

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369432 (CHEMBL1627465) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Irreversible inhibition of human steroid sulfatase expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by liquid scintillation counting... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115368 BindingDB Entry DOI: 10.7270/Q2V98CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50545255 (CHEMBL4643348) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Irreversible inhibition of human steroid sulfatase expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by liquid scintillation counting... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115368 BindingDB Entry DOI: 10.7270/Q2V98CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369432 (CHEMBL1627465) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115368 BindingDB Entry DOI: 10.7270/Q2V98CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50545254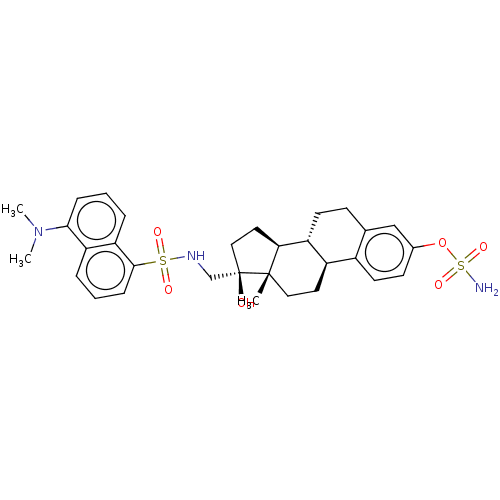 (CHEMBL4639657) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Irreversible inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by scintillation coun... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115368 BindingDB Entry DOI: 10.7270/Q2V98CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chromatin remodeling regulator CECR2 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of BAZ2A (unknown origin) after 30 mins by AlphaScreen assay | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366532 (CHEMBL518966) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Reversible inhibition of steroid sulfatase in human JEG3 cells using [3H] E1S as substrate by scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115368 BindingDB Entry DOI: 10.7270/Q2V98CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366532 (CHEMBL518966) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115368 BindingDB Entry DOI: 10.7270/Q2V98CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of H3K14Ac from BAZ2B (unknown origin) preincubated for 30 mins with non-biotinylated peptide followed by addition of biotinylated pepti... | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chromatin remodeling regulator CECR2 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of BAZ2A (unknown origin) after 30 mins by AlphaScreen assay | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50545253 (CHEMBL4632735) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Reversible inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by scintillation counti... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115368 BindingDB Entry DOI: 10.7270/Q2V98CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chromatin remodeling regulator CECR2 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of BAZ2A (unknown origin) after 30 mins by AlphaScreen assay | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of BAZ2A (unknown origin) after 30 mins by AlphaScreen assay | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peregrin (Homo sapiens (Human)) | BDBM50157570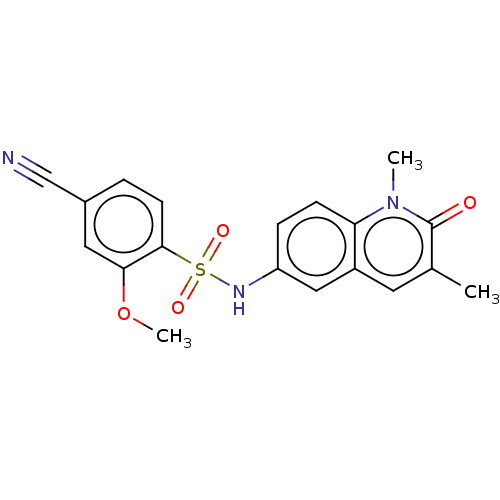 (4-cyano-N-(1,3-dimethyl-2-oxoquinolin-6-yl)-2-meth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of H3K14Ac from BAZ2B (unknown origin) preincubated for 30 mins with non-biotinylated peptide followed by addition of biotinylated pepti... | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peregrin (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of BAZ2A (unknown origin) after 30 mins by AlphaScreen assay | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peregrin (Homo sapiens (Human)) | BDBM50032927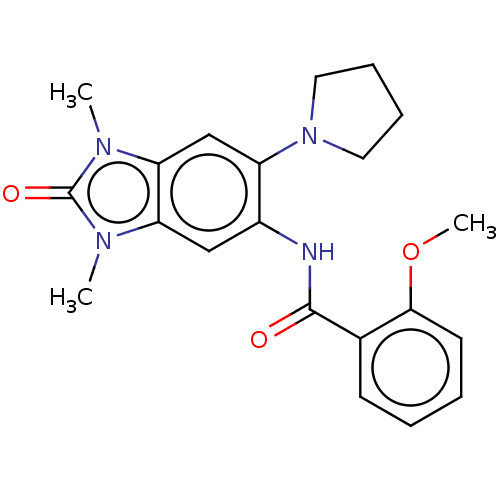 (CHEMBL3356143 | N-[2,3-Dihydro-1,3-dimethyl-2-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peregrin (Homo sapiens (Human)) | BDBM50148549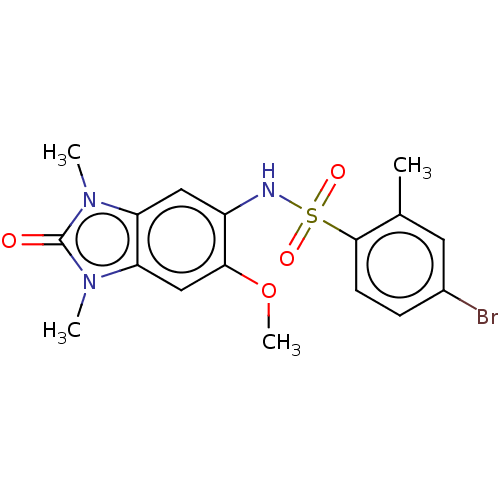 (4-Bromo-N-(2,3-dihydro-6-methoxy-1,3-dimethyl-2-ox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of BAZ2A (unknown origin) after 30 mins by AlphaScreen assay | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB PubMed | n/a | n/a | 311 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of BAZ2A (unknown origin) after 30 mins by AlphaScreen assay | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB PubMed | n/a | n/a | 468 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of BAZ2A (unknown origin) after 30 mins by AlphaScreen assay | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 1 (Homo sapiens (Human)) | BDBM50157570 (4-cyano-N-(1,3-dimethyl-2-oxoquinolin-6-yl)-2-meth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 619 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB PubMed | n/a | n/a | 701 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of BAZ2A (unknown origin) after 30 mins by AlphaScreen assay | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain and PHD finger-containing protein 3 (Homo sapiens (Human)) | BDBM50157570 (4-cyano-N-(1,3-dimethyl-2-oxoquinolin-6-yl)-2-meth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 1 (Homo sapiens (Human)) | BDBM50148549 (4-Bromo-N-(2,3-dihydro-6-methoxy-1,3-dimethyl-2-ox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PubMed | n/a | n/a | 2.