 Found 103 hits with Last Name = 'kornmeier' and Initial = 'cm'
Found 103 hits with Last Name = 'kornmeier' and Initial = 'cm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50064015
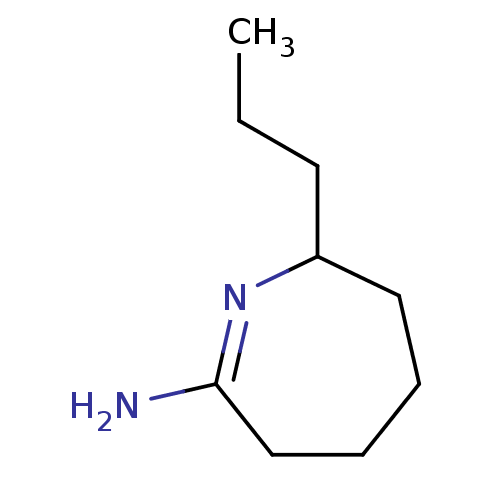
(7-Propyl-azepan-(2Z)-ylideneamine; hydrochloride |...)Show InChI InChI=1S/C9H18N2/c1-2-5-8-6-3-4-7-9(10)11-8/h8H,2-7H2,1H3,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against L-arginine binding to Inducible nitric oxide synthase |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50385150
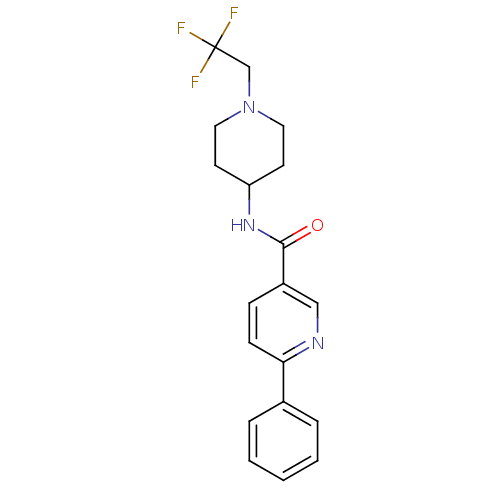
(CHEMBL2035650)Show SMILES FC(F)(F)CN1CCC(CC1)NC(=O)c1ccc(nc1)-c1ccccc1 Show InChI InChI=1S/C19H20F3N3O/c20-19(21,22)13-25-10-8-16(9-11-25)24-18(26)15-6-7-17(23-12-15)14-4-2-1-3-5-14/h1-7,12,16H,8-11,13H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HPGDS |
Bioorg Med Chem Lett 22: 3795-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.004
BindingDB Entry DOI: 10.7270/Q28C9X82 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50385142
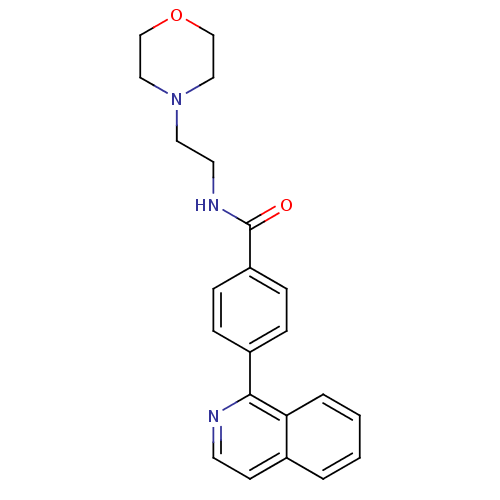
(CHEMBL2035651)Show InChI InChI=1S/C22H23N3O2/c26-22(24-11-12-25-13-15-27-16-14-25)19-7-5-18(6-8-19)21-20-4-2-1-3-17(20)9-10-23-21/h1-10H,11-16H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.34 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HPGDS using PGH2 as substrate assessed as production of PGD2 preincubated for 10 mins prior substrate addition measur... |
Bioorg Med Chem Lett 22: 3795-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.004
BindingDB Entry DOI: 10.7270/Q28C9X82 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50385144
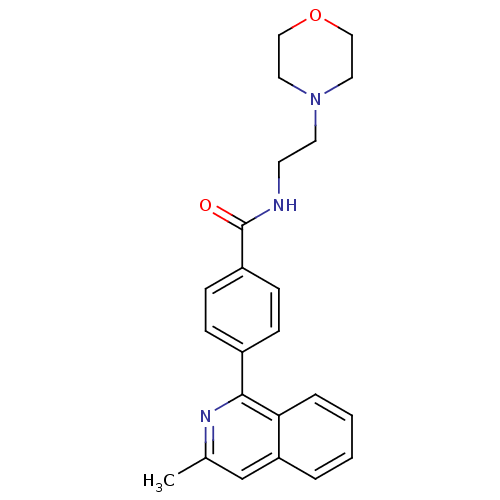
(CHEMBL2035653)Show SMILES Cc1cc2ccccc2c(n1)-c1ccc(cc1)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C23H25N3O2/c1-17-16-20-4-2-3-5-21(20)22(25-17)18-6-8-19(9-7-18)23(27)24-10-11-26-12-14-28-15-13-26/h2-9,16H,10-15H2,1H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HPGDS using PGH2 as substrate assessed as production of PGD2 preincubated for 10 mins prior substrate addition measur... |
Bioorg Med Chem Lett 22: 3795-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.004
BindingDB Entry DOI: 10.7270/Q28C9X82 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50064015

