

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50105033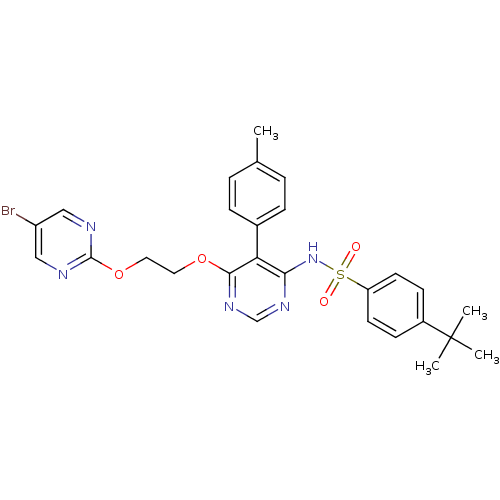 (CHEMBL112531 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50369953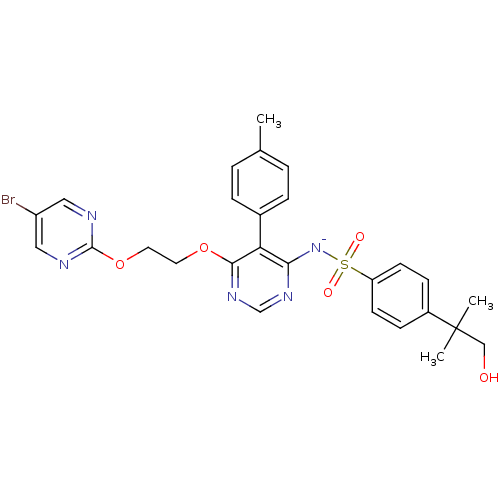 (CHEMBL1627022) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM430 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM430 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50409174 (CHEMBL169119) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM472 (5-tert-butyl-4-{[(6S)-4-hydroxy-6-[2-(4-hydroxyphe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM472 (5-tert-butyl-4-{[(6S)-4-hydroxy-6-[2-(4-hydroxyphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50093142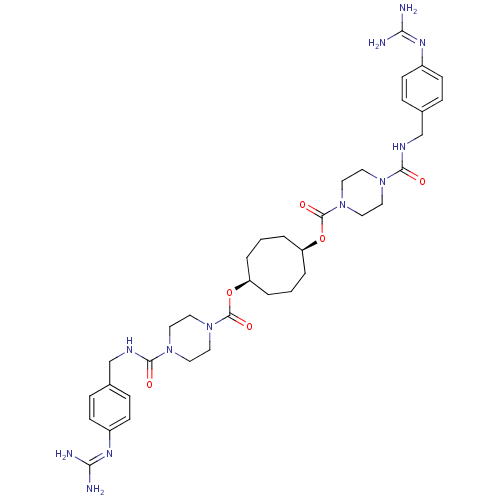 (1,5-di{4-[4-amino(imino)methylaminobenzylcarbamoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human tryptase enzyme | Bioorg Med Chem Lett 11: 2325-30 (2001) BindingDB Entry DOI: 10.7270/Q21835S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50218667 (APC-1390 | CHEMBL46809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5-HT3 serotonin receptor in NG 108-15 neuroblastoma glioma cells using [3H]-GR-65,630 radioligand. | Bioorg Med Chem Lett 11: 1629-33 (2001) BindingDB Entry DOI: 10.7270/Q2R78DHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM465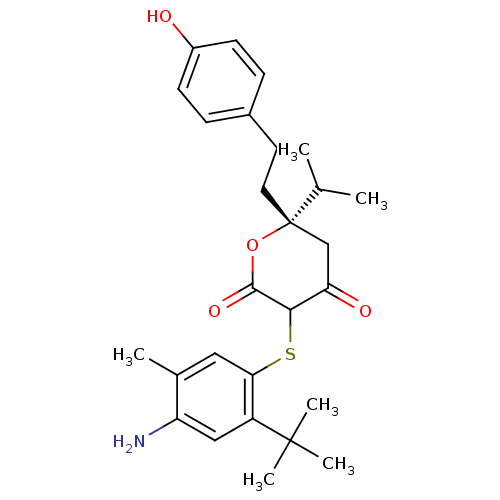 ((6S)-3-[(4-amino-2-tert-butyl-5-methylphenyl)sulfa...