 Found 1549 hits with Last Name = 'lai' and Initial = 'h'
Found 1549 hits with Last Name = 'lai' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM50025935
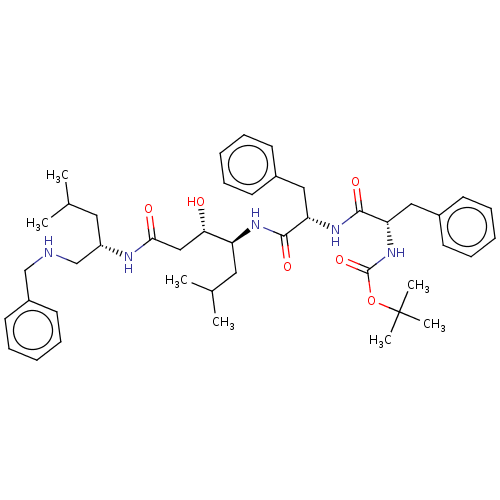
(CHEMBL3144404 | {1-[1-(1-{2-[1-(Benzylamino-methyl...)Show SMILES CC(C)C[C@@H](CNCc1ccccc1)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C44H63N5O6/c1-30(2)23-35(29-45-28-34-21-15-10-16-22-34)46-40(51)27-39(50)36(24-31(3)4)47-41(52)37(25-32-17-11-8-12-18-32)48-42(53)38(26-33-19-13-9-14-20-33)49-43(54)55-44(5,6)7/h8-22,30-31,35-39,45,50H,23-29H2,1-7H3,(H,46,51)(H,47,52)(H,48,53)(H,49,54) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of hog kidney renin |
J Med Chem 28: 1755-6 (1986)
BindingDB Entry DOI: 10.7270/Q2P26X56 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 50: 2391-8 (2007)
Article DOI: 10.1021/jm061321+
BindingDB Entry DOI: 10.7270/Q2NC6407 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025934
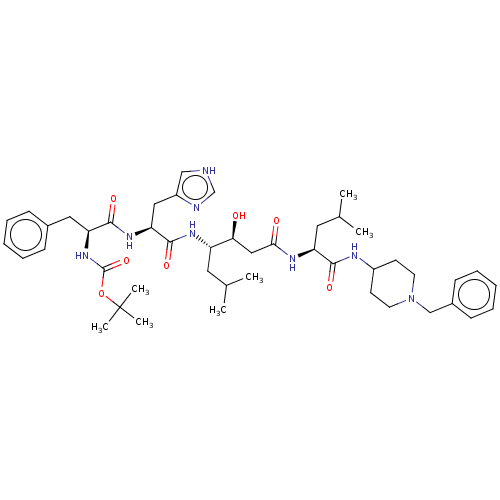
(CHEMBL3144405 | {1-[1-(1-{2-[1-(1-Benzyl-piperidin...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)NC1CCN(Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C46H68N8O7/c1-30(2)22-36(40(55)26-41(56)50-37(23-31(3)4)42(57)49-34-18-20-54(21-19-34)28-33-16-12-9-13-17-33)51-44(59)39(25-35-27-47-29-48-35)52-43(58)38(24-32-14-10-8-11-15-32)53-45(60)61-46(5,6)7/h8-17,27,29-31,34,36-40,55H,18-26,28H2,1-7H3,(H,47,48)(H,49,57)(H,50,56)(H,51,59)(H,52,58)(H,53,60) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human kidney renin |
J Med Chem 28: 1755-6 (1986)
BindingDB Entry DOI: 10.7270/Q2P26X56 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405190
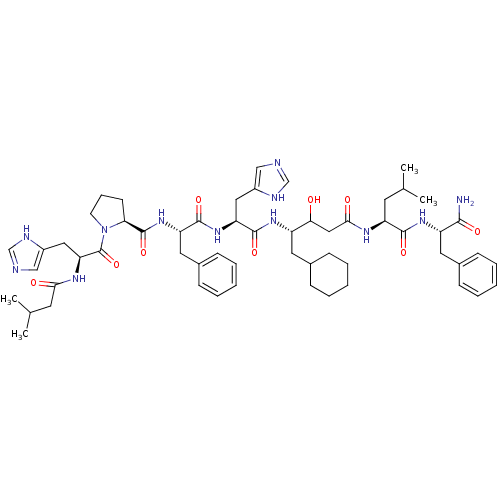
(CHEMBL2028991)Show SMILES CC(C)C[C@H](NC(=O)CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C57H80N12O9/c1-35(2)23-44(53(74)66-43(52(58)73)26-38-17-10-6-11-18-38)63-51(72)30-49(70)42(25-37-15-8-5-9-16-37)65-55(76)46(28-40-31-59-33-61-40)67-54(75)45(27-39-19-12-7-13-20-39)68-56(77)48-21-14-22-69(48)57(78)47(29-41-32-60-34-62-41)64-50(71)24-36(3)4/h6-7,10-13,17-20,31-37,42-49,70H,5,8-9,14-16,21-30H2,1-4H3,(H2,58,73)(H,59,61)(H,60,62)(H,63,72)(H,64,71)(H,65,76)(H,66,74)(H,67,75)(H,68,77)/t42-,43-,44-,45-,46-,47-,48-,49?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 50: 2391-8 (2007)
Article DOI: 10.1021/jm061321+
BindingDB Entry DOI: 10.7270/Q2NC6407 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405190