(7-Propyl-azepan-(2Z)-ylideneamine; hydrochloride |...)Show InChI InChI=1S/C9H18N2/c1-2-5-8-6-3-4-7-9(10)11-8/h8H,2-7H2,1H3,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated for soluble cell extract of human Inducible nitric oxide synthase and partially purified by DEAE-sepharose chromatograp... |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066778
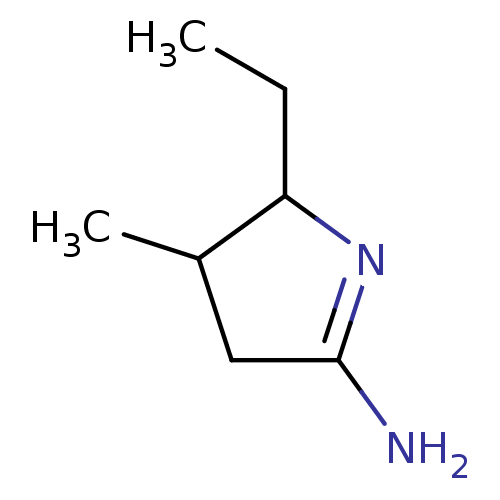
(5-Ethyl-4-methyl-pyrrolidin-(2E)-ylideneamine; hyd...)Show InChI InChI=1S/C7H14N2/c1-3-6-5(2)4-7(8)9-6/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human inducible nitric oxide synthase (hiNOS) |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50066778

(5-Ethyl-4-methyl-pyrrolidin-(2E)-ylideneamine; hyd...)Show InChI InChI=1S/C7H14N2/c1-3-6-5(2)4-7(8)9-6/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human Neuronal nitric oxide synthase |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50064018

