

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards Wild-type kappa opioid receptor expressed in HEK cells | J Med Chem 43: 1251-2 (2001) BindingDB Entry DOI: 10.7270/Q270824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50089779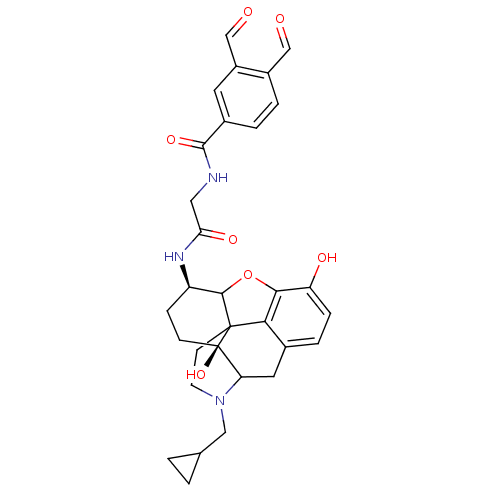 (4-cyclopropylmethyl-14-(3,4-diformylphenylcarboxam...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]- diprenorphine binding to Opioid receptor mu 1 (83 fmol/mg protein) stably expressed in membranes from CHO cells | J Med Chem 43: 2489-92 (2000) BindingDB Entry DOI: 10.7270/Q2XW4KH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding constant against E203Q,D204N,D206N EL-2 Opioid receptor kappa 1 using [3H]diprenorphine as radioligand expressed in HEK cells | J Med Chem 43: 1251-2 (2001) BindingDB Entry DOI: 10.7270/Q270824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50089779 (4-cyclopropylmethyl-14-(3,4-diformylphenylcarboxam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to Opioid receptor kappa 1 | J Med Chem 43: 2489-92 (2000) BindingDB Entry DOI: 10.7270/Q2XW4KH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50115095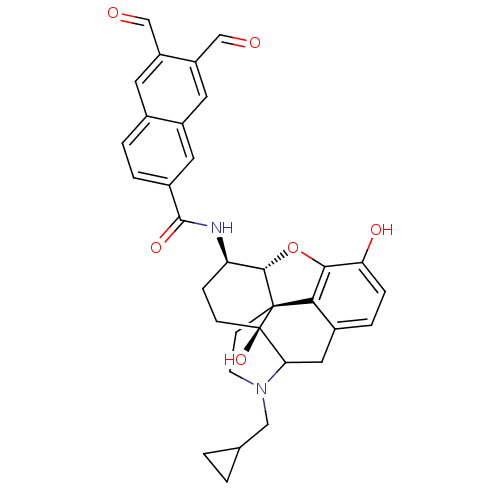 (4-cyclopropylmethyl-14-(6,7-diformyl-2-naphthylcar...