(CHEMBL2028991)Show SMILES CC(C)C[C@H](NC(=O)CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C57H80N12O9/c1-35(2)23-44(53(74)66-43(52(58)73)26-38-17-10-6-11-18-38)63-51(72)30-49(70)42(25-37-15-8-5-9-16-37)65-55(76)46(28-40-31-59-33-61-40)67-54(75)45(27-39-19-12-7-13-20-39)68-56(77)48-21-14-22-69(48)57(78)47(29-41-32-60-34-62-41)64-50(71)24-36(3)4/h6-7,10-13,17-20,31-37,42-49,70H,5,8-9,14-16,21-30H2,1-4H3,(H2,58,73)(H,59,61)(H,60,62)(H,63,72)(H,64,71)(H,65,76)(H,66,74)(H,67,75)(H,68,77)/t42-,43-,44-,45-,46-,47-,48-,49?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of rhesus monkey plasma renin. |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50600964
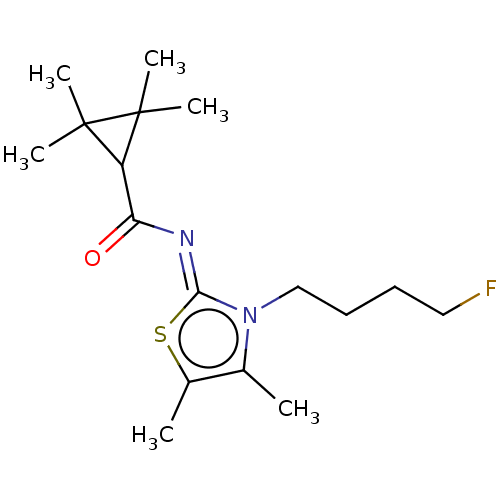
(CHEMBL5181335)Show SMILES Cc1s\c(=N/C(=O)C2C(C)(C)C2(C)C)n(CCCC[18F])c1C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00256
BindingDB Entry DOI: 10.7270/Q2Z89HGZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050511
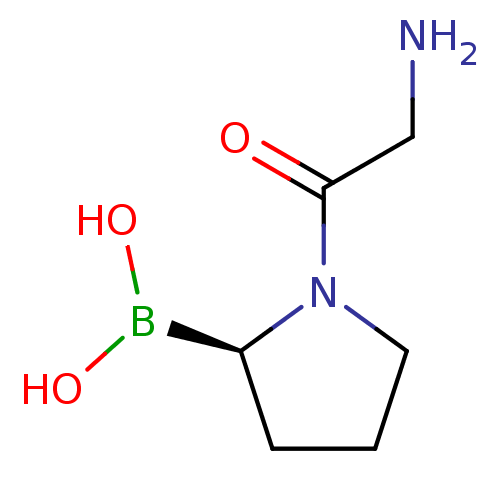
((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...)Show InChI InChI=1S/C6H13BN2O3/c8-4-6(10)9-3-1-2-5(9)7(11)12/h5,11-12H,1-4,8H2/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50050511

((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...)Show InChI InChI=1S/C6H13BN2O3/c8-4-6(10)9-3-1-2-5(9)7(11)12/h5,11-12H,1-4,8H2/t5-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP9 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025937
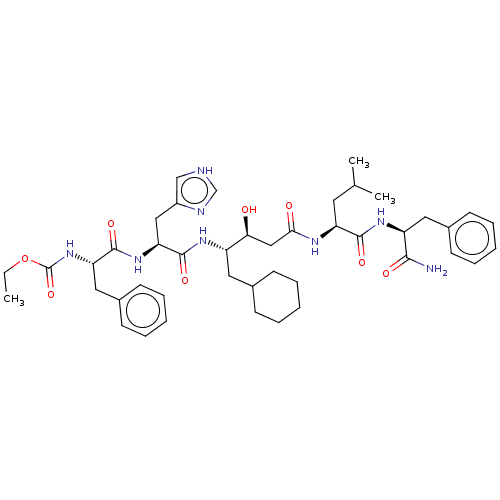
(CHEMBL3144417 | {1-[1-{3-[1-(1-Carbamoyl-2-phenyl-...)Show SMILES CCOC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C44H62N8O8/c1-4-60-44(59)52-36(23-31-18-12-7-13-19-31)42(57)51-37(24-32-26-46-27-47-32)43(58)49-33(21-29-14-8-5-9-15-29)38(53)25-39(54)48-35(20-28(2)3)41(56)50-34(40(45)55)22-30-16-10-6-11-17-30/h6-7,10-13,16-19,26-29,33-38,53H,4-5,8-9,14-15,20-25H2,1-3H3,(H2,45,55)(H,46,47)(H,48,54)(H,49,58)(H,50,56)(H,51,57)(H,52,59) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP9 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405190

(CHEMBL2028991)Show SMILES CC(C)C[C@H](NC(=O)CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C57H80N12O9/c1-35(2)23-44(53(74)66-43(52(58)73)26-38-17-10-6-11-18-38)63-51(72)30-49(70)42(25-37-15-8-5-9-16-37)65-55(76)46(28-40-31-59-33-61-40)67-54(75)45(27-39-19-12-7-13-20-39)68-56(77)48-21-14-22-69(48)57(78)47(29-41-32-60-34-62-41)64-50(71)24-36(3)4/h6-7,10-13,17-20,31-37,42-49,70H,5,8-9,14-16,21-30H2,1-4H3,(H2,58,73)(H,59,61)(H,60,62)(H,63,72)(H,64,71)(H,65,76)(H,66,74)(H,67,75)(H,68,77)/t42-,43-,44-,45-,46-,47-,48-,49?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50600961