(7-Allyl-azepan-(2Z)-ylideneamine; hydrochloride | ...)Show InChI InChI=1S/C9H16N2/c1-2-5-8-6-3-4-7-9(10)11-8/h2,8H,1,3-7H2,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated for soluble cell extract of human Inducible nitric oxide synthase and partially purified by DEAE-sepharose chromatograp... |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066775
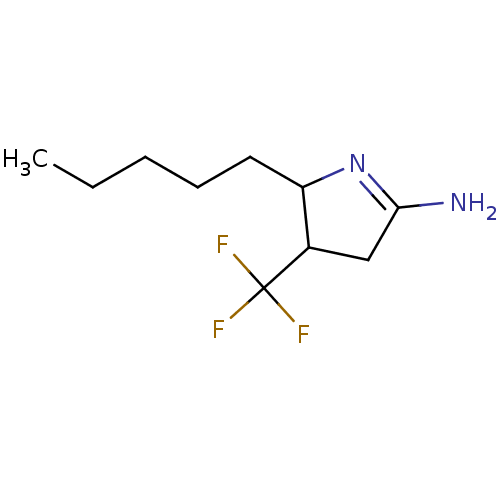
(5-Pentyl-4-trifluoromethyl-pyrrolidin-(2E)-ylidene...)Show InChI InChI=1S/C10H17F3N2/c1-2-3-4-5-8-7(10(11,12)13)6-9(14)15-8/h7-8H,2-6H2,1H3,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human inducible nitric oxide synthase (hiNOS) |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50385143
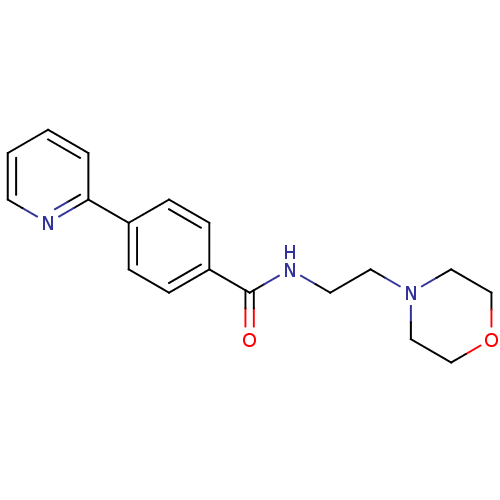
(CHEMBL2035652)Show InChI InChI=1S/C18H21N3O2/c22-18(20-9-10-21-11-13-23-14-12-21)16-6-4-15(5-7-16)17-3-1-2-8-19-17/h1-8H,9-14H2,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 274 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HPGDS using PGH2 as substrate assessed as production of PGD2 preincubated for 10 mins prior substrate addition measur... |
Bioorg Med Chem Lett 22: 3795-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.004
BindingDB Entry DOI: 10.7270/Q28C9X82 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50385149
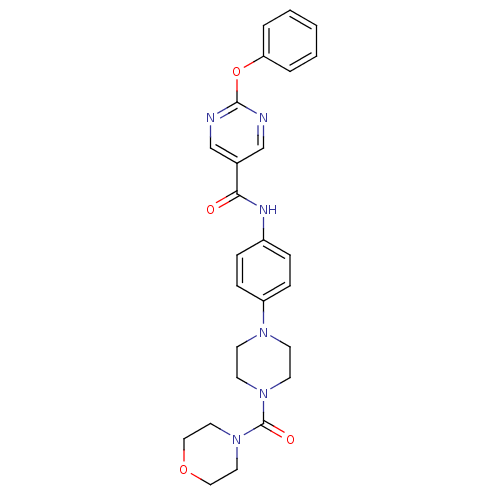
(CHEMBL2035649)Show SMILES O=C(Nc1ccc(cc1)N1CCN(CC1)C(=O)N1CCOCC1)c1cnc(Oc2ccccc2)nc1 Show InChI InChI=1S/C26H28N6O4/c33-24(20-18-27-25(28-19-20)36-23-4-2-1-3-5-23)29-21-6-8-22(9-7-21)30-10-12-31(13-11-30)26(34)32-14-16-35-17-15-32/h1-9,18-19H,10-17H2,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HPGDS |
Bioorg Med Chem Lett 22: 3795-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.004
BindingDB Entry DOI: 10.7270/Q28C9X82 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066785
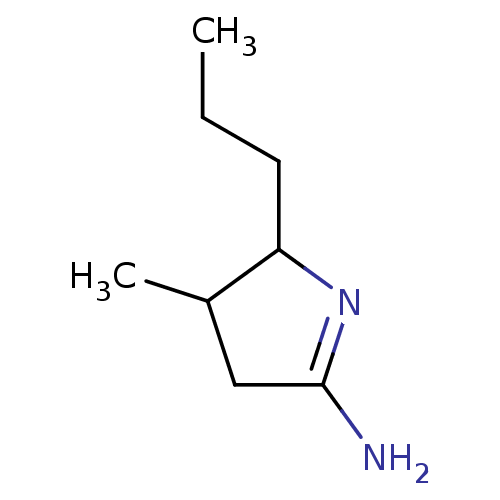
(4-Methyl-5-propyl-pyrrolidin-(2E)-ylideneamine; hy...)Show InChI InChI=1S/C8H16N2/c1-3-4-7-6(2)5-8(9)10-7/h6-7H,3-5H2,1-2H3,(H2,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human inducible nitric oxide synthase (hiNOS) |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066774