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.744 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]- diprenorphine binding on Opioid receptor mu 1 expressed in human embryonic kidney (HEK) cells | J Med Chem 45: 2887-90 (2002) BindingDB Entry DOI: 10.7270/Q23N243V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding constant against wild type EL-2 Opioid receptor kappa 1 using [3H]diprenorphine as radioligand expressed in HEK cells | J Med Chem 43: 1251-2 (2001) BindingDB Entry DOI: 10.7270/Q270824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding constant against E203Q,D204N,D206N,E209Q EL-2 Opioid receptor kappa 1 using [3H]diprenorphine as radioligand expressed in HEK cells | J Med Chem 43: 1251-2 (2001) BindingDB Entry DOI: 10.7270/Q270824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50098209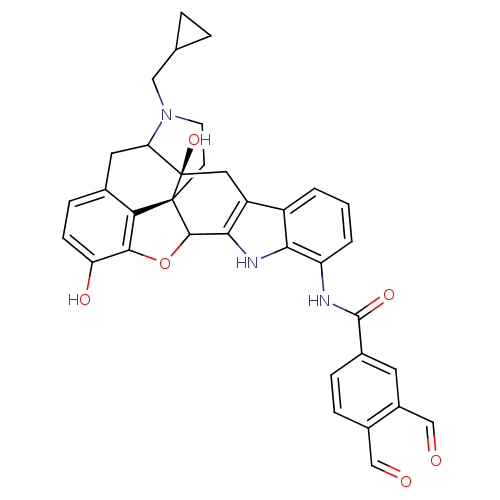 (22-cyclopropylmethyl-9-(3,4-diformylphenylcarboxam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards delta-opioid receptor from CHO cells | J Med Chem 44: 1017-20 (2001) BindingDB Entry DOI: 10.7270/Q2QF8TK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50005429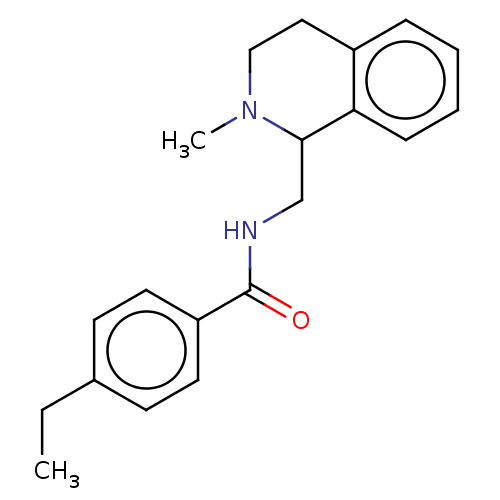 (CHEMBL4070288) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50089779 (4-cyclopropylmethyl-14-(3,4-diformylphenylcarboxam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]- diprenorphine binding to Opioid receptor delta 1 | J Med Chem 43: 2489-92 (2000) BindingDB Entry DOI: 10.7270/Q2XW4KH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding constant against D216N,D217N,E218Q EL-2 Opioid receptor kappa 1 using [3H]diprenorphine as radioligand expressed in HEK cells | J Med Chem 43: 1251-2 (2001) BindingDB Entry DOI: 10.7270/Q270824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50007985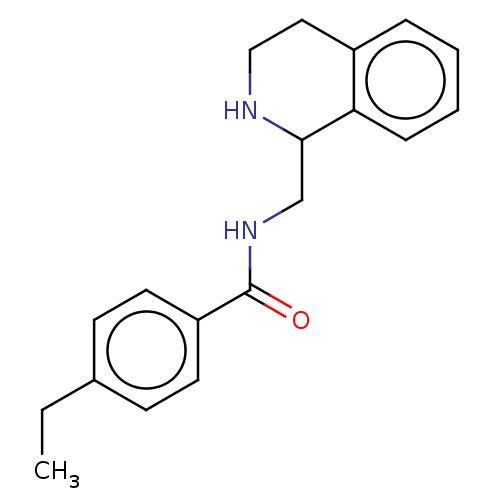 (CHEMBL4097865) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50042064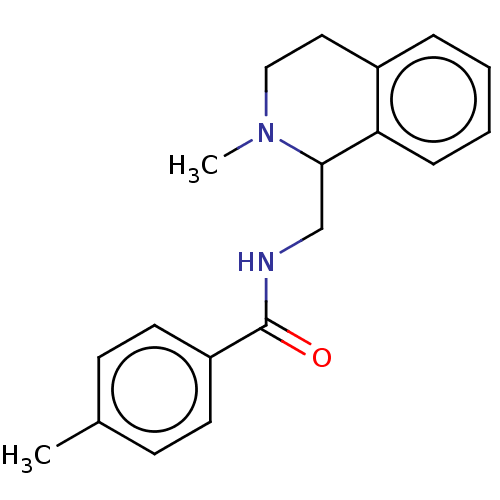 (CHEMBL4097466) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015426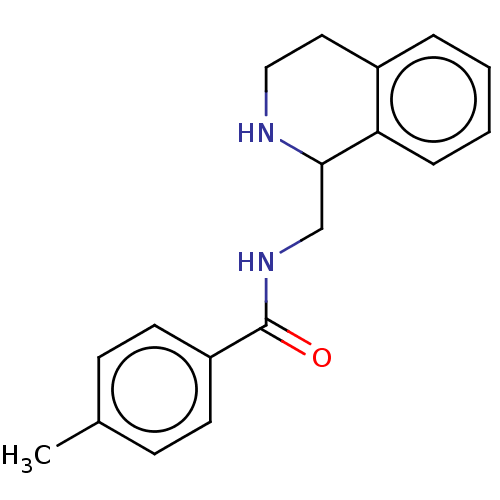 (CHEMBL4070750) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50009239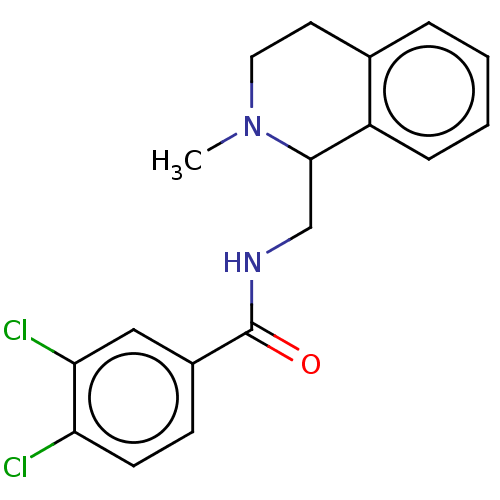 (CHEMBL4092125) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50098209 (22-cyclopropylmethyl-9-(3,4-diformylphenylcarboxam...