(CHEMBL5198892)Show SMILES FCCCCn1c2ncccc2cc(C(=O)NC23CC4CC(CC(C4)C2)C3)c1=O |TLB:16:17:20:23.24.22,THB:25:17:20:23.24.22,25:23:20:26.17.18,22:21:18:25.23.24,22:23:20.21.26:18| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00256
BindingDB Entry DOI: 10.7270/Q2Z89HGZ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50600966
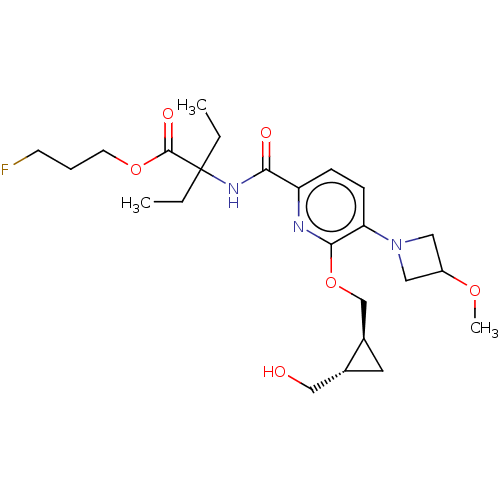
(CHEMBL5205138)Show SMILES [2H]C([2H])([18F])C([2H])([2H])C([2H])([2H])OC(=O)C(CC)(CC)NC(=O)c1ccc(N2CC(C2)OC)c(OC[C@H]2C[C@@H]2CO)n1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00256
BindingDB Entry DOI: 10.7270/Q2Z89HGZ |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50431227
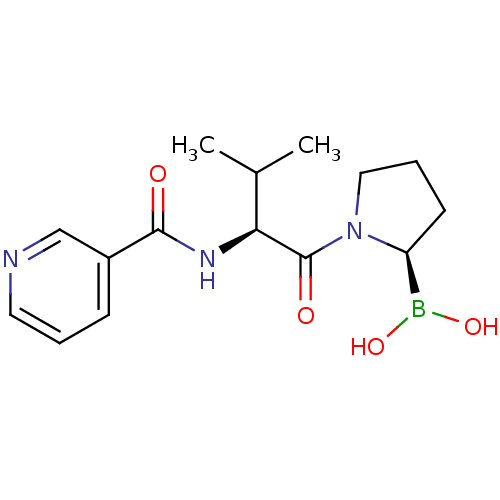
(CHEMBL2333024)Show SMILES CC(C)[C@H](NC(=O)c1cccnc1)C(=O)N1CCC[C@H]1B(O)O |r| Show InChI InChI=1S/C15H22BN3O4/c1-10(2)13(18-14(20)11-5-3-7-17-9-11)15(21)19-8-4-6-12(19)16(22)23/h3,5,7,9-10,12-13,22-23H,4,6,8H2,1-2H3,(H,18,20)/t12-,13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PREP using Suc-GP-AMC as substrate by Lineweaver-Burk plot analysis |
J Med Chem 56: 3467-77 (2013)
Article DOI: 10.1021/jm400351a
BindingDB Entry DOI: 10.7270/Q2C53N7W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP9 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025945
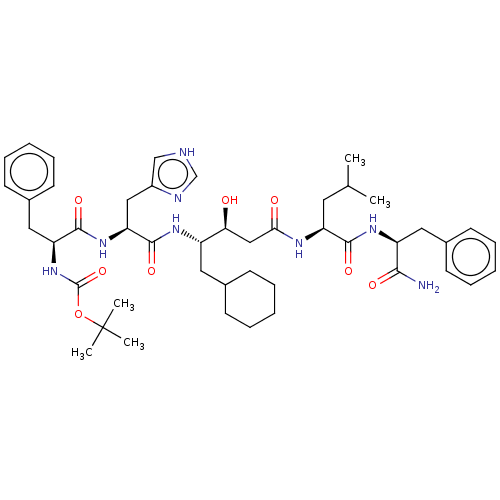
(CHEMBL3144416 | {1-[1-{3-[1-(1-Carbamoyl-2-phenyl-...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C46H66N8O8/c1-29(2)21-36(42(58)52-35(41(47)57)23-31-17-11-7-12-18-31)50-40(56)26-39(55)34(22-30-15-9-6-10-16-30)51-44(60)38(25-33-27-48-28-49-33)53-43(59)37(24-32-19-13-8-14-20-32)54-45(61)62-46(3,4)5/h7-8,11-14,17-20,27-30,34-39,55H,6,9-10,15-16,21-26H2,1-5H3,(H2,47,57)(H,48,49)(H,50,56)(H,51,60)(H,52,58)(H,53,59)(H,54,61) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human kidney renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50600965