(4-Methyl-5-pentyl-pyrrolidin-(2E)-ylideneamine; hy...)Show InChI InChI=1S/C10H20N2/c1-3-4-5-6-9-8(2)7-10(11)12-9/h8-9H,3-7H2,1-2H3,(H2,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human inducible nitric oxide synthase (hiNOS) |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066774

(4-Methyl-5-pentyl-pyrrolidin-(2E)-ylideneamine; hy...)Show InChI InChI=1S/C10H20N2/c1-3-4-5-6-9-8(2)7-10(11)12-9/h8-9H,3-7H2,1-2H3,(H2,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human inducible nitric oxide synthase (hiNOS) |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50066785

(4-Methyl-5-propyl-pyrrolidin-(2E)-ylideneamine; hy...)Show InChI InChI=1S/C8H16N2/c1-3-4-7-6(2)5-8(9)10-7/h6-7H,3-5H2,1-2H3,(H2,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human Neuronal nitric oxide synthase |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50064015

(7-Propyl-azepan-(2Z)-ylideneamine; hydrochloride |...)Show InChI InChI=1S/C9H18N2/c1-2-5-8-6-3-4-7-9(10)11-8/h8H,2-7H2,1H3,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated from soluble cell extract of human Neuronal nitric oxide synthase and partially purified by DEAE-sepharose chromatograp... |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50225106
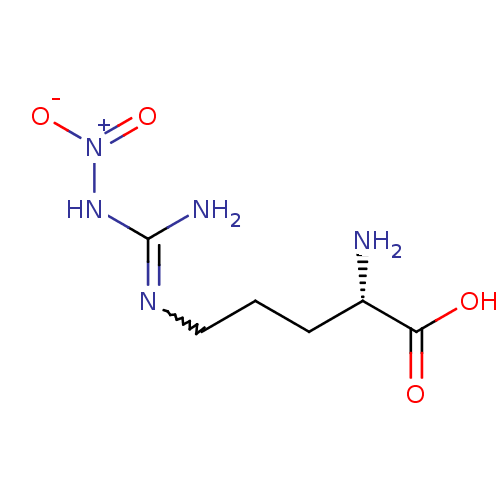
((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(O)=O |r,w:8.8| Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human Neuronal nitric oxide synthase |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50064011
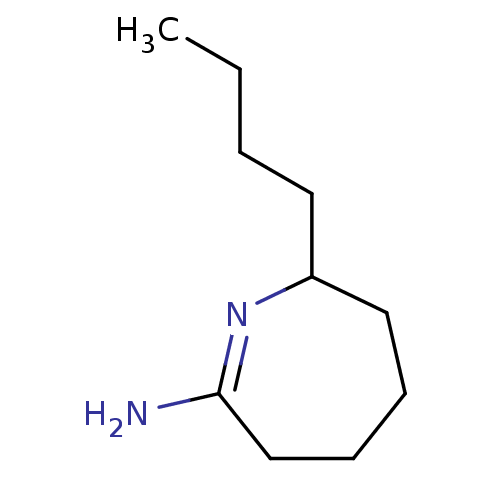
(7-Butyl-azepan-(2Z)-ylideneamine; hydrochloride | ...)Show InChI InChI=1S/C10H20N2/c1-2-3-6-9-7-4-5-8-10(11)12-9/h9H,2-8H2,1H3,(H2,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated for soluble cell extract of human Inducible nitric oxide synthase and partially purified by DEAE-sepharose chromatograp... |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50225106

((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(O)=O |r,w:8.8| Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50064018

(7-Allyl-azepan-(2Z)-ylideneamine; hydrochloride | ...)Show InChI InChI=1S/C9H16N2/c1-2-5-8-6-3-4-7-9(10)11-8/h2,8H,1,3-7H2,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
The ability of compound to inhibit mouse Inducible nitric oxide synthase in LPS stimulated mouse RAW cells was determined |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50064015

