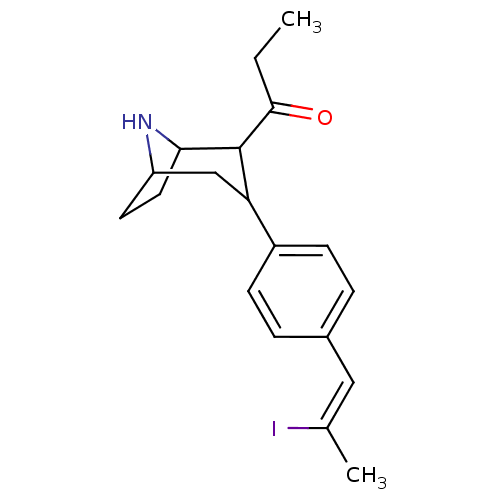 (1-{3-[4-((Z)-2-Iodo-propenyl)-phenyl]-8-aza-bicycl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]- paroxetine binding in rat frontal cortex membrane against Serotonin transporter | Bioorg Med Chem Lett 12: 845-7 (2002) BindingDB Entry DOI: 10.7270/Q25M6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM193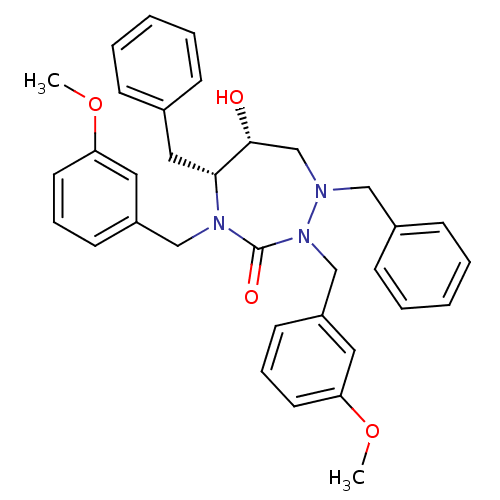 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(3-methoxyp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | -56.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM189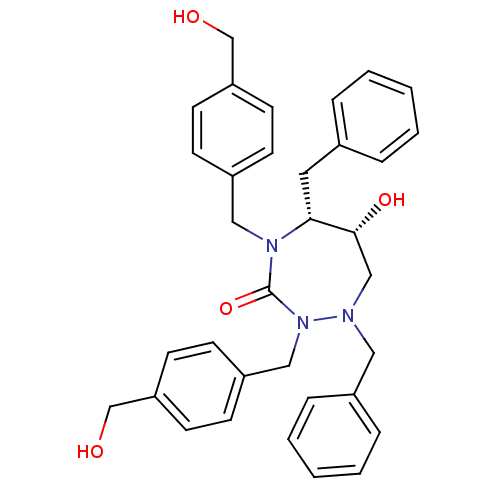 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis({[4-(hydrox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | -56.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM190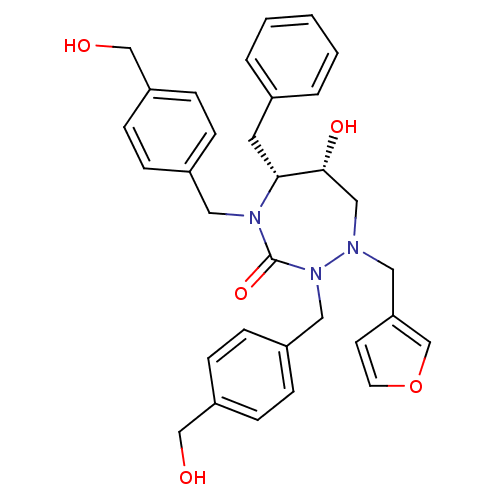 ((5R,6R)-2,4-Bis[4-(hydroxymethyl)benzyl]-1-(3-fura...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.490 | -54.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50111894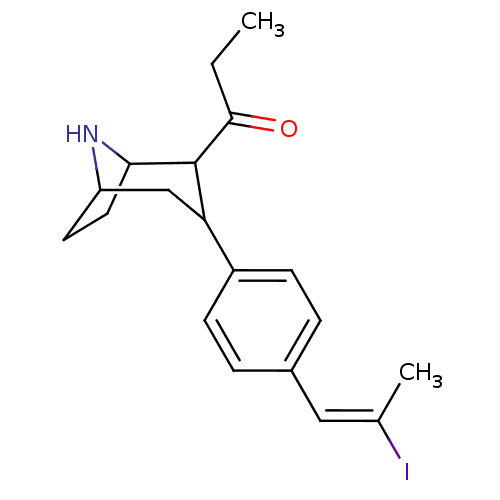 (1-{3-[4-((E)-2-Iodo-propenyl)-phenyl]-8-aza-bicycl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]- paroxetine binding in rat frontal cortex membrane against Serotonin transporter | Bioorg Med Chem Lett 12: 845-7 (2002) BindingDB Entry DOI: 10.7270/Q25M6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304826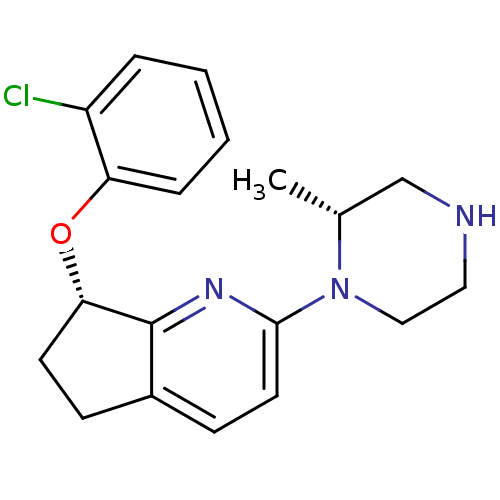 ((S)-7-(2-chlorophenoxy)-2-((R)-2-methylpiperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]5HT from human 5HT2C receptor expressed in mouse 3T3 cells by scintillation counting | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50111881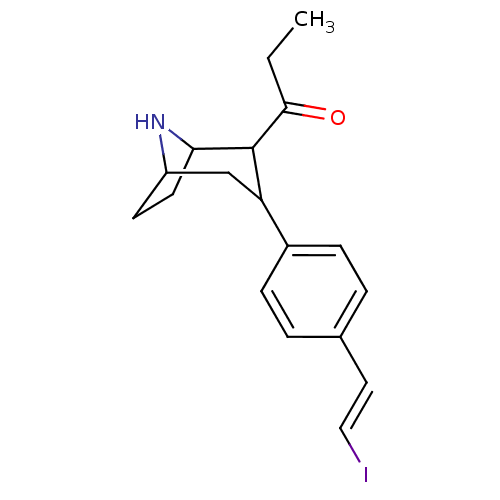 (1-{3-[4-((E)-2-Iodo-vinyl)-phenyl]-8-aza-bicyclo[3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]- paroxetine binding in rat frontal cortex membrane against Serotonin transporter | Bioorg Med Chem Lett 12: 845-7 (2002) BindingDB Entry DOI: 10.7270/Q25M6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50519598 (CHEMBL4436406) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of ALK L1196M (unknown origin) | J Med Chem 62: 10927-10954 (2019) Article DOI: 10.1021/acs.jmedchem.9b00446 BindingDB Entry DOI: 10.7270/Q2S185WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304805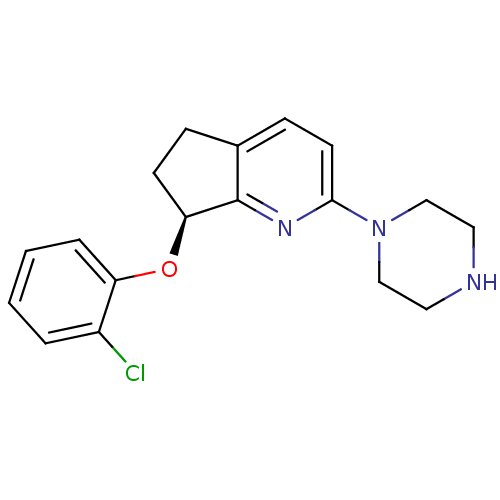 ((S)-7-(2-chlorophenoxy)-2-(piperazin-1-yl)-6,7-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50304802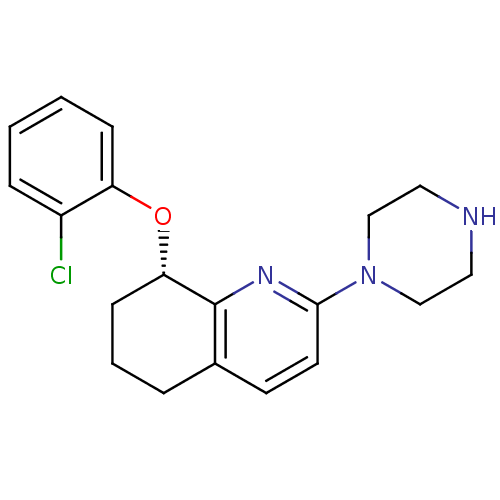 ((S)-8-(2-chlorophenoxy)-2-(piperazin-1-yl)-5,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM192752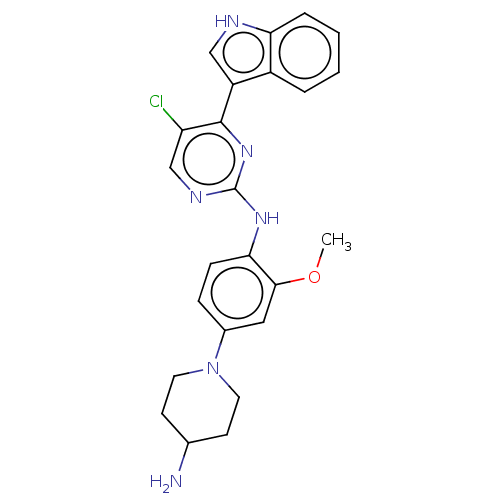 (AZD3463) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of ALK (unknown origin) | J Med Chem 62: 10927-10954 (2019) Article DOI: 10.1021/acs.jmedchem.9b00446 