(CHEMBL5182619)Show SMILES COc1cccc2c1n(CCOCCC[18F])cc(C(=O)NC13CC4CC(CC(C4)C1)C3)c2=O |TLB:24:25:23.22.28:29,THB:24:23:29:30.25.26,26:25:22:28.27.29,26:27:30.25.24:22| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00256
BindingDB Entry DOI: 10.7270/Q2Z89HGZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025941
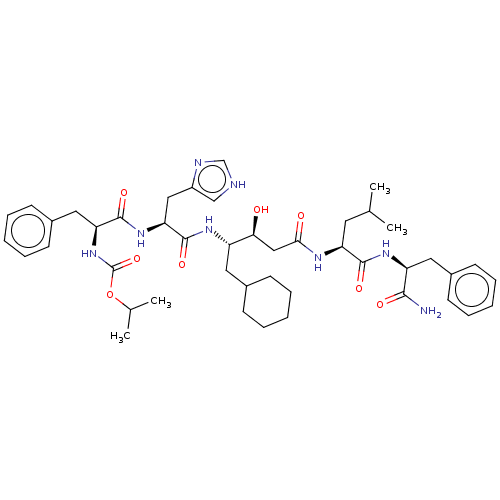
(CHEMBL3144415 | {1-[1-{3-[1-(1-Carbamoyl-2-phenyl-...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C45H64N8O8/c1-28(2)20-36(42(57)51-35(41(46)56)22-31-16-10-6-11-17-31)49-40(55)25-39(54)34(21-30-14-8-5-9-15-30)50-44(59)38(24-33-26-47-27-48-33)52-43(58)37(53-45(60)61-29(3)4)23-32-18-12-7-13-19-32/h6-7,10-13,16-19,26-30,34-39,54H,5,8-9,14-15,20-25H2,1-4H3,(H2,46,56)(H,47,48)(H,49,55)(H,50,59)(H,51,57)(H,52,58)(H,53,60) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP8 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50600962
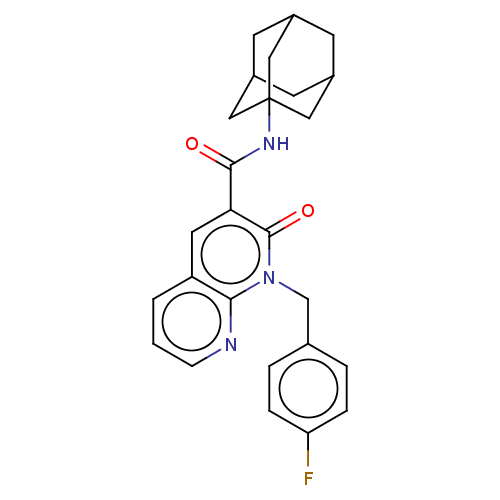
(CHEMBL5186632)Show SMILES Fc1ccc(Cn2c3ncccc3cc(C(=O)NC34CC5CC(CC(C5)C3)C4)c2=O)cc1 |TLB:21:22:20.19.25:26,THB:21:20:26:27.22.23,23:22:19:25.24.26,23:24:27.22.21:19| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00256
BindingDB Entry DOI: 10.7270/Q2Z89HGZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507688
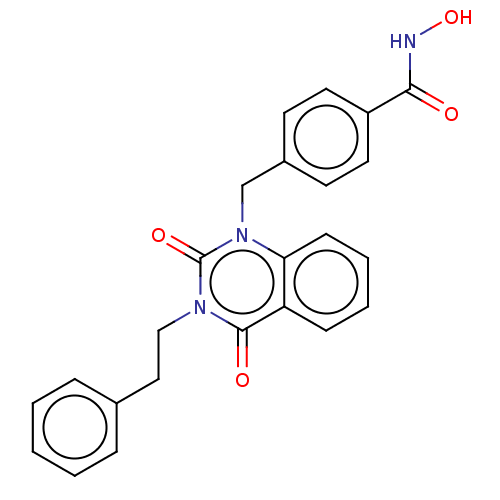
(CHEMBL4550214 | US11535598, Compound 5)Show SMILES ONC(=O)c1ccc(Cn2c3ccccc3c(=O)n(CCc3ccccc3)c2=O)cc1 Show InChI InChI=1S/C24H21N3O4/c28-22(25-31)19-12-10-18(11-13-19)16-27-21-9-5-4-8-20(21)23(29)26(24(27)30)15-14-17-6-2-1-3-7-17/h1-13,31H,14-16H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human full-length recombinant HDAC6 expressed in baculovirus infected Sf9 insect cells using Boc-Lys (Ac)-AMC as substrate ... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50029958
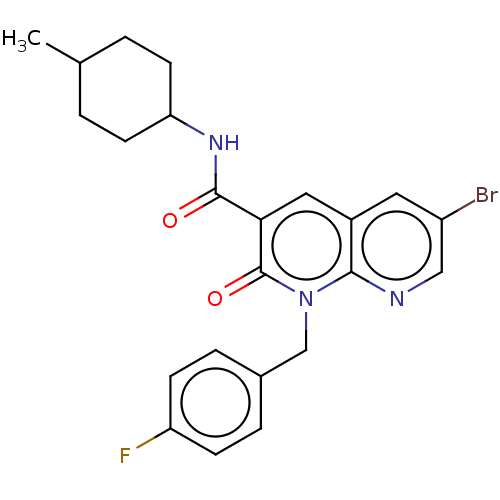
(CHEMBL3353439)Show SMILES CC1CCC(CC1)NC(=O)c1cc2cc(Br)cnc2n(Cc2ccc(F)cc2)c1=O |(16.02,-5.47,;14.69,-6.25,;14.69,-7.79,;13.37,-8.56,;12.03,-7.79,;12.02,-6.26,;13.35,-5.48,;10.7,-8.57,;9.36,-7.8,;9.35,-6.26,;8.03,-8.58,;6.69,-7.82,;5.37,-8.6,;4.03,-7.83,;2.7,-8.6,;1.37,-7.83,;2.7,-10.15,;4.04,-10.92,;5.37,-10.14,;6.7,-10.91,;6.71,-12.45,;8.04,-13.21,;8.04,-14.75,;9.37,-15.52,;10.71,-14.74,;12.04,-15.51,;10.7,-13.19,;9.36,-12.43,;8.04,-10.13,;9.38,-10.9,)| Show InChI InChI=1S/C23H23BrFN3O2/c1-14-2-8-19(9-3-14)27-22(29)20-11-16-10-17(24)12-26-21(16)28(23(20)30)13-15-4-6-18(25)7-5-15/h4-7,10-12,14,19H,2-3,8-9,13H2,1H3,(H,27,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00256
BindingDB Entry DOI: 10.7270/Q2Z89HGZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50253621
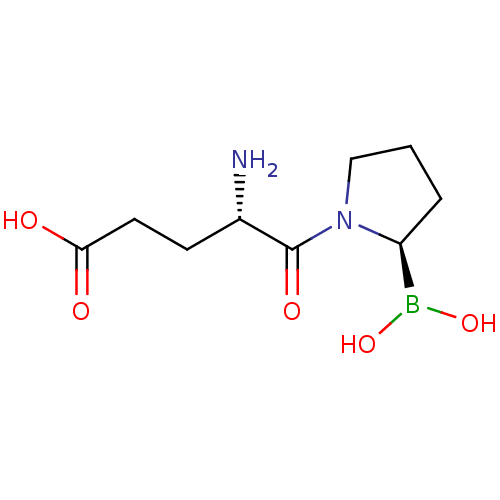
((S)-4-amino-5-((R)-2-boronopyrrolidin-1-yl)-5-oxop...)Show InChI InChI=1S/C9H17BN2O5/c11-6(3-4-8(13)14)9(15)12-5-1-2-7(12)10(16)17/h6-7,16-17H,1-5,11H2,(H,13,14)/t6-,7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP8 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507695
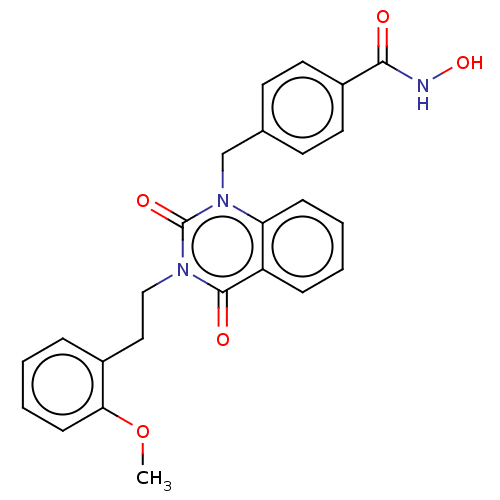
(CHEMBL4554270 | US11535598, Compound 15)Show SMILES COc1ccccc1CCn1c(=O)n(Cc2ccc(cc2)C(=O)NO)c2ccccc2c1=O Show InChI InChI=1S/C25H23N3O5/c1-33-22-9-5-2-6-18(22)14-15-27-24(30)20-7-3-4-8-21(20)28(25(27)31)16-17-10-12-19(13-11-17)23(29)26-32/h2-13,32H,14-16H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length recombinant HDAC6 expressed in baculovirus infected Sf9 insect cells using Boc-Lys (Ac)-AMC as substrate preincubated... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50476305
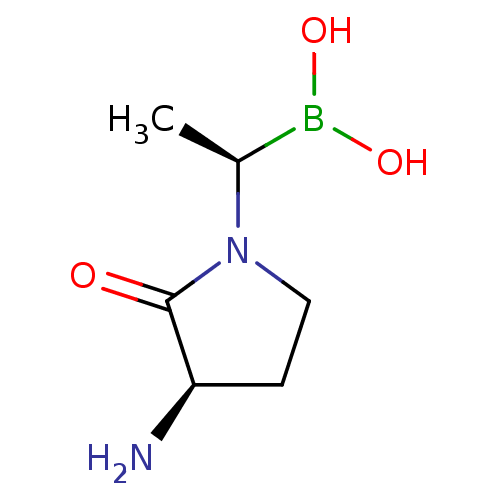
(CHEMBL2068511)Show InChI InChI=1S/C6H13BN2O3/c1-4(7(11)12)9-3-2-5(8)6(9)10/h4-5,11-12H,2-3,8H2,1H3/t4-,5+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 50: 2391-8 (2007)
Article DOI: 10.1021/jm061321+
BindingDB Entry DOI: 10.7270/Q2NC6407 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50253621