(7-Propyl-azepan-(2Z)-ylideneamine; hydrochloride |...)Show InChI InChI=1S/C9H18N2/c1-2-5-8-6-3-4-7-9(10)11-8/h8H,2-7H2,1H3,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
The ability of compound to inhibit mouse Inducible nitric oxide synthase in LPS stimulated mouse RAW cells was determined |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066779
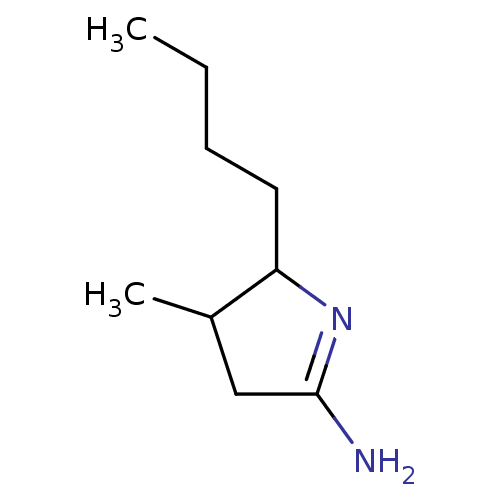
(5-Butyl-4-methyl-pyrrolidin-(2E)-ylideneamine; hyd...)Show InChI InChI=1S/C9H18N2/c1-3-4-5-8-7(2)6-9(10)11-8/h7-8H,3-6H2,1-2H3,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human inducible nitric oxide synthase (hiNOS) |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50385145
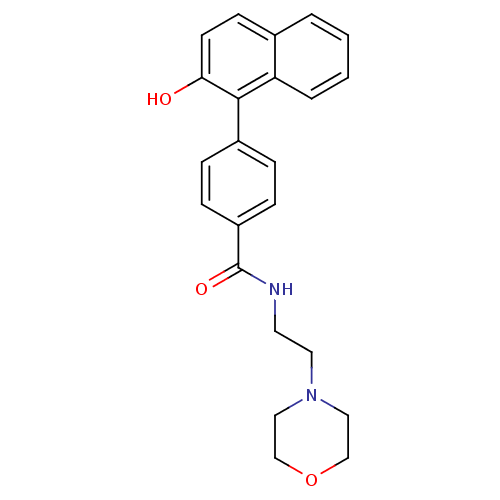
(CHEMBL2035654)Show InChI InChI=1S/C23H24N2O3/c26-21-10-9-17-3-1-2-4-20(17)22(21)18-5-7-19(8-6-18)23(27)24-11-12-25-13-15-28-16-14-25/h1-10,26H,11-16H2,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 662 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HPGDS using PGH2 as substrate assessed as production of PGD2 preincubated for 10 mins prior substrate addition measur... |
Bioorg Med Chem Lett 22: 3795-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.004
BindingDB Entry DOI: 10.7270/Q28C9X82 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50385147

(CHEMBL2035656)Show SMILES NCc1ccc2ccccc2c1-c1ccc(cc1)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C24H27N3O2/c25-17-21-10-5-18-3-1-2-4-22(18)23(21)19-6-8-20(9-7-19)24(28)26-11-12-27-13-15-29-16-14-27/h1-10H,11-17,25H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 845 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HPGDS using PGH2 as substrate assessed as production of PGD2 preincubated for 10 mins prior substrate addition measur... |
Bioorg Med Chem Lett 22: 3795-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.004
BindingDB Entry DOI: 10.7270/Q28C9X82 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50066779

(5-Butyl-4-methyl-pyrrolidin-(2E)-ylideneamine; hyd...)Show InChI InChI=1S/C9H18N2/c1-3-4-5-8-7(2)6-9(10)11-8/h7-8H,3-6H2,1-2H3,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human Neuronal nitric oxide synthase |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50064011

(7-Butyl-azepan-(2Z)-ylideneamine; hydrochloride | ...)Show InChI InChI=1S/C10H20N2/c1-2-3-6-9-7-4-5-8-10(11)12-9/h9H,2-8H2,1H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated from soluble cell extract of human nNeuronal nitric oxide synthase and partially purified by DEAE-sepharose chromatogra... |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50064018