BindingDB Entry DOI: 10.7270/Q2S185WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304804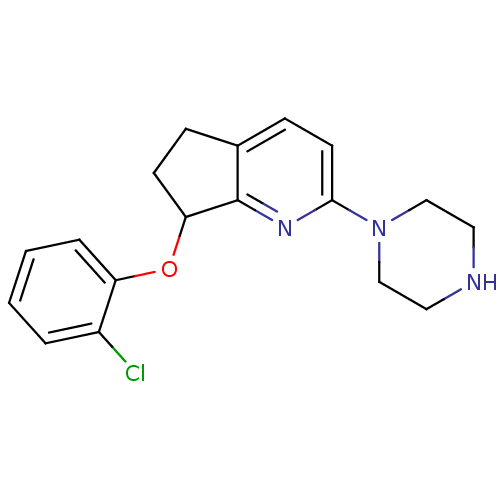 (7-(2-chlorophenoxy)-2-(piperazin-1-yl)-6,7-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50314613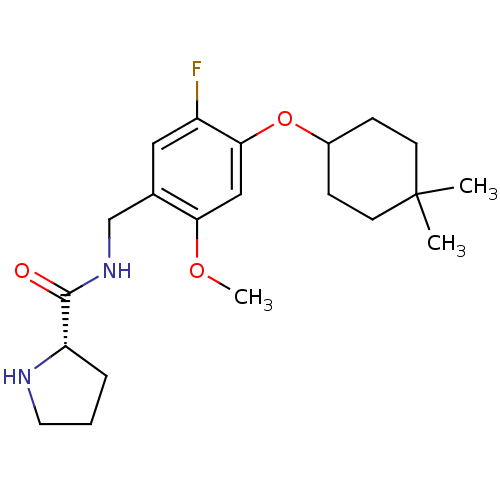 ((S)-N-(4-(4,4-dimethylcyclohexyloxy)-5-fluoro-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity to 5HT2C receptor | Bioorg Med Chem Lett 20: 2365-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.107 BindingDB Entry DOI: 10.7270/Q24M94QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50591671 (CHEMBL5191603) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00056 BindingDB Entry DOI: 10.7270/Q26977J9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304808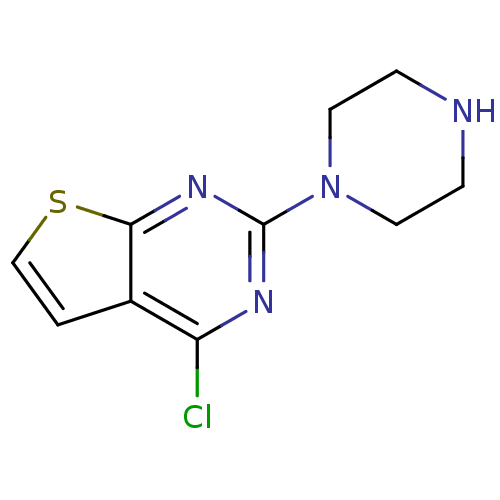 (4-chloro-2-(piperazin-1-yl)thieno[2,3-d]pyrimidine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50304805 ((S)-7-(2-chlorophenoxy)-2-(piperazin-1-yl)-6,7-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50111893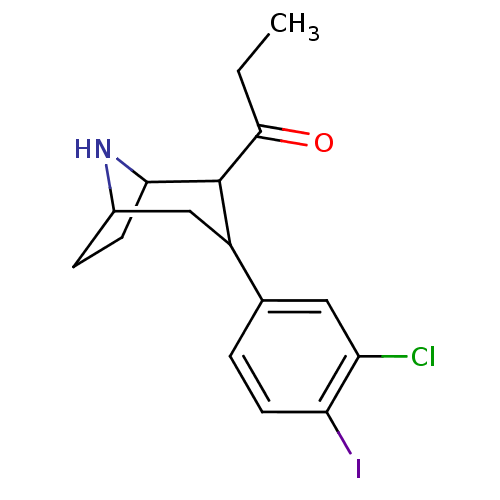 (1-[3-(3-Chloro-4-iodo-phenyl)-8-aza-bicyclo[3.2.1]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]- paroxetine binding in rat frontal cortex membrane against Serotonin transporter | Bioorg Med Chem Lett 12: 845-7 (2002) BindingDB Entry DOI: 10.7270/Q25M6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50111889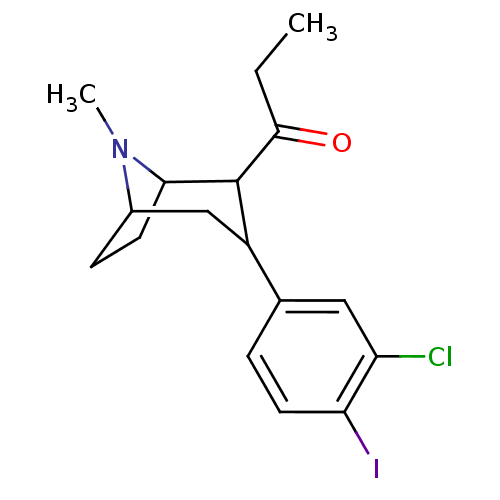 (1-[3-(3-Chloro-4-iodo-phenyl)-8-methyl-8-aza-bicyc...