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor from CHO cells | J Med Chem 44: 1017-20 (2001) BindingDB Entry DOI: 10.7270/Q2QF8TK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50098209 (22-cyclopropylmethyl-9-(3,4-diformylphenylcarboxam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards opioid receptor kappa 1 from CHO cells | J Med Chem 44: 1017-20 (2001) BindingDB Entry DOI: 10.7270/Q2QF8TK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008523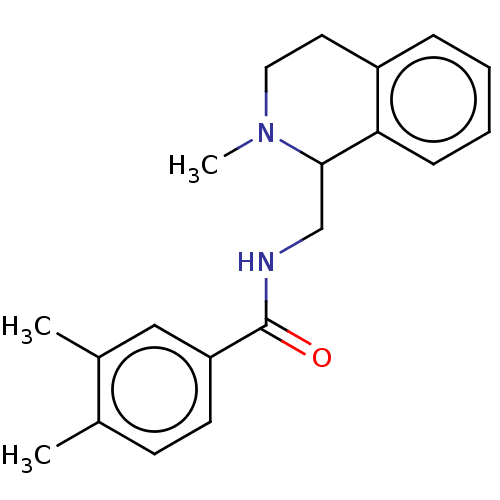 (CHEMBL4071332) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015433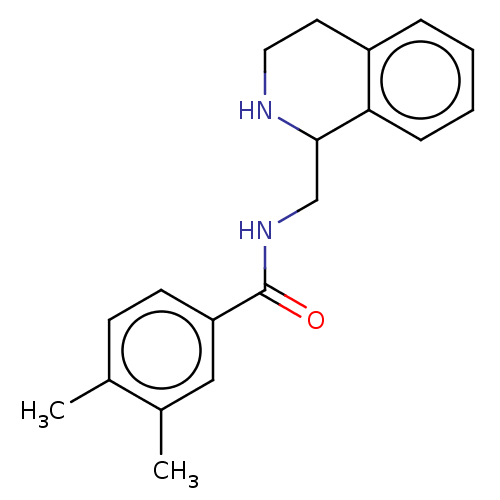 (CHEMBL4105599) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008003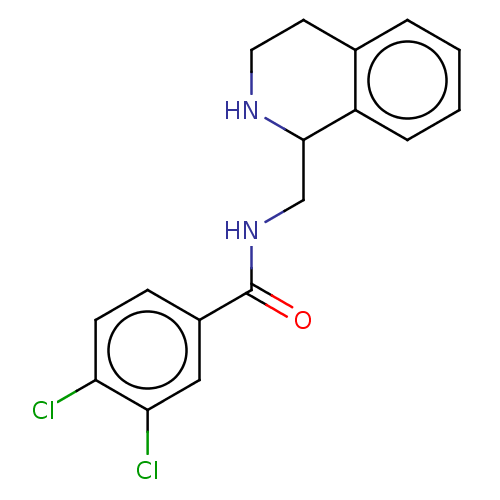 (CHEMBL4065924) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055967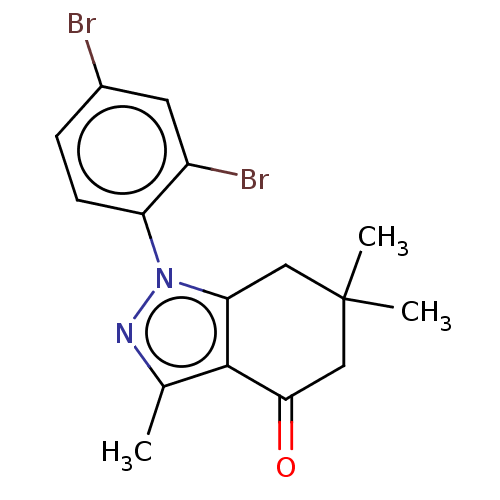 (CHEMBL3325714) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055966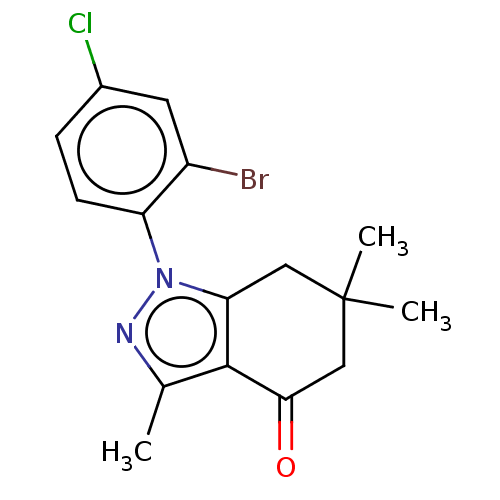 (CHEMBL3325715) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055965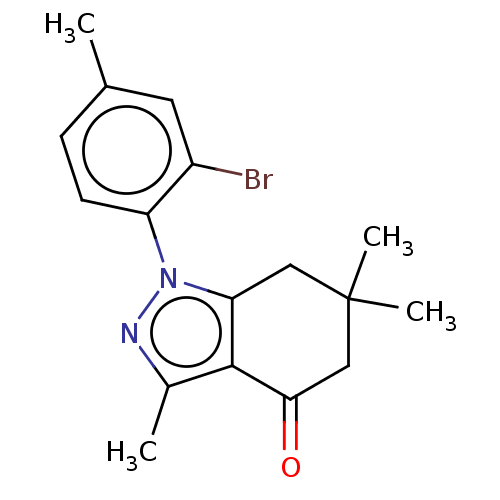 (CHEMBL3325716) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055969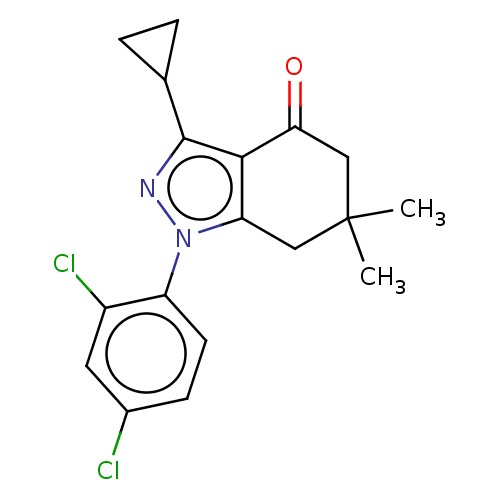 (CHEMBL3325719) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055973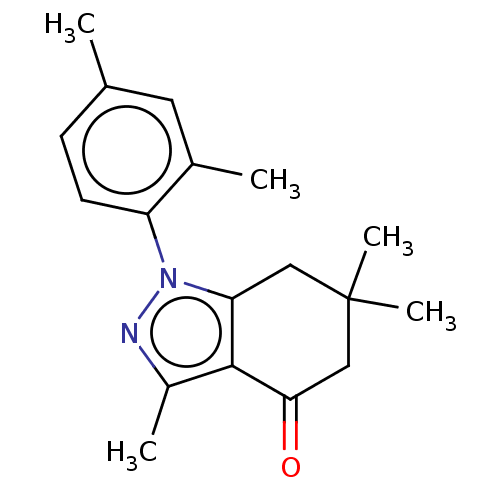 (CHEMBL3325707) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055972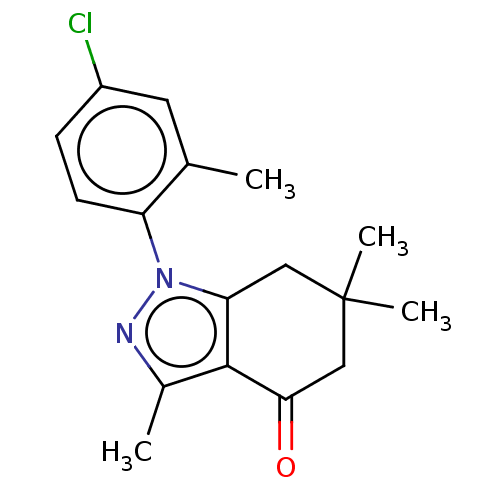 (CHEMBL3325710) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055964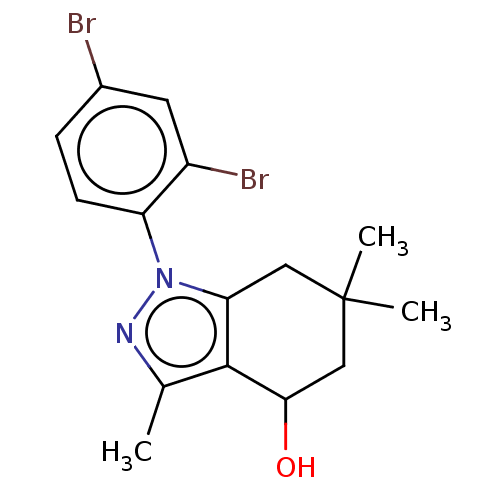 (CHEMBL3325841) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21008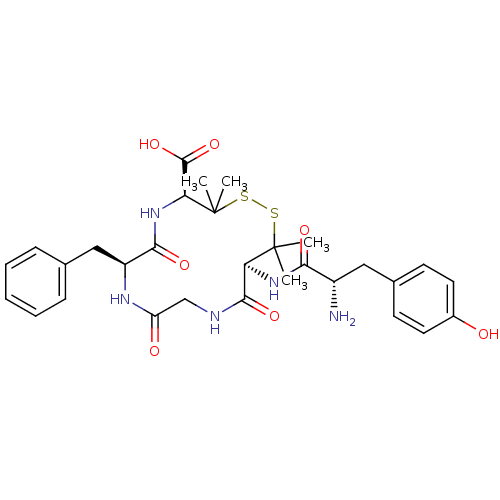 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human DOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins b... | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human delta opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins b... | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins by H... | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50013629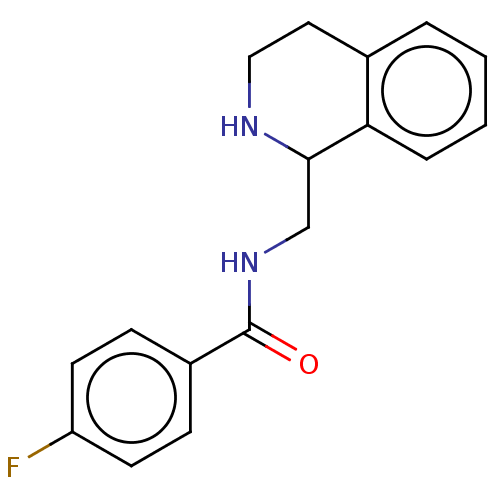 (CHEMBL4062124) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human DOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50003238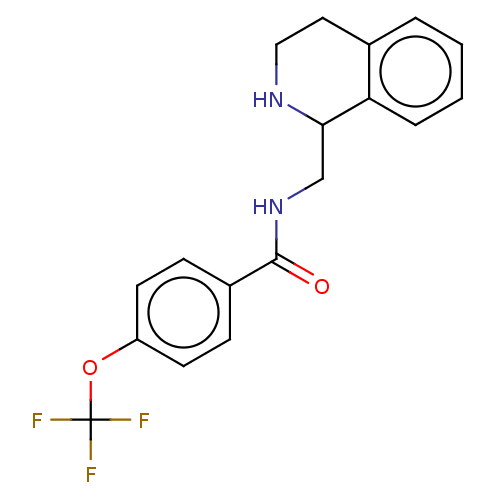 (CHEMBL4082299) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human DOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50007981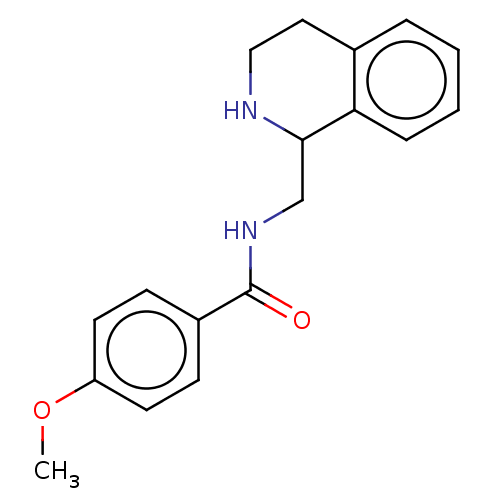 (CHEMBL4100317) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50009239 (CHEMBL4092125) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008523 (CHEMBL4071332) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50008523 (CHEMBL4071332) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50003327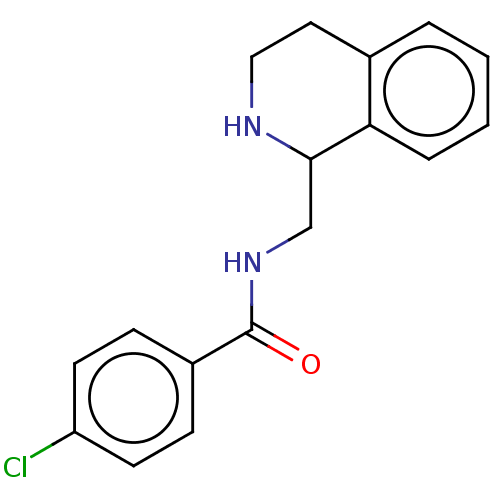 (CHEMBL4090148) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50005557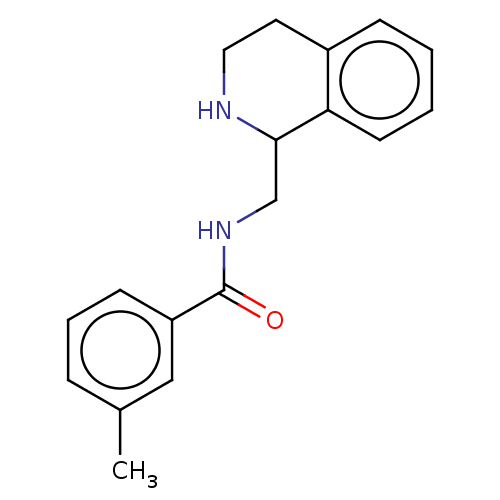 (CHEMBL4087761) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50042064 (CHEMBL4097466) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50003169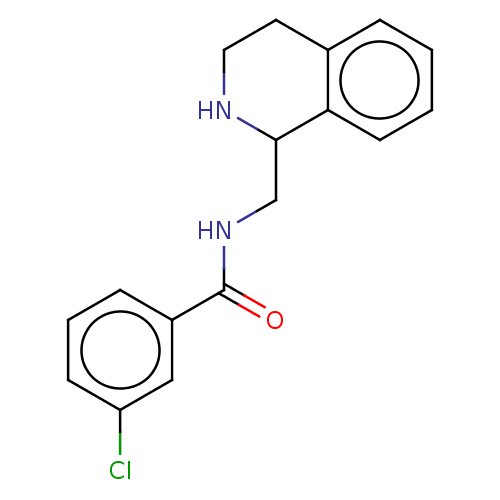 (CHEMBL4066436) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human DOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50005429 (CHEMBL4070288) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human DOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008521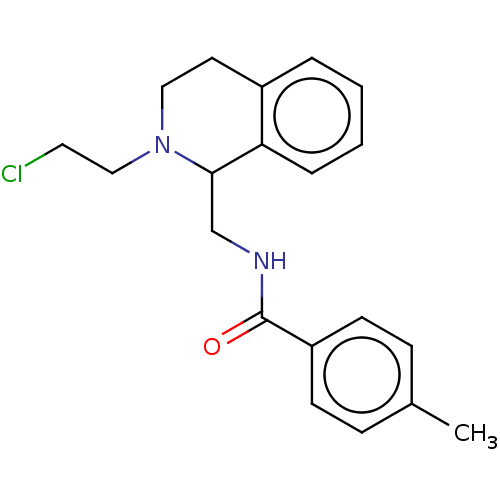 (CHEMBL4061620) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50003327 (CHEMBL4090148) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008003 (CHEMBL4065924) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50003238 (CHEMBL4082299) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015433 (CHEMBL4105599) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 182 total ) | Next | Last >> |