(7-Allyl-azepan-(2Z)-ylideneamine; hydrochloride | ...)Show InChI InChI=1S/C9H16N2/c1-2-5-8-6-3-4-7-9(10)11-8/h2,8H,1,3-7H2,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated from soluble cell extract of human Neuronal nitric oxide synthase and partially purified by DEAE-sepharose chromatograp... |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066780

(4-Methyl-pyrrolidin-(2E)-ylideneamine; hydrochlori...)Show InChI InChI=1S/C5H10N2/c1-4-2-5(6)7-3-4/h4H,2-3H2,1H3,(H2,6,7) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human inducible nitric oxide synthase (hiNOS) |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50385146
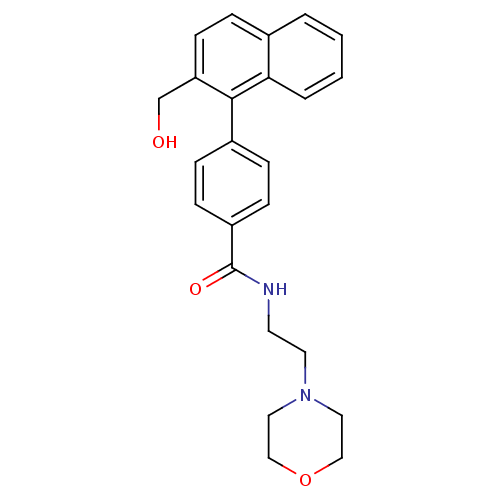
(CHEMBL2035655)Show SMILES OCc1ccc2ccccc2c1-c1ccc(cc1)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C24H26N2O3/c27-17-21-10-5-18-3-1-2-4-22(18)23(21)19-6-8-20(9-7-19)24(28)25-11-12-26-13-15-29-16-14-26/h1-10,27H,11-17H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HPGDS using PGH2 as substrate assessed as production of PGD2 preincubated for 10 mins prior substrate addition measur... |
Bioorg Med Chem Lett 22: 3795-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.004
BindingDB Entry DOI: 10.7270/Q28C9X82 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50064010
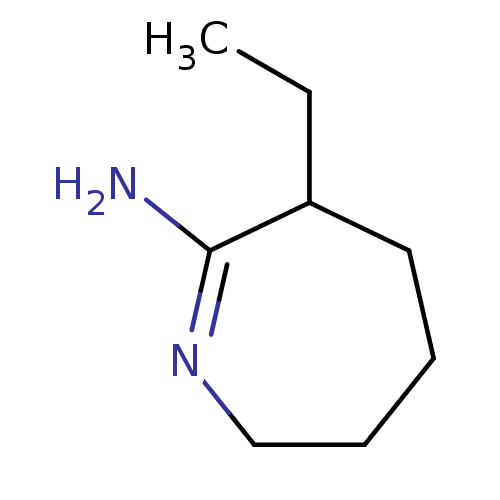
(3-Ethyl-azepan-(2Z)-ylideneamine; hydrochloride | ...)Show InChI InChI=1S/C8H16N2/c1-2-7-5-3-4-6-10-8(7)9/h7H,2-6H2,1H3,(H2,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated for soluble cell extract of human Inducible nitric oxide synthase and partially purified by DEAE-sepharose chromatograp... |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50066778

(5-Ethyl-4-methyl-pyrrolidin-(2E)-ylideneamine; hyd...)Show InChI InChI=1S/C7H14N2/c1-3-6-5(2)4-7(8)9-6/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50064011

(7-Butyl-azepan-(2Z)-ylideneamine; hydrochloride | ...)Show InChI InChI=1S/C10H20N2/c1-2-3-6-9-7-4-5-8-10(11)12-9/h9H,2-8H2,1H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
The ability of compound to inhibit mouse Inducible nitric oxide synthase in LPS stimulated mouse RAW cells was determined |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50064013

(7-(2-Ethyl-butyl)-azepan-(2Z)-ylideneamine; hydroc...)Show InChI InChI=1S/C12H24N2/c1-3-10(4-2)9-11-7-5-6-8-12(13)14-11/h10-11H,3-9H2,1-2H3,(H2,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated for soluble cell extract of human Inducible nitric oxide synthase and partially purified by DEAE-sepharose chromatograp... |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50049257