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]- paroxetine binding in rat frontal cortex membrane against Serotonin transporter | Bioorg Med Chem Lett 12: 845-7 (2002) BindingDB Entry DOI: 10.7270/Q25M6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304807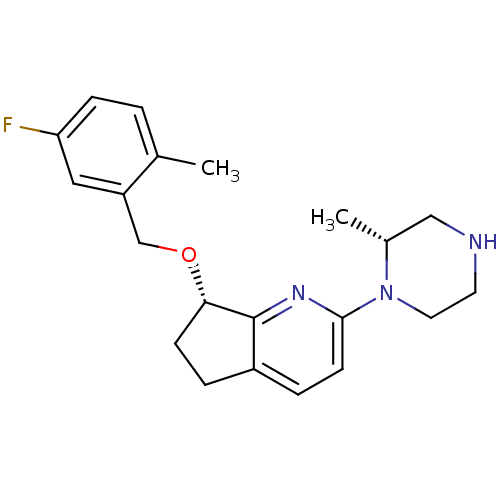 ((S)-7-(5-fluoro-2-methylbenzyloxy)-2-((R)-2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304802 ((S)-8-(2-chlorophenoxy)-2-(piperazin-1-yl)-5,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50314616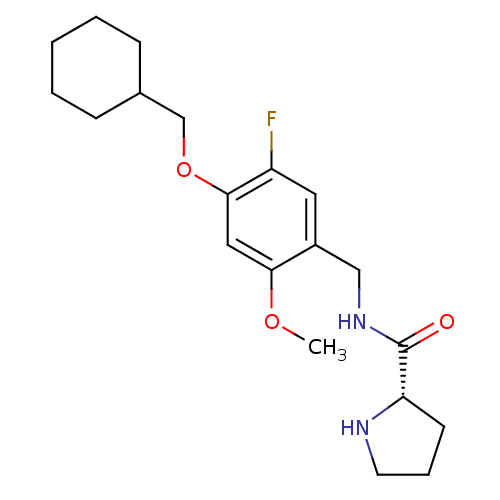 ((S)-N-(4-(cyclohexylmethoxy)-5-fluoro-2-methoxyben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity to 5HT2C receptor | Bioorg Med Chem Lett 20: 2365-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.107 BindingDB Entry DOI: 10.7270/Q24M94QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50314614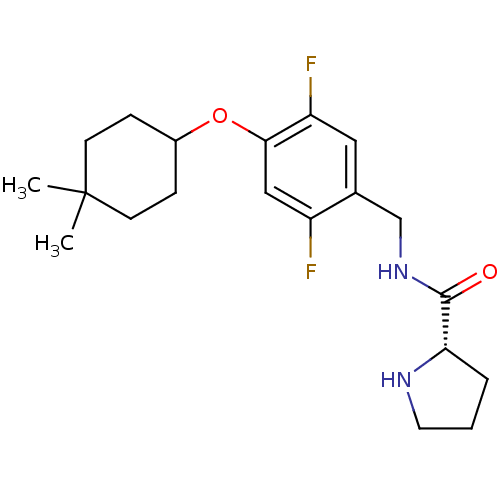 ((S)-N-(4-(4,4-dimethylcyclohexyloxy)-2,5-difluorob...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity to 5HT2C receptor | Bioorg Med Chem Lett 20: 2365-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.107 BindingDB Entry DOI: 10.7270/Q24M94QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50111891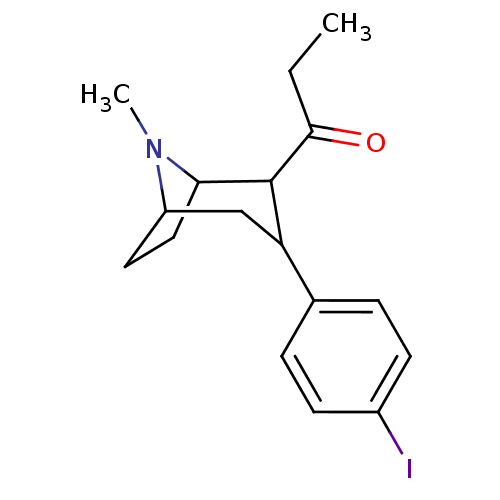 (1-[3-(4-Iodo-phenyl)-8-methyl-8-aza-bicyclo[3.2.1]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]- paroxetine binding in rat frontal cortex membrane against Serotonin transporter | Bioorg Med Chem Lett 12: 845-7 (2002) BindingDB Entry DOI: 10.7270/Q25M6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM191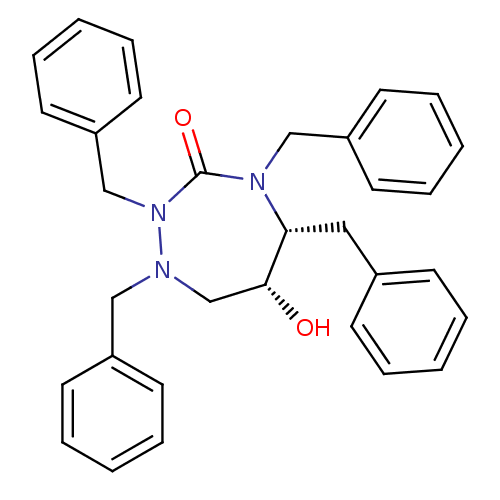 ((5R,6R)-1,2,4,5-tetrabenzyl-6-hydroxy-1,2,4-triaze...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -50.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50304803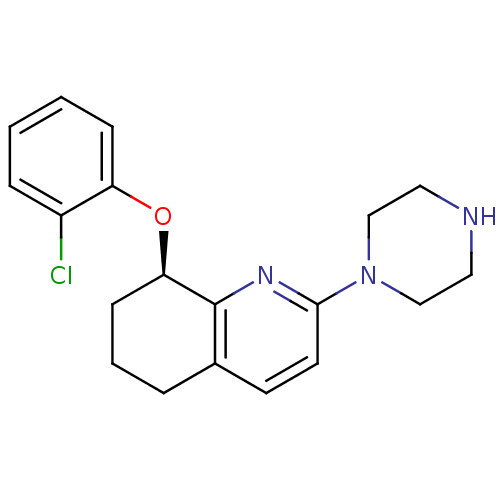 ((R)-8-(2-chlorophenoxy)-2-(piperazin-1-yl)-5,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50306682 ((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) | J Med Chem 62: 10927-10954 (2019) Article DOI: 10.1021/acs.jmedchem.9b00446 BindingDB Entry DOI: 10.7270/Q2S185WV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50591673 (CHEMBL5198400) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00056 BindingDB Entry DOI: 10.7270/Q26977J9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50111883 (1-[3-(4-Iodo-phenyl)-8-aza-bicyclo[3.2.1]oct-2-yl]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]- paroxetine binding in rat frontal cortex membrane against Serotonin transporter | Bioorg Med Chem Lett 12: 845-7 (2002) BindingDB Entry DOI: 10.7270/Q25M6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50591672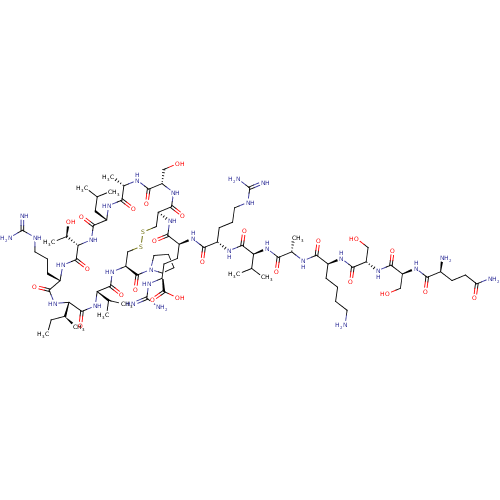 (CHEMBL5184798) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00056 BindingDB Entry DOI: 10.7270/Q26977J9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50591674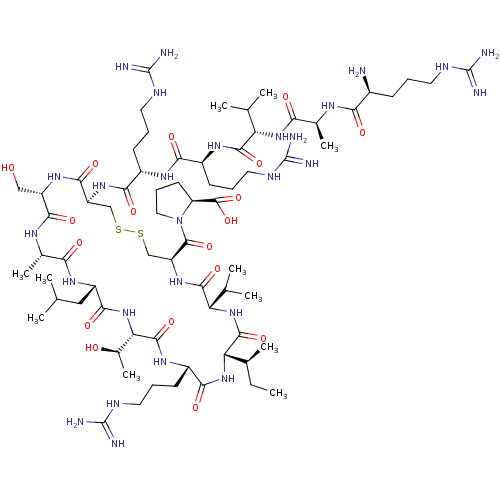 (CHEMBL5173140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00056 BindingDB Entry DOI: 10.7270/Q26977J9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50111890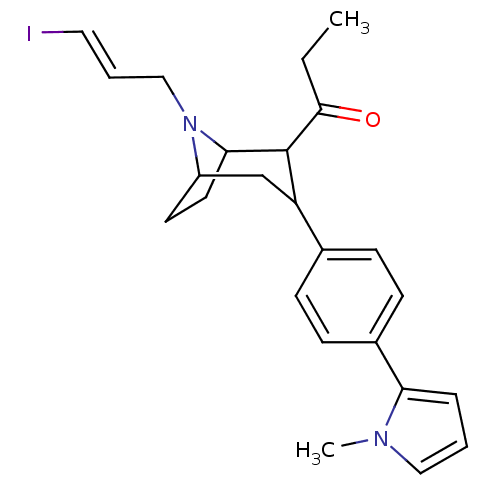 (1-{8-((E)-3-Iodo-allyl)-3-[4-(1-methyl-1H-pyrrol-2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]- paroxetine binding in rat frontal cortex membrane against Serotonin transporter | Bioorg Med Chem Lett 12: 845-7 (2002) BindingDB Entry DOI: 10.7270/Q25M6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304827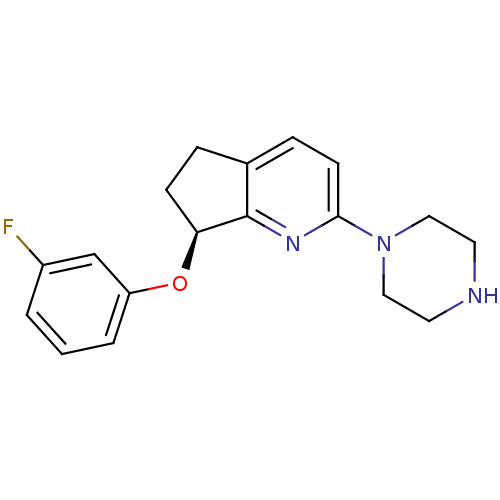 ((S)-7-(3-fluorophenoxy)-2-(piperazin-1-yl)-6,7-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]5HT from human 5HT2C receptor expressed in mouse 3T3 cells by scintillation counting | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50304804 (7-(2-chlorophenoxy)-2-(piperazin-1-yl)-6,7-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304809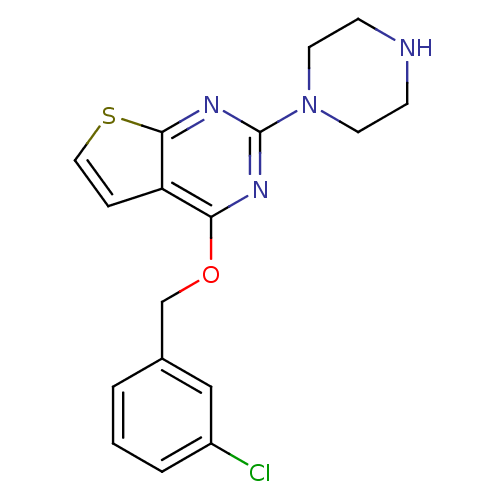 (4-(3-chlorobenzyloxy)-2-(piperazin-1-yl)thieno[2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Memphis/1/1971 H3N2)) | BDBM50365357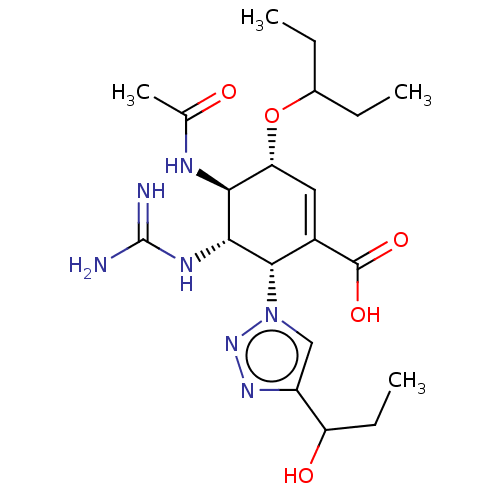 (CHEMBL4168935) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (H3N2) neuraminidase activity | J Med Chem 61: 6379-6397 (2018) Article DOI: 10.1021/acs.jmedchem.8b00929 BindingDB Entry DOI: 10.7270/Q2H41V0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50314618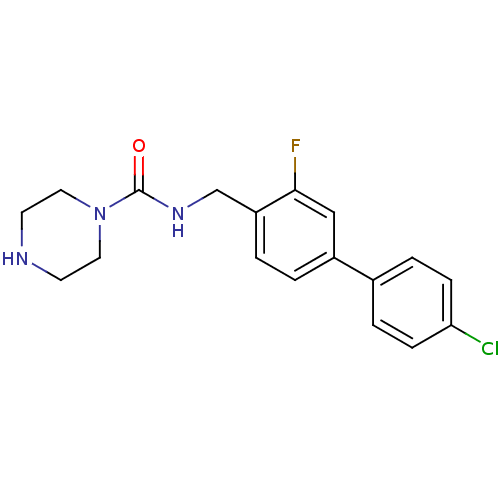 (CHEMBL1090397 | N-((4'-chloro-3-fluorobiphenyl-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity to 5HT2C receptor | Bioorg Med Chem Lett 20: 2365-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.107 BindingDB Entry DOI: 10.7270/Q24M94QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50314623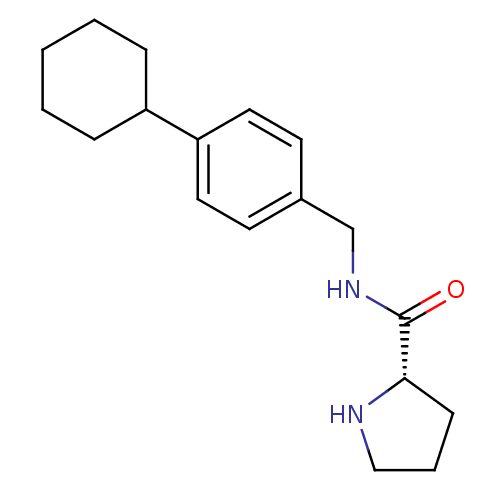 ((S)-N-(4-cyclohexylbenzyl)pyrrolidine-2-carboxamid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity to 5HT2C receptor | Bioorg Med Chem Lett 20: 2365-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.107 BindingDB Entry DOI: 10.7270/Q24M94QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50304807 ((S)-7-(5-fluoro-2-methylbenzyloxy)-2-((R)-2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304801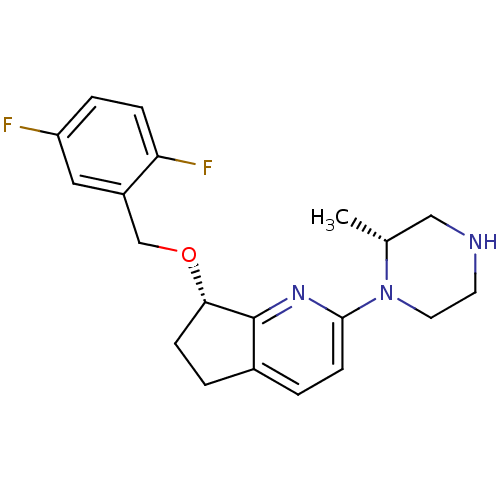 ((S)-7-(2,5-difluorobenzyloxy)-2-((R)-2-methylpiper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50111886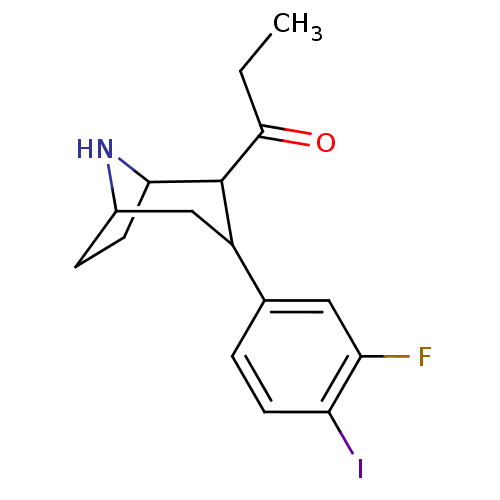 (1-[3-(3-Fluoro-4-iodo-phenyl)-8-aza-bicyclo[3.2.1]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]- paroxetine binding in rat frontal cortex membrane