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2208 ((6S)-3-{[2-tert-butyl-4-(hydroxymethyl)-5-methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2204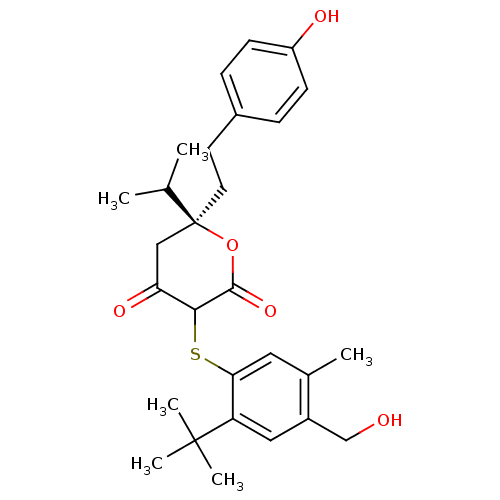 ((3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-phenylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50093157 (CHEMBL431969 | Derivative of piperazine-1-carboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50093175 (CHEMBL311655 | Derivative of APC-2059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2206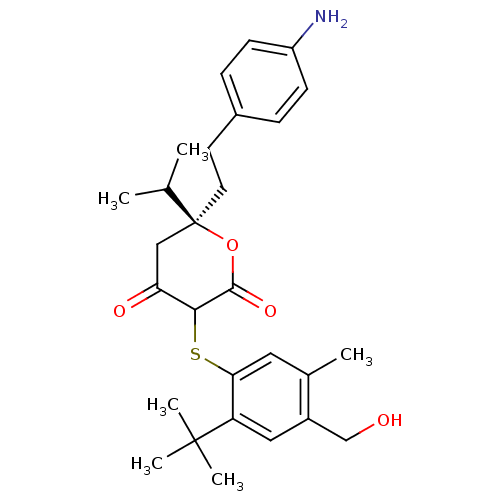 ((6S)-6-[2-(4-aminophenyl)ethyl]-3-{[2-tert-butyl-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50171394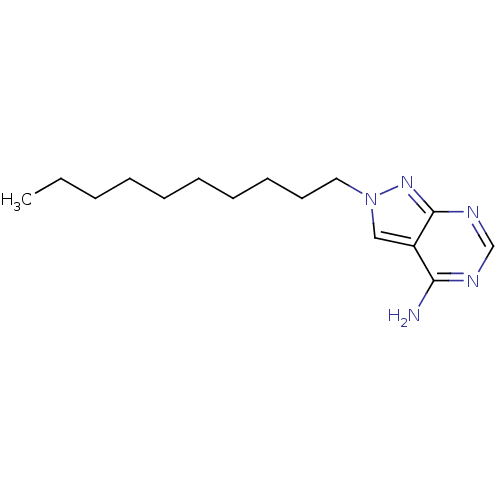 (2-Decyl-2H-pyrazolo[3,4-d]pyrimidin-4-ylamine | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chaminade University of Honolulu Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged VEGFR2 assessed as infrared absorption | Bioorg Med Chem Lett 21: 1371-5 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.037 BindingDB Entry DOI: 10.7270/Q21J9B1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034267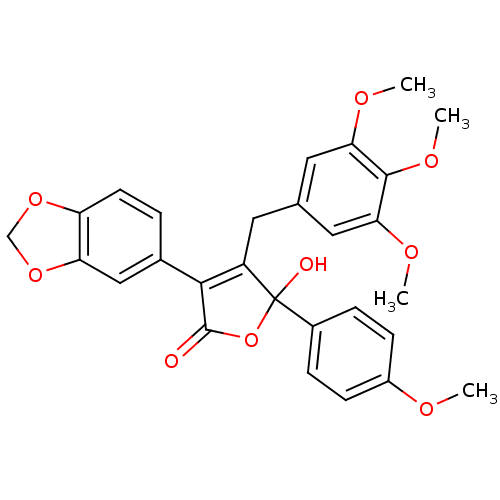 (3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-5-(4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by PDSP Ki Database | J Pharmacol Exp Ther 280: 1102-8 (1997) BindingDB Entry DOI: 10.7270/Q2N0152V | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2533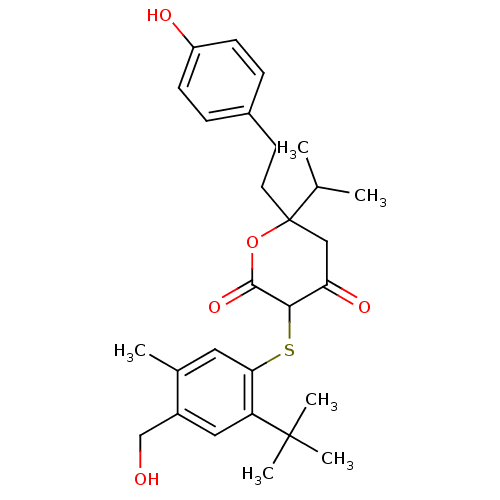 (3-{[2-tert-butyl-4-(hydroxymethyl)-5-methylphenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.170 | -58.0 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29321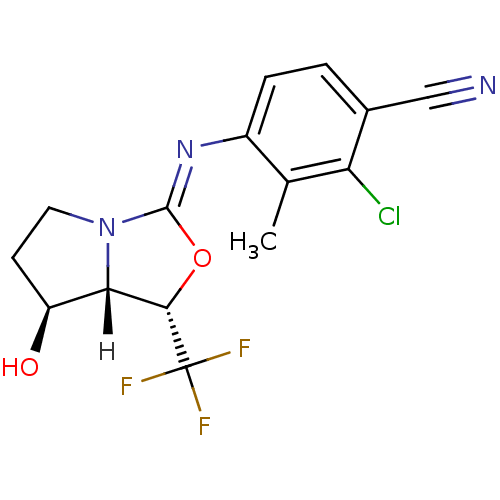 (oxazolidin-2-imine, 6d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -54.8 | n/a | n/a | 19 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM469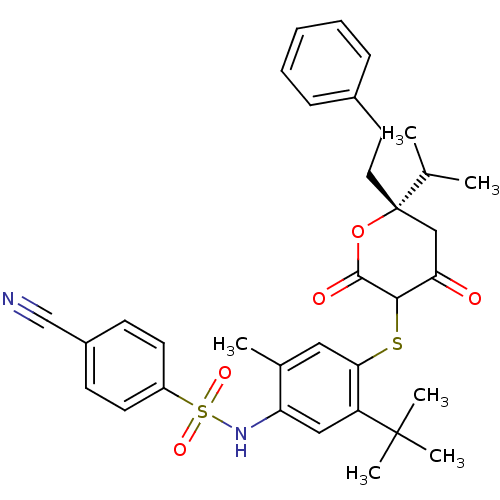 (CHEMBL2110206 | Dihydropyran-2-one deriv. 74 | N-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50001450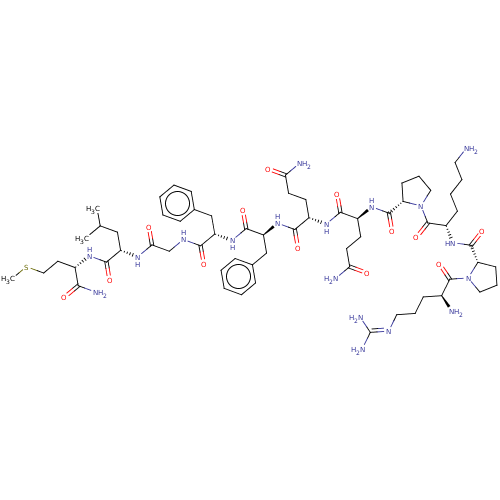 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Curated by PDSP Ki Database | J Pharmacol Exp Ther 277: 840-51 (1996) BindingDB Entry DOI: 10.7270/Q2KD1WF0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50093192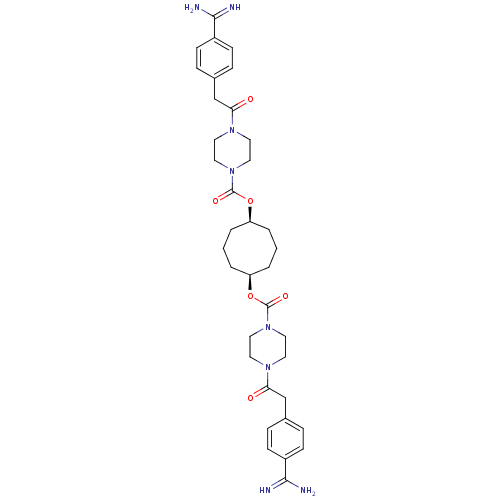 (CHEMBL311482 | Derivative of piperazine-1-carboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM469 (CHEMBL2110206 | Dihydropyran-2-one deriv. 74 | N-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM402 (CHEMBL354027 | Dihydropyran-2-one deriv. 7 | N-[5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM402 (CHEMBL354027 | Dihydropyran-2-one deriv. 7 | N-[5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | -57.5 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Curated by PDSP Ki Database | J Pharmacol Exp Ther 277: 840-51 (1996) BindingDB Entry DOI: 