(Azepan-(2Z)-ylideneamine | CHEMBL315857 | CHEMBL54...)Show InChI InChI=1S/C6H12N2/c7-6-4-2-1-3-5-8-6/h1-5H2,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated for soluble cell extract of human Inducible nitric oxide synthase and partially purified by DEAE-sepharose chromatograp... |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50064010

(3-Ethyl-azepan-(2Z)-ylideneamine; hydrochloride | ...)Show InChI InChI=1S/C8H16N2/c1-2-7-5-3-4-6-10-8(7)9/h7H,2-6H2,1H3,(H2,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated from soluble cell extract of Neuronal nitric oxide synthase and partially purified by DEAE-sepharose chromatography |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066777
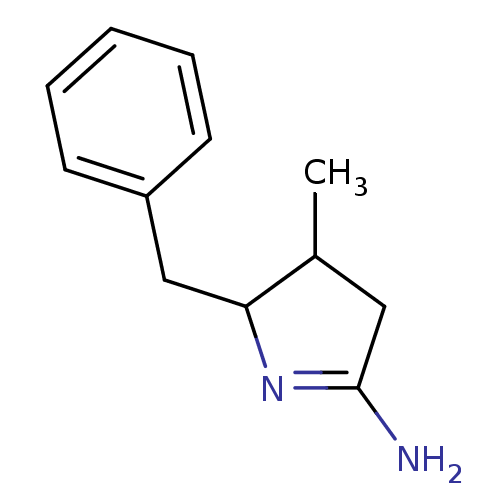
(5-Benzyl-4-methyl-pyrrolidin-(2E)-ylideneamine; hy...)Show InChI InChI=1S/C12H16N2/c1-9-7-12(13)14-11(9)8-10-5-3-2-4-6-10/h2-6,9,11H,7-8H2,1H3,(H2,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human inducible nitric oxide synthase (hiNOS) |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50066781

(3-Methyl-pyrrolidin-(2E)-ylideneamine; hydrochlori...)Show InChI InChI=1S/C5H10N2/c1-4-2-3-7-5(4)6/h4H,2-3H2,1H3,(H2,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human Neuronal nitric oxide synthase |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066777

(5-Benzyl-4-methyl-pyrrolidin-(2E)-ylideneamine; hy...)Show InChI InChI=1S/C12H16N2/c1-9-7-12(13)14-11(9)8-10-5-3-2-4-6-10/h2-6,9,11H,7-8H2,1H3,(H2,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human inducible nitric oxide synthase (hiNOS) |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50064013

(7-(2-Ethyl-butyl)-azepan-(2Z)-ylideneamine; hydroc...)Show InChI InChI=1S/C12H24N2/c1-3-10(4-2)9-11-7-5-6-8-12(13)14-11/h10-11H,3-9H2,1-2H3,(H2,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
The ability of compound to inhibit mouse Inducible nitric oxide synthase in LPS stimulated mouse RAW cells was determined |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066783
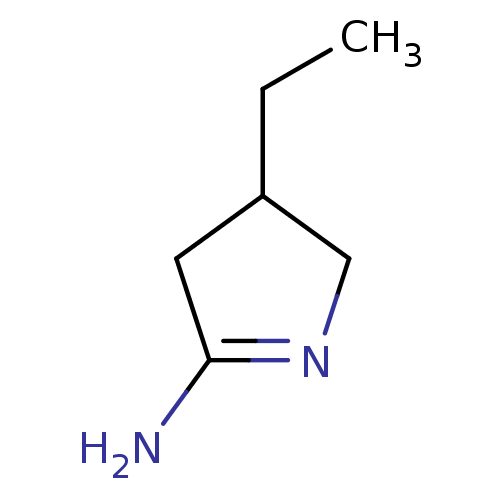
(4-Ethyl-pyrrolidin-(2E)-ylideneamine; hydrochlorid...)Show InChI InChI=1S/C6H12N2/c1-2-5-3-6(7)8-4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human inducible nitric oxide synthase (hiNOS) |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50049257

(Azepan-(2Z)-ylideneamine | CHEMBL315857 | CHEMBL54...)Show InChI InChI=1S/C6H12N2/c7-6-4-2-1-3-5-8-6/h1-5H2,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated from soluble cell extract of human Neuronal nitric oxide synthase and partially purified by DEAE-sepharose chromatograp... |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50066785