((S)-4-amino-5-((R)-2-boronopyrrolidin-1-yl)-5-oxop...)Show InChI InChI=1S/C9H17BN2O5/c11-6(3-4-8(13)14)9(15)12-5-1-2-7(12)10(16)17/h6-7,16-17H,1-5,11H2,(H,13,14)/t6-,7-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP9 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507695

(CHEMBL4554270 | US11535598, Compound 15)Show SMILES COc1ccccc1CCn1c(=O)n(Cc2ccc(cc2)C(=O)NO)c2ccccc2c1=O Show InChI InChI=1S/C25H23N3O5/c1-33-22-9-5-2-6-18(22)14-15-27-24(30)20-7-3-4-8-21(20)28(25(27)31)16-17-10-12-19(13-11-17)23(29)26-32/h2-13,32H,14-16H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 CD2 expressed in Escherichia coli BL21 (RIL) using Boc-Lys (Ac)-AMC as substrate preincubated for 10 mins followed by subst... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50600942
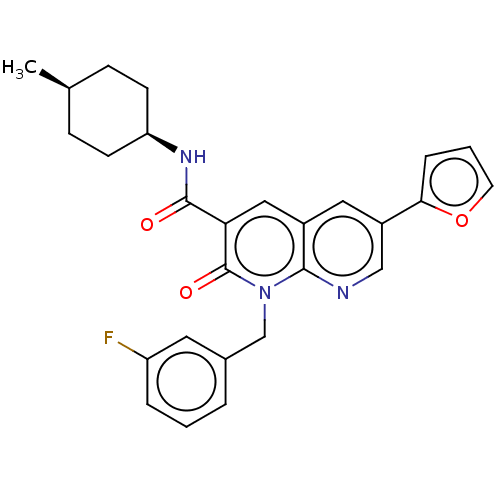
(CHEMBL5173419)Show SMILES C[C@H]1CC[C@H](CC1)NC(=O)c1cc2cc(cnc2n(Cc2cccc(F)c2)c1=O)-c1ccco1 |r,wD:4.7,1.0,(8.58,5,;7.24,4.23,;5.91,5,;4.57,4.23,;4.57,2.69,;5.91,1.92,;7.24,2.69,;3.24,1.92,;1.91,2.69,;1.91,4.23,;.58,1.92,;-.76,2.69,;-2.09,1.93,;-3.42,2.7,;-4.75,1.93,;-4.75,.38,;-3.42,-.38,;-2.09,.39,;-.75,-.39,;-.75,-1.93,;.58,-2.7,;.58,-4.24,;1.92,-5,;3.25,-4.24,;3.25,-2.69,;4.58,-1.93,;1.92,-1.93,;.58,.39,;1.91,-.38,;-6.09,2.7,;-6.42,4.19,;-7.96,4.36,;-8.58,2.99,;-7.42,1.93,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00256
BindingDB Entry DOI: 10.7270/Q2Z89HGZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50050511

((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...)Show InChI InChI=1S/C6H13BN2O3/c8-4-6(10)9-3-1-2-5(9)7(11)12/h5,11-12H,1-4,8H2/t5-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP8 expressed in HEK293T cells |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50600948
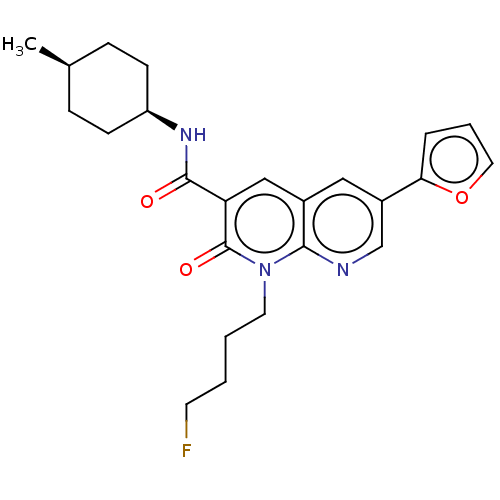
(CHEMBL5199801)Show SMILES C[C@H]1CC[C@H](CC1)NC(=O)c1cc2cc(cnc2n(CCCCF)c1=O)-c1ccco1 |r,wD:4.7,1.0,(8.55,5.78,;7.22,5.01,;5.88,5.78,;4.55,5.01,;4.55,3.47,;5.88,2.7,;7.22,3.47,;3.21,2.7,;1.88,3.47,;1.88,5.01,;.55,2.7,;-.79,3.46,;-2.12,2.7,;-3.45,3.47,;-4.78,2.7,;-4.78,1.15,;-3.44,.39,;-2.12,1.16,;-.78,.39,;-.78,-1.15,;-2.11,-1.92,;-2.11,-3.47,;-3.45,-4.24,;-3.45,-5.78,;.55,1.16,;1.88,.39,;-6.11,3.47,;-6.27,5,;-7.78,5.32,;-8.55,3.99,;-7.52,2.84,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00256
BindingDB Entry DOI: 10.7270/Q2Z89HGZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length recombinant HDAC6 expressed in baculovirus infected Sf9 insect cells using Boc-Lys (Ac)-AMC as substrate preincubated... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025932