against Serotonin transporter | Bioorg Med Chem Lett 12: 845-7 (2002) BindingDB Entry DOI: 10.7270/Q25M6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50591676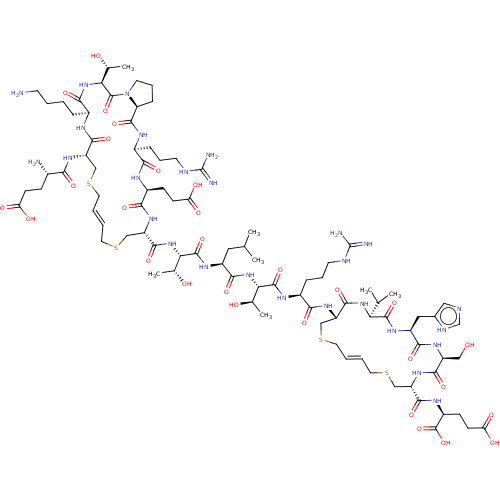 (CHEMBL5202369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00056 BindingDB Entry DOI: 10.7270/Q26977J9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50304807 ((S)-7-(5-fluoro-2-methylbenzyloxy)-2-((R)-2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human 5HT2B receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50314624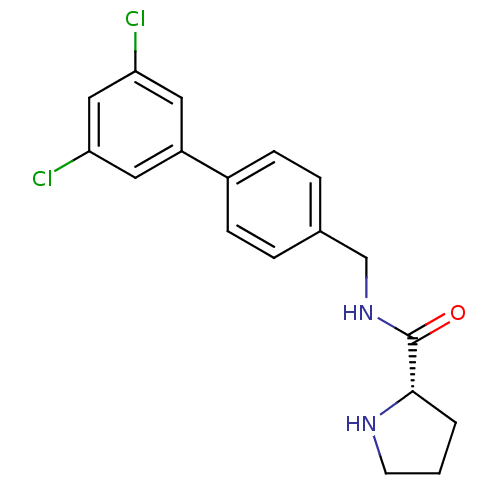 ((S)-N-((3',5'-dichlorobiphenyl-4-yl)methyl)pyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity to 5HT2C receptor | Bioorg Med Chem Lett 20: 2365-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.107 BindingDB Entry DOI: 10.7270/Q24M94QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50111888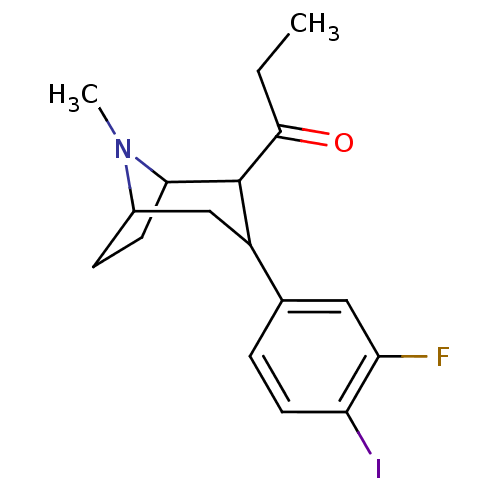 (1-[3-(3-Fluoro-4-iodo-phenyl)-8-methyl-8-aza-bicyc...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]- paroxetine binding in rat frontal cortex membrane against Serotonin transporter | Bioorg Med Chem Lett 12: 845-7 (2002) BindingDB Entry DOI: 10.7270/Q25M6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304803 ((R)-8-(2-chlorophenoxy)-2-(piperazin-1-yl)-5,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1242 total ) | Next | Last >> |