10.7270/Q2KD1WF0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50171396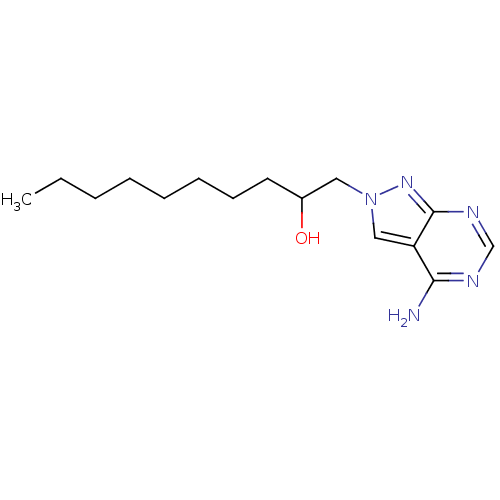 (1-((R)-4-Amino-pyrazolo[3,4-d]pyrimidin-2-yl)-deca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chaminade University of Honolulu Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged VEGFR2 assessed as infrared absorption | Bioorg Med Chem Lett 21: 1371-5 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.037 BindingDB Entry DOI: 10.7270/Q21J9B1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Binding affinity to human cloned 5HT2A receptor | Bioorg Med Chem 16: 7291-301 (2008) Article DOI: 10.1016/j.bmc.2008.06.030 BindingDB Entry DOI: 10.7270/Q2ZP45XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | -53.8 | n/a | n/a | 2.80 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 49: 7596-9 (2006) Article DOI: 10.1021/jm061101w BindingDB Entry DOI: 10.7270/Q2862DQ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29323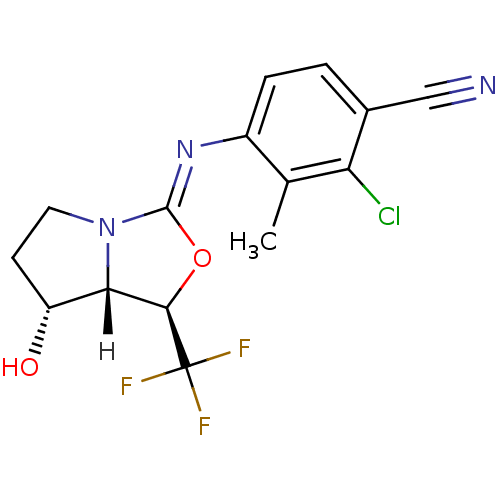 (oxazolidin-2-imine, 6f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | -53.8 | n/a | n/a | 1.40 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29320 (BMS-665139 | oxazolidin-2-imine, 6c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | -53.8 | n/a | n/a | 0.200 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29319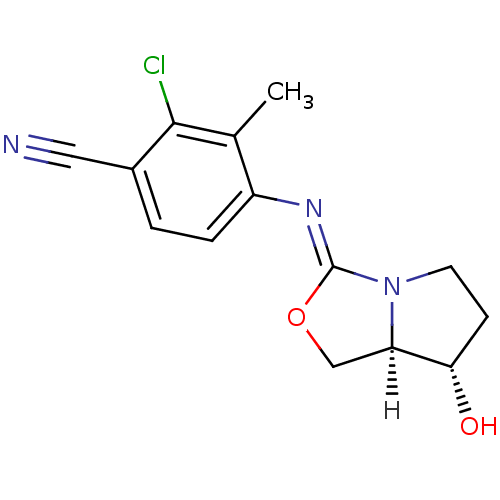 (oxazolidin-2-imine, 6b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | -53.8 | n/a | n/a | 14 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM470 (CHEMBL2110205 | Dihydropyran-2-one deriv. 