

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50157911 (13a-hydroxy-10-(4-methoxyphenyl)-5,5,5a,7a,13b-pen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Binding affinity for Acetylcholinesterase | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50094903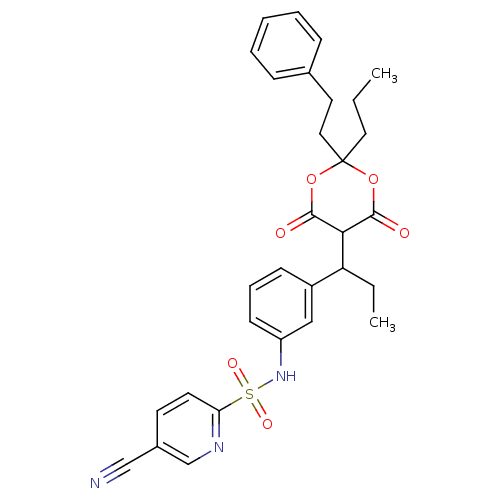 (5-Cyano-pyridine-2-sulfonic acid {3-[1-(6-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity towards HIV protease | Bioorg Med Chem Lett 10: 2625-7 (2000) BindingDB Entry DOI: 10.7270/Q20R9NPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50094906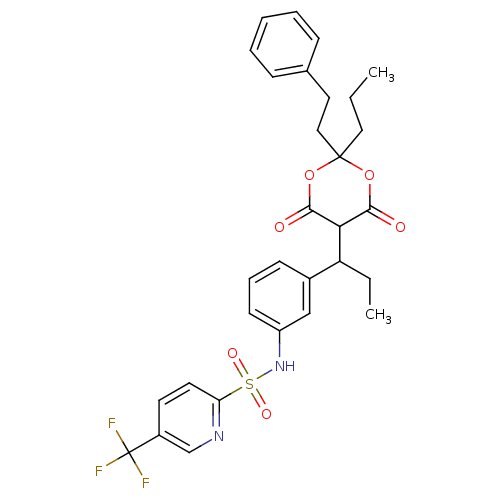 (5-Trifluoromethyl-pyridine-2-sulfonic acid {3-[1-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity towards HIV protease | Bioorg Med Chem Lett 10: 2625-7 (2000) BindingDB Entry DOI: 10.7270/Q20R9NPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Butyrylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50094901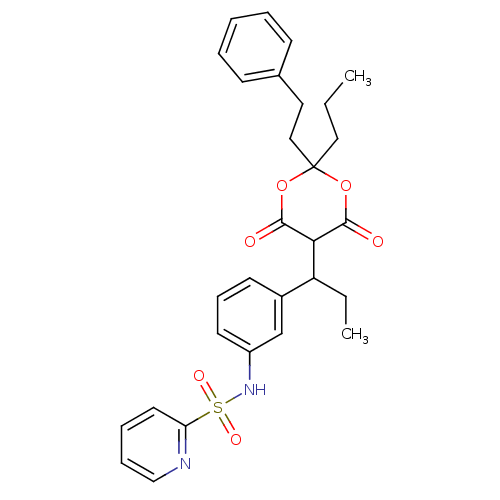 (CHEMBL90449 | Pyridine-2-sulfonic acid {3-[1-(6-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity towards HIV protease | Bioorg Med Chem Lett 10: 2625-7 (2000) BindingDB Entry DOI: 10.7270/Q20R9NPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50094902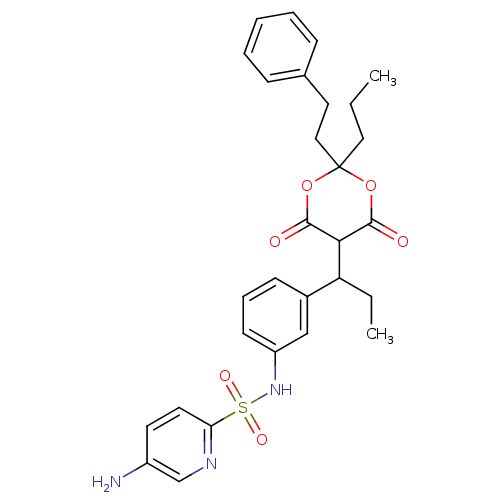 (5-Amino-pyridine-2-sulfonic acid {3-[1-(6-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity towards HIV protease | Bioorg Med Chem Lett 10: 2625-7 (2000) BindingDB Entry DOI: 10.7270/Q20R9NPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50094907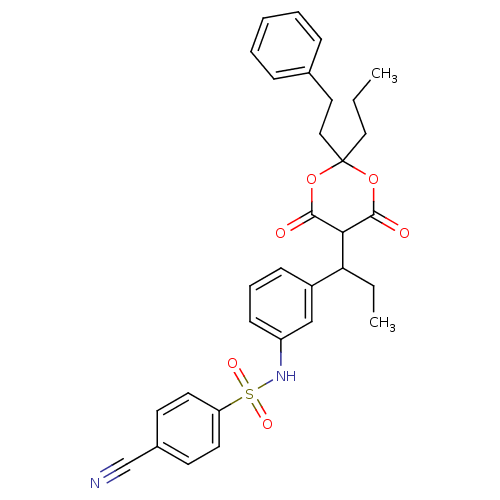 (4-Cyano-N-{3-[1-(6-hydroxy-4-oxo-2-phenethyl-2-pro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity towards HIV protease | Bioorg Med Chem Lett 10: 2625-7 (2000) BindingDB Entry DOI: 10.7270/Q20R9NPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50094904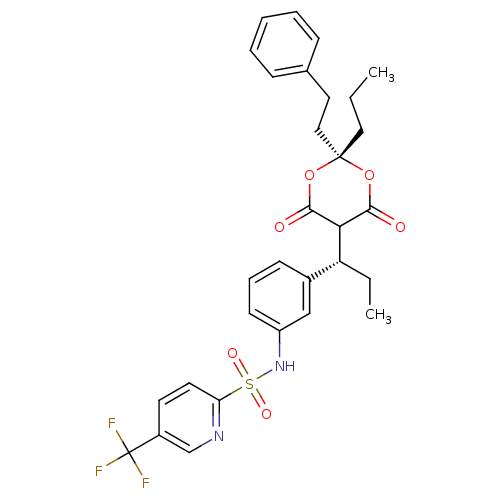 (5-Trifluoromethyl-pyridine-2-sulfonic acid {3-[(R)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity towards HIV protease | Bioorg Med Chem Lett 10: 2625-7 (2000) BindingDB Entry DOI: 10.7270/Q20R9NPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50157914 (4a,12a-dihydroxy-9-(4-methoxyphenyl)-4,4,6a,12b-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Acetylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50094905 (5-Nitro-pyridine-2-sulfonic acid {3-[1-(6-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity towards HIV protease | Bioorg Med Chem Lett 10: 2625-7 (2000) BindingDB Entry DOI: 