(4-Methyl-5-propyl-pyrrolidin-(2E)-ylideneamine; hy...)Show InChI InChI=1S/C8H16N2/c1-3-4-7-6(2)5-8(9)10-7/h6-7H,3-5H2,1-2H3,(H2,9,10) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human endothelial constitutive Endothelial nitric oxide synthase (heNOS) |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50064010

(3-Ethyl-azepan-(2Z)-ylideneamine; hydrochloride | ...)Show InChI InChI=1S/C8H16N2/c1-2-7-5-3-4-6-10-8(7)9/h7H,2-6H2,1H3,(H2,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
The ability of compound to inhibit mouse Inducible nitric oxide synthase in LPS stimulated mouse RAW cells was determined |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50064014
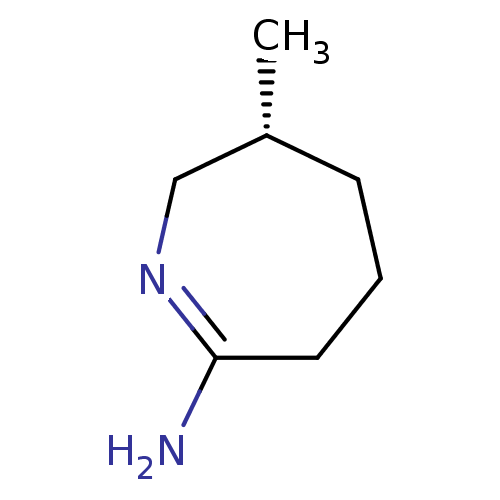
((R)-6-Methyl-azepan-(2Z)-ylideneamine; hydrochlori...)Show InChI InChI=1S/C7H14N2/c1-6-3-2-4-7(8)9-5-6/h6H,2-5H2,1H3,(H2,8,9)/t6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated from soluble cell extract of human nNeuronal nitric oxide synthase and partially purified by DEAE-sepharose chromatogra... |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50066780

(4-Methyl-pyrrolidin-(2E)-ylideneamine; hydrochlori...)Show InChI InChI=1S/C5H10N2/c1-4-2-5(6)7-3-4/h4H,2-3H2,1H3,(H2,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human Neuronal nitric oxide synthase |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50066774

(4-Methyl-5-pentyl-pyrrolidin-(2E)-ylideneamine; hy...)Show InChI InChI=1S/C10H20N2/c1-3-4-5-6-9-8(2)7-10(11)12-9/h8-9H,3-7H2,1-2H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human Neuronal nitric oxide synthase |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50066774

(4-Methyl-5-pentyl-pyrrolidin-(2E)-ylideneamine; hy...)Show InChI InChI=1S/C10H20N2/c1-3-4-5-6-9-8(2)7-10(11)12-9/h8-9H,3-7H2,1-2H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human Neuronal nitric oxide synthase |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50064013

(7-(2-Ethyl-butyl)-azepan-(2Z)-ylideneamine; hydroc...)Show InChI InChI=1S/C12H24N2/c1-3-10(4-2)9-11-7-5-6-8-12(13)14-11/h10-11H,3-9H2,1-2H3,(H2,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated from soluble cell extract of human Neuronal nitric oxide synthase and partially purified by DEAE-sepharose chromatograp... |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50066783

(4-Ethyl-pyrrolidin-(2E)-ylideneamine; hydrochlorid...)Show InChI InChI=1S/C6H12N2/c1-2-5-3-6(7)8-4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cloned (from RNA) human Neuronal nitric oxide synthase |
J Med Chem 41: 3675-83 (1998)
Article DOI: 10.1021/jm970840x
BindingDB Entry DOI: 10.7270/Q2XP75MP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50064019

(5-Methyl-azepan-(2Z)-ylideneamine; hydrochloride |...)Show InChI InChI=1S/C7H14N2/c1-6-2-3-7(8)9-5-4-6/h6H,2-5H2,1H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated for soluble cell extract of human Inducible nitric oxide synthase and partially purified by DEAE-sepharose chromatograp... |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data