75 | N-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50469804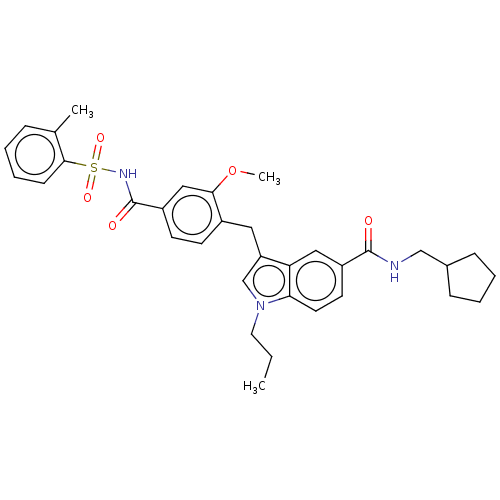 (CHEMBL339277) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Binding affinity to the leukotriene receptor (LTD4) from guinea pig lung parenchymal membranes assayed using [3H]LTD4 as radioligand | J Med Chem 36: 394-409 (1993) Article DOI: 10.1021/jm00055a011 BindingDB Entry DOI: 10.7270/Q2FX7D5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50009073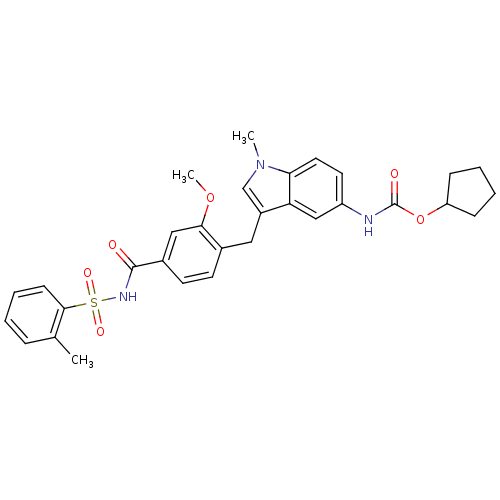 (4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 37: 1282-97 (1994) BindingDB Entry DOI: 10.7270/Q2SN0812 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50041356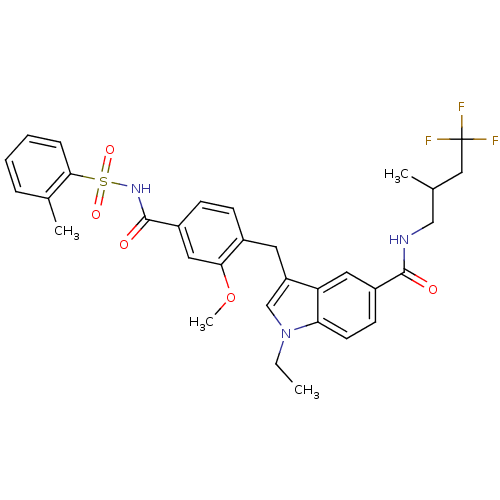 (1-Ethyl-3-[2-methoxy-4-(toluene-2-sulfonylaminocar...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 37: 1282-97 (1994) BindingDB Entry DOI: 10.7270/Q2SN0812 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM467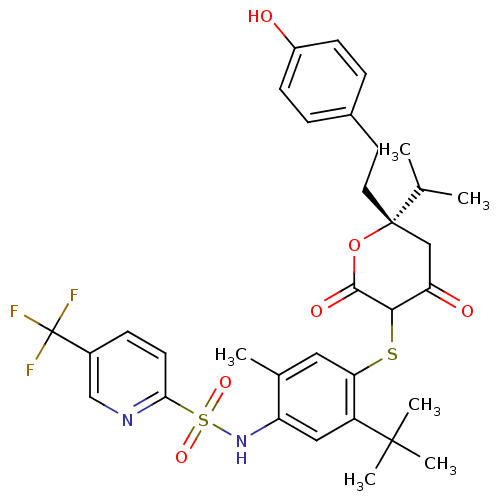 ((S)-N-(5-tert-Butyl-4-{4-hydroxy-6-[2-(4-hydroxyph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50048803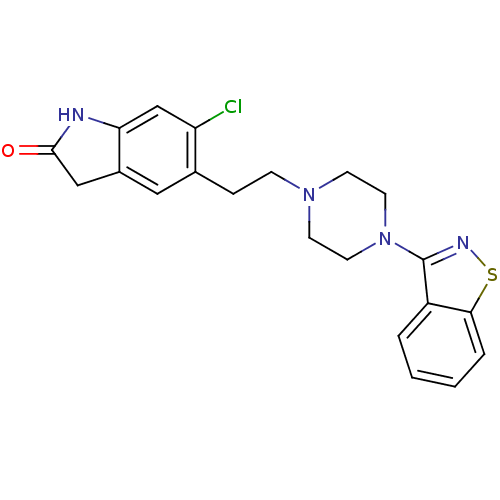 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Binding affinity to human cloned 5HT2A receptor | Bioorg Med Chem 16: 7291-301 (2008) Article DOI: 10.1016/j.bmc.2008.06.030 BindingDB Entry DOI: 