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of H3K14Ac from BAZ2B (unknown origin) preincubated for 30 mins with non-biotinylated peptide followed by addition of biotinylated pepti... | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 1 (Homo sapiens (Human)) | BDBM50032927 (CHEMBL3356143 | N-[2,3-Dihydro-1,3-dimethyl-2-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.52E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134329 (CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center Curated by ChEMBL | Assay Description Reversible inhibition of steroid sulfatase in human JEG3 cells using [3H] E1S as substrate by scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115368 BindingDB Entry DOI: 10.7270/Q2V98CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription intermediary factor 1-alpha (Homo sapiens (Human)) | BDBM50032927 (CHEMBL3356143 | N-[2,3-Dihydro-1,3-dimethyl-2-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription intermediary factor 1-alpha (Homo sapiens (Human)) | BDBM50148549 (4-Bromo-N-(2,3-dihydro-6-methoxy-1,3-dimethyl-2-ox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription intermediary factor 1-alpha (Homo sapiens (Human)) | BDBM50157570 (4-cyano-N-(1,3-dimethyl-2-oxoquinolin-6-yl)-2-meth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50032927 (CHEMBL3356143 | N-[2,3-Dihydro-1,3-dimethyl-2-oxo-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50157570 (4-cyano-N-(1,3-dimethyl-2-oxoquinolin-6-yl)-2-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50148549 (4-Bromo-N-(2,3-dihydro-6-methoxy-1,3-dimethyl-2-ox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein polybromo-1 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PubMed | n/a | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Binding affinity to CECR2 (unknown origin) by isothermal titration calorimetric analysis | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription intermediary factor 1-alpha (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PubMed | n/a | n/a | n/a | 8.48E+3 | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Reverse ITC (compound as receptor). Domain start/stop: G861-E979 | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1-like (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB PubMed | n/a | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Reverse ITC (compound as receptor). Domain start/stop: M1401-D1522 | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB PubMed | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Reverse ITC (compound as receptor). Domain start/stop: D1522-D1656 | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB PubMed | n/a | n/a | n/a | 5.53E+3 | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Reverse ITC (compound as receptor). Domain start/stop: R1398-D1524 | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transcription activator BRG1 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PubMed | n/a | n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Reverse ITC (compound as receptor). Domain start/stop: L1451-E1580 | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2B (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PubMed | n/a | n/a | n/a | 4.76E+3 | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Reverse ITC (compound as receptor). Domain start/stop: G715-D831 | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain adjacent to zinc finger domain protein 2A (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PubMed | n/a | n/a | n/a | 3.75E+3 | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of H3K14Ac from BAZ2B (unknown origin) preincubated for 30 mins with non-biotinylated peptide followed by addition of biotinylated pepti... | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nucleosome-remodeling factor subunit BPTF (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PubMed | n/a | n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Binding affinity to BAZ2B (unknown origin) by isothermal titration calorimetric analysis | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chromatin remodeling regulator CECR2 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PubMed | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Binding affinity to BAZ2A (unknown origin) by isothermal titration calorimetric analysis | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain and PHD finger-containing protein 3 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PubMed | n/a | n/a | n/a | 8.62E+3 | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of H3K14Ac from BAZ2B (unknown origin) preincubated for 30 mins with non-biotinylated peptide followed by addition of biotinylated pepti... | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB PubMed | n/a | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of H3K14Ac from BAZ2B (unknown origin) preincubated for 30 mins with non-biotinylated peptide followed by addition of biotinylated pepti... | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 1 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PubMed | n/a | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of H3K14Ac from BAZ2B (unknown origin) preincubated for 30 mins with non-biotinylated peptide followed by addition of biotinylated pepti... | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PubMed | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of H3K14Ac from BAZ2B (unknown origin) preincubated for 30 mins with non-biotinylated peptide followed by addition of biotinylated pepti... | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50504160 (CHEMBL3133807 | US11773085, Compound B25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB PubMed | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of H3K14Ac from BAZ2B (unknown origin) preincubated for 30 mins with non-biotinylated peptide followed by addition of biotinylated pepti... | Sci Adv 2: (2016) BindingDB Entry DOI: 10.7270/Q24J0JPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||