(CHEMBL3144406 | Nitrate salt of {1-[1-(1-{2-[1-(1-...)Show SMILES O[N+]([O-])=O.CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)NCc1cccc(CN=C(N)N)c1 |r,wU:12.11,44.45,38.39,wD:22.22,8.6,(-1.54,,;;.77,1.33,;.77,-1.33,;18.37,2.44,;19.8,3.03,;20,4.56,;21.02,2.09,;20.81,.56,;22.03,-.38,;23.46,.21,;23.66,1.74,;24.68,-.73,;24.47,-2.25,;23.05,-2.84,;22.69,-4.34,;21.15,-4.46,;20.57,-3.03,;21.74,-2.03,;26.1,-.14,;27.32,-1.08,;27.12,-2.61,;28.75,-.49,;28.95,1.03,;30.37,1.62,;30.58,3.15,;32,3.73,;33.22,2.8,;33.02,1.27,;31.59,.68,;29.97,-1.43,;29.76,-2.96,;30.98,-3.9,;28.34,-3.55,;28.14,-5.07,;27.93,-6.6,;26.61,-4.87,;29.66,-5.28,;19.39,-.02,;19.19,-1.55,;18.17,.92,;16.75,.33,;16.54,-1.2,;15.53,1.27,;14.1,.68,;13.9,-.84,;12.48,-1.43,;12.27,-2.96,;11.25,-.49,;12.88,1.62,;13.09,3.15,;11.46,1.03,;10.24,1.97,;8.81,1.39,;8.61,-.14,;7.19,-.73,;5.97,.21,;6.17,1.74,;4.95,2.68,;5.15,4.2,;6.58,4.79,;6.78,6.32,;7.8,3.85,;7.59,2.33,)| Show InChI InChI=1S/C43H64N10O7/c1-26(2)16-32(36(54)21-37(55)50-33(17-27(3)4)38(56)47-22-29-14-11-15-30(18-29)23-48-41(44)45)51-40(58)35(20-31-24-46-25-49-31)52-39(57)34(19-28-12-9-8-10-13-28)53-42(59)60-43(5,6)7/h8-15,18,24-27,32-36,54H,16-17,19-23H2,1-7H3,(H,46,49)(H,47,56)(H,50,55)(H,51,58)(H,52,57)(H,53,59)(H4,44,45,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human plasma renin |
J Med Chem 28: 1755-6 (1986)
BindingDB Entry DOI: 10.7270/Q2P26X56 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507688

(CHEMBL4550214 | US11535598, Compound 5)Show SMILES ONC(=O)c1ccc(Cn2c3ccccc3c(=O)n(CCc3ccccc3)c2=O)cc1 Show InChI InChI=1S/C24H21N3O4/c28-22(25-31)19-12-10-18(11-13-19)16-27-21-9-5-4-8-20(21)23(29)26(24(27)30)15-14-17-6-2-1-3-7-17/h1-13,31H,14-16H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human HDAC6 CD2 expressed in Escherichia coli BL21 (RIL) using Boc-Lys (Ac)-AMC as substrate preincubated for 10 mins follo... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405190

(CHEMBL2028991)Show SMILES CC(C)C[C@H](NC(=O)CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C57H80N12O9/c1-35(2)23-44(53(74)66-43(52(58)73)26-38-17-10-6-11-18-38)63-51(72)30-49(70)42(25-37-15-8-5-9-16-37)65-55(76)46(28-40-31-59-33-61-40)67-54(75)45(27-39-19-12-7-13-20-39)68-56(77)48-21-14-22-69(48)57(78)47(29-41-32-60-34-62-41)64-50(71)24-36(3)4/h6-7,10-13,17-20,31-37,42-49,70H,5,8-9,14-16,21-30H2,1-4H3,(H2,58,73)(H,59,61)(H,60,62)(H,63,72)(H,64,71)(H,65,76)(H,66,74)(H,67,75)(H,68,77)/t42-,43-,44-,45-,46-,47-,48-,49?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined against Nicotinic acetylcholine receptor using [3H]-(-)-nicotine radioligand |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50171556

((R)-1-((S)-2-amino-3-methylbutanamido)ethylboronic...)Show InChI InChI=1S/C7H17BN2O3/c1-4(2)6(9)7(11)10-5(3)8(12)13/h4-6,12-13H,9H2,1-3H3,(H,10,11)/t5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50600963