10.7270/Q2ZP45XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM467 ((S)-N-(5-tert-Butyl-4-{4-hydroxy-6-[2-(4-hydroxyph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50469821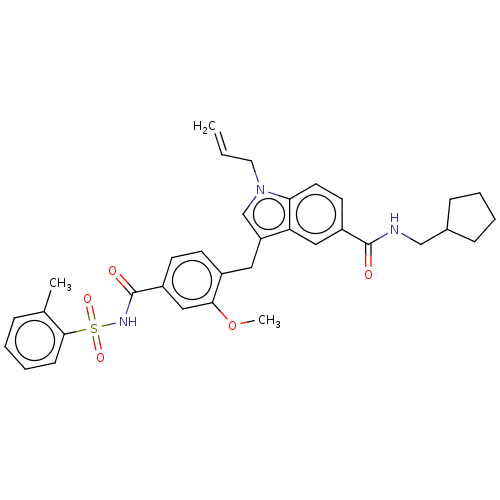 (CHEMBL130463) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Binding affinity to the leukotriene receptor (LTD4) from guinea pig lung parenchymal membranes assayed using [3H]LTD4 as radioligand | J Med Chem 36: 394-409 (1993) Article DOI: 10.1021/jm00055a011 BindingDB Entry DOI: 10.7270/Q2FX7D5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50101018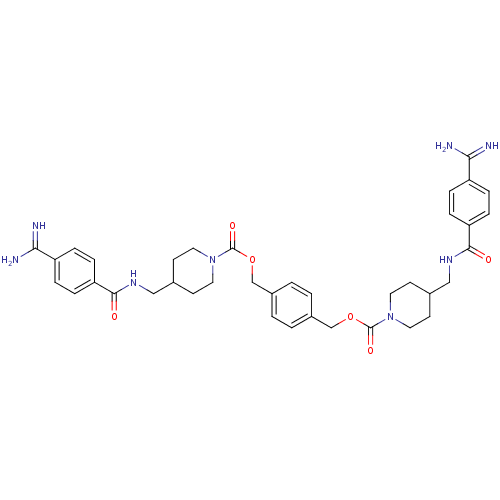 (1,4-di{4-[4-amino(imino)methylphenylcarboxamidomet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human tryptase enzyme | Bioorg Med Chem Lett 11: 2325-30 (2001) BindingDB Entry DOI: 10.7270/Q21835S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50093156 (CHEMBL432172 | Derivative of APC-2059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50213599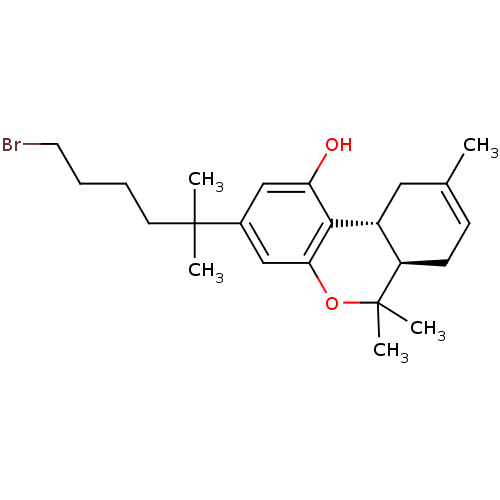 ((6aR,10aR)-3-(6-bromo-2-methylhexan-2-yl)-6,6,9-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chaminade University of Honolulu Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged VEGFR2 assessed as infrared absorption | Bioorg Med Chem Lett 21: 1371-5 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.037 BindingDB Entry DOI: 10.7270/Q21J9B1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50101018 (1,4-di{4-[4-amino(imino)methylphenylcarboxamidomet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5-HT3 serotonin receptor in NG 108-15 neuroblastoma glioma cells using [3H]-GR-65,630 radioligand. | Bioorg Med Chem Lett 11: 1629-33 (2001) BindingDB Entry DOI: 10.7270/Q2R78DHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50093154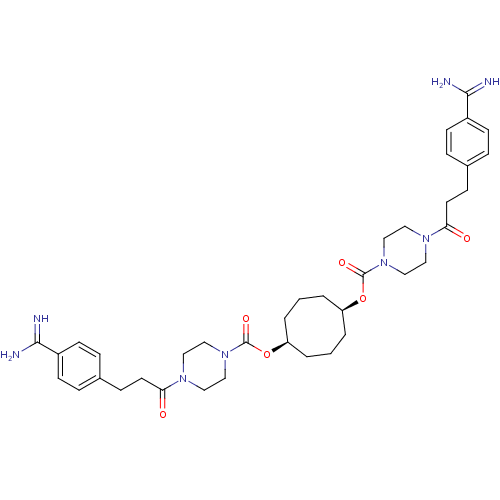 (CHEMBL448786 | Derivative of piperazine-1-carboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50093167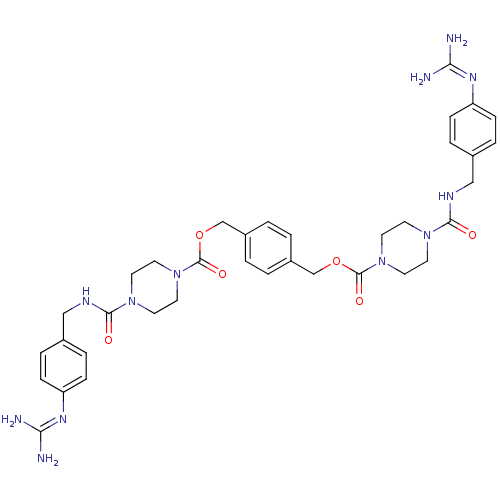 (CHEMBL75750 | Derivative of APC-2059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Evaluated for its inhibitory potency against tryptase | Bioorg Med Chem Lett 10: 2361-6 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM403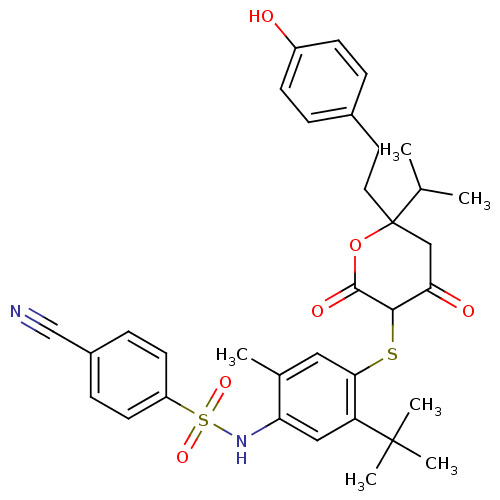 (CHEMBL169391 | Dihydropyran-2-one deriv. 8 | N-[5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | -55.7 | 2.20 | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM403 (CHEMBL169391 | Dihydropyran-2-one deriv. 8 | N-[5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50041349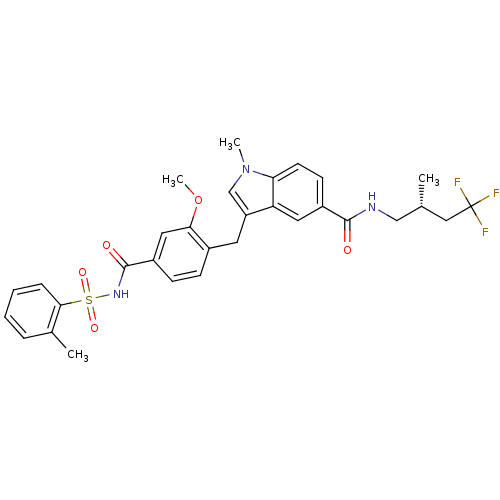 (3-[2-Methoxy-4-(toluene-2-sulfonylaminocarbonyl)-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 37: 1282-97 (1994) BindingDB Entry DOI: 10.7270/Q2SN0812 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2536 (6-Alkyl-6-phenethyldihydropyrone 13y | 6-[2-(4-ami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5411 total ) | Next | Last >> |