(CHEMBL5183565)Show SMILES FCCOCCn1c2ncccc2cc(C(=O)NC23CC4CC(CC(C4)C2)C3)c1=O |TLB:21:22:20.19.25:26,THB:21:20:26:27.22.23,23:22:19:25.24.26,23:24:27.22.21:19| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00256
BindingDB Entry DOI: 10.7270/Q2Z89HGZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507698
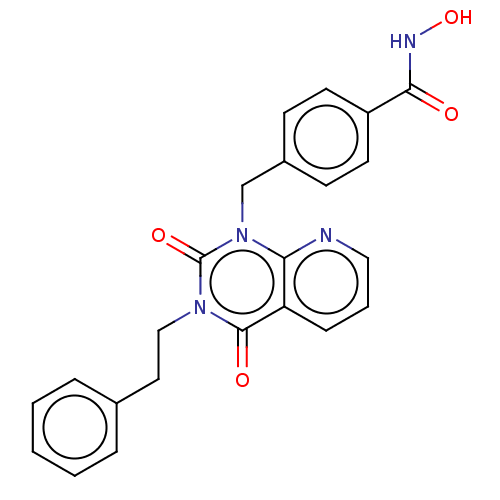
(CHEMBL4438057 | US11535598, Compound 12)Show SMILES ONC(=O)c1ccc(Cn2c3ncccc3c(=O)n(CCc3ccccc3)c2=O)cc1 Show InChI InChI=1S/C23H20N4O4/c28-21(25-31)18-10-8-17(9-11-18)15-27-20-19(7-4-13-24-20)22(29)26(23(27)30)14-12-16-5-2-1-3-6-16/h1-11,13,31H,12,14-15H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length recombinant HDAC6 expressed in baculovirus infected Sf9 insect cells using Boc-Lys (Ac)-AMC as substrate preincubated... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507698

(CHEMBL4438057 | US11535598, Compound 12)Show SMILES ONC(=O)c1ccc(Cn2c3ncccc3c(=O)n(CCc3ccccc3)c2=O)cc1 Show InChI InChI=1S/C23H20N4O4/c28-21(25-31)18-10-8-17(9-11-18)15-27-20-19(7-4-13-24-20)22(29)26(23(27)30)14-12-16-5-2-1-3-6-16/h1-11,13,31H,12,14-15H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 CD2 expressed in Escherichia coli BL21 (RIL) using Boc-Lys (Ac)-AMC as substrate preincubated for 10 mins followed by subst... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50600950
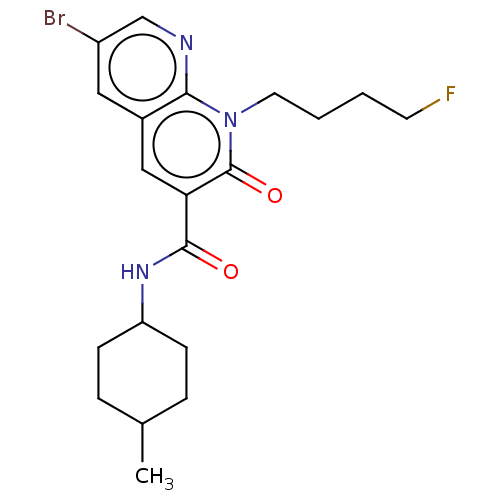
(CHEMBL5195027)Show SMILES CC1CCC(CC1)NC(=O)c1cc2cc(Br)cnc2n(CCCCF)c1=O |(7.33,5.77,;6,5.01,;4.66,5.77,;3.33,5.01,;3.33,3.46,;4.66,2.69,;6,3.46,;2,2.69,;.66,3.46,;.66,5.01,;-.67,2.69,;-2.01,3.46,;-3.34,2.7,;-4.66,3.47,;-6,2.7,;-7.33,3.47,;-6,1.15,;-4.66,.39,;-3.34,1.16,;-2,.39,;-2,-1.15,;-3.33,-1.92,;-3.33,-3.46,;-4.66,-4.24,;-4.66,-5.77,;-.67,1.16,;.67,.39,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00256
BindingDB Entry DOI: 10.7270/Q2Z89HGZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50023050

(CHEMBL40928 | {1-[1-(1-{2-[1-(1-Carbamoyl-2-phenyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C46H64N6O8/c1-29(2)23-34(39(53)28-40(54)48-36(24-30(3)4)42(56)50-35(41(47)55)25-31-17-11-8-12-18-31)49-43(57)37(26-32-19-13-9-14-20-32)51-44(58)38(27-33-21-15-10-16-22-33)52-45(59)60-46(5,6)7/h8-22,29-30,34-39,53H,23-28H2,1-7H3,(H2,47,55)(H,48,54)(H,49,57)(H,50,56)(H,51,58)(H,52,59)/t34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human kidney renin |
J Med Chem 28: 1755-6 (1986)
BindingDB Entry DOI: 10.7270/Q2P26X56 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50029962
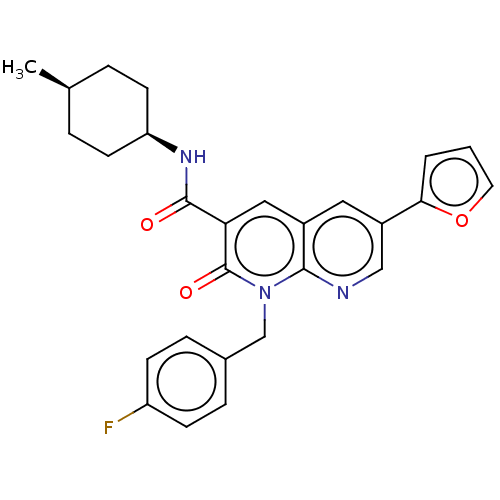
(CHEMBL3353450)Show SMILES C[C@H]1CC[C@H](CC1)NC(=O)c1cc2cc(cnc2n(Cc2ccc(F)cc2)c1=O)-c1ccco1 |r,wU:4.7,1.0,(23.36,-18.24,;22.03,-19.01,;22.03,-20.55,;20.71,-21.32,;19.37,-20.55,;19.36,-19.02,;20.69,-18.24,;18.04,-21.33,;16.7,-20.57,;16.69,-19.03,;15.37,-21.34,;14.04,-20.58,;12.71,-21.36,;11.37,-20.59,;10.05,-21.36,;10.04,-22.91,;11.38,-23.68,;12.71,-22.9,;14.05,-23.67,;14.05,-25.21,;15.38,-25.97,;15.38,-27.51,;16.71,-28.28,;18.05,-27.5,;19.38,-28.27,;18.04,-25.96,;16.71,-25.2,;15.38,-22.89,;16.72,-23.66,;8.71,-20.59,;7.3,-21.22,;6.26,-20.08,;7.03,-18.74,;8.54,-19.06,)| Show InChI InChI=1S/C27H26FN3O3/c1-17-4-10-22(11-5-17)30-26(32)23-14-19-13-20(24-3-2-12-34-24)15-29-25(19)31(27(23)33)16-18-6-8-21(28)9-7-18/h2-3,6-9,12-15,17,22H,4-5,10-11,16H2,1H3,(H,30,32)/t17-,22+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00256
BindingDB Entry DOI: 10.7270/Q2Z89HGZ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50600941

(CHEMBL5173890)Show SMILES C[C@H]1CC[C@H](CC1)NC(=O)c1cc2cccnc2n(CCCCF)c1=O |r,wD:4.7,1.0,(6.66,5.77,;5.33,5,;4,5.77,;2.66,5,;2.66,3.46,;4,2.69,;5.33,3.46,;1.33,2.69,;-0,3.46,;-0,5,;-1.33,2.69,;-2.67,3.46,;-4,2.7,;-5.33,3.47,;-6.66,2.7,;-6.66,1.15,;-5.33,.39,;-4,1.16,;-2.66,.39,;-2.66,-1.15,;-4,-1.92,;-4,-3.46,;-5.33,-4.23,;-5.33,-5.77,;-1.33,1.16,;-0,.39,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00256
BindingDB Entry DOI: 10.7270/Q2Z89HGZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405191
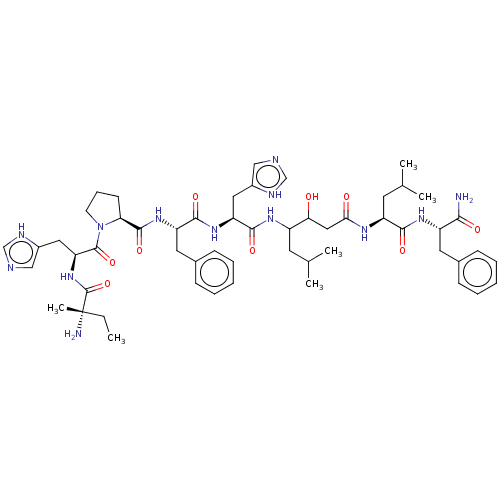
(CHEMBL269752)Show SMILES CC[C@](C)(N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)NC(CC(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H77N13O9/c1-7-54(6,56)53(76)66-43(26-37-29-58-31-60-37)52(75)67-20-14-19-44(67)51(74)65-41(24-35-17-12-9-13-18-35)49(72)64-42(25-36-28-57-30-59-36)50(73)62-38(21-32(2)3)45(68)27-46(69)61-40(22-33(4)5)48(71)63-39(47(55)70)23-34-15-10-8-11-16-34/h8-13,15-18,28-33,38-45,68H,7,14,19-27,56H2,1-6H3,(H2,55,70)(H,57,59)(H,58,60)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)(H,66,76)/t38?,39-,40-,41-,42-,43-,44-,45?,54-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of rabbit plasma renin. |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50271443
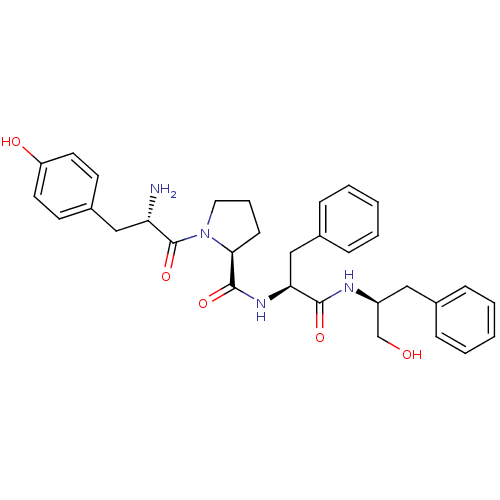
(CHEMBL522293 | Tyr-Pro-Phe-Phe-OCH2OH)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](CO)Cc1ccccc1 |r| Show InChI InChI=1S/C32H38N4O5/c33-27(19-24-13-15-26(38)16-14-24)32(41)36-17-7-12-29(36)31(40)35-28(20-23-10-5-2-6-11-23)30(39)34-25(21-37)18-22-8-3-1-4-9-22/h1-6,8-11,13-16,25,27-29,37-38H,7,12,17-21,33H2,(H,34,39)(H,35,40)/t25-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025946
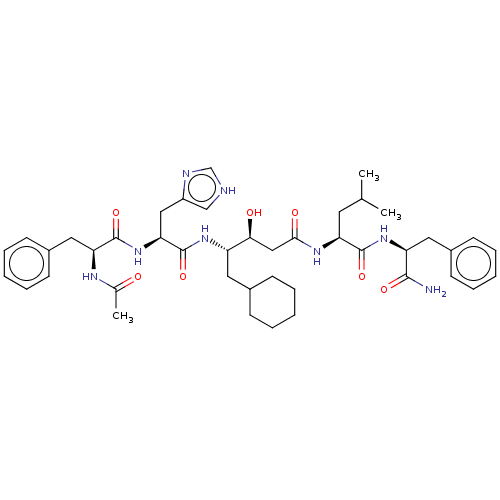
(4-[2-(2-Acetylamino-3-phenyl-propionylamino)-3-(1H...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C43H60N8O7/c1-27(2)19-35(41(56)50-34(40(44)55)21-30-15-9-5-10-16-30)48-39(54)24-38(53)33(20-29-13-7-4-8-14-29)49-43(58)37(23-32-25-45-26-46-32)51-42(57)36(47-28(3)52)22-31-17-11-6-12-18-31/h5-6,9-12,15-18,25-27,29,33-38,53H,4,7-8,13-14,19-24H2,1-3H3,(H2,44,55)(H,45,46)(H,47,52)(H,48,54)(H,49,58)(H,50,56)(H,51,57) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human kidney renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 16: 6415-22 (2008)
Article DOI: 10.1016/j.bmc.2008.05.001
BindingDB Entry DOI: 10.7270/Q2RR1Z1F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data