10.7270/Q20R9NPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Acetylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50157915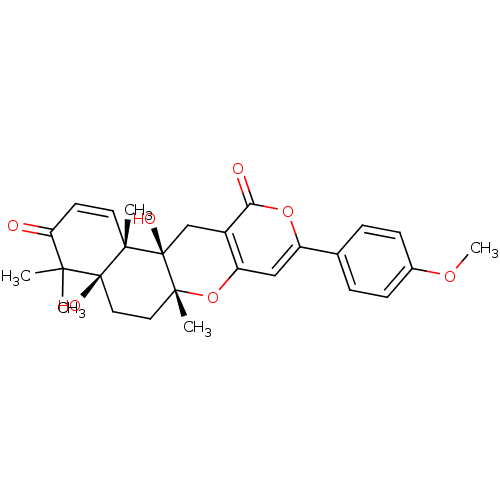 (4a,12a-dihydroxy-9-(4-methoxyphenyl)-4,4,6a,12b-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Acetylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50157912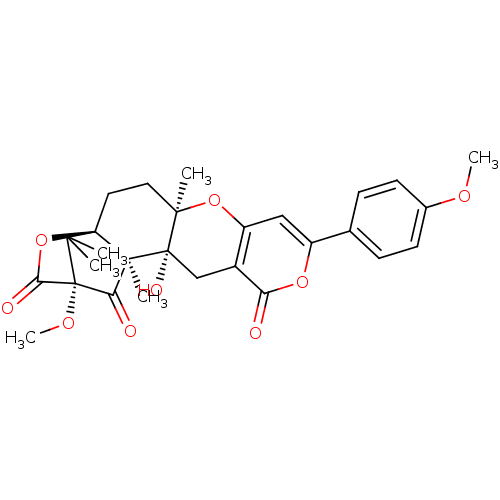 (13-hydroxy-16-methoxy-8-(4-methoxyphenyl)-4,14,19,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Acetylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090474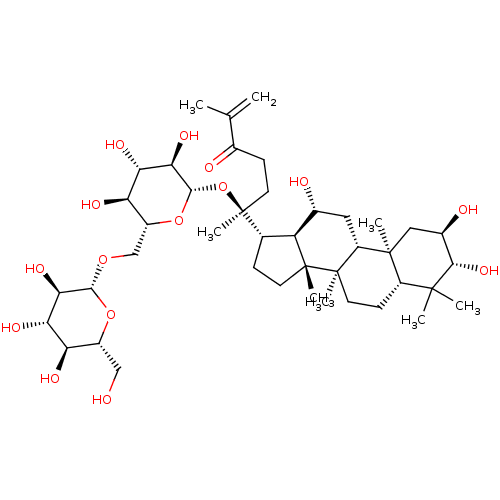 (CHEMBL3581710) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50157913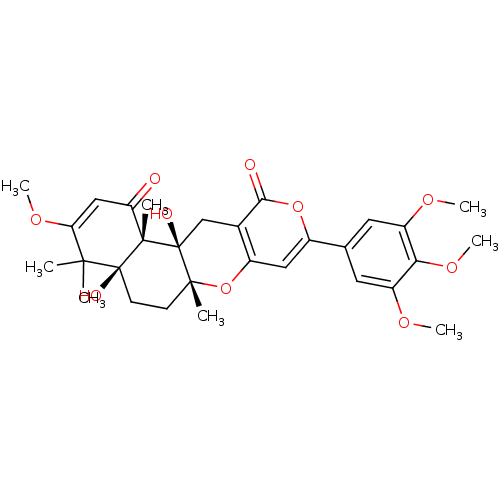 (4a,12a-dihydroxy-3-methoxy-4,4,6a,12b-tetramethyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Acetylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090504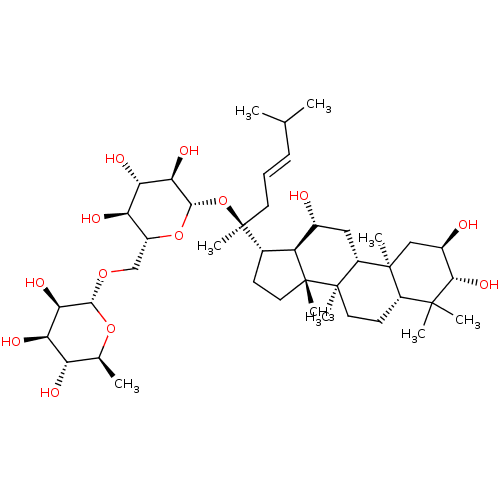 (CHEMBL3581714) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462020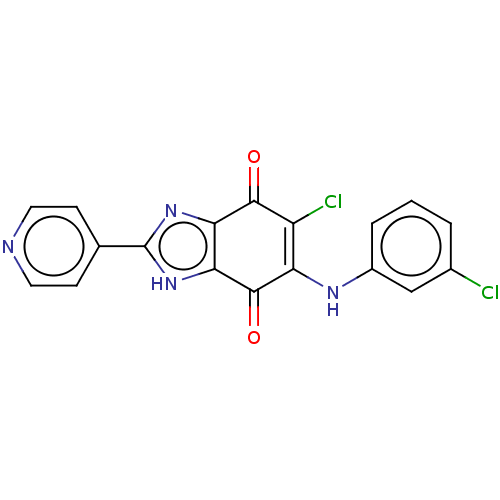 (CHEMBL515513) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462028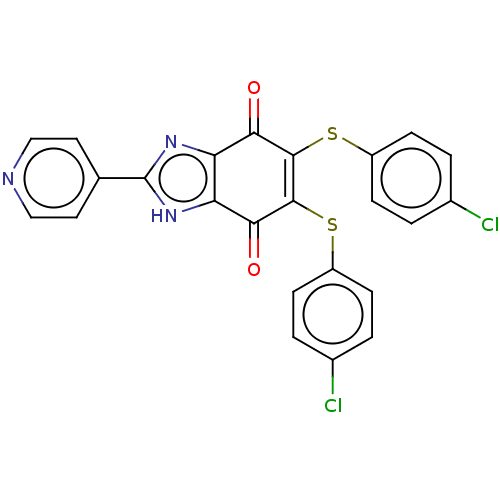 (CHEMBL4226173) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090483 (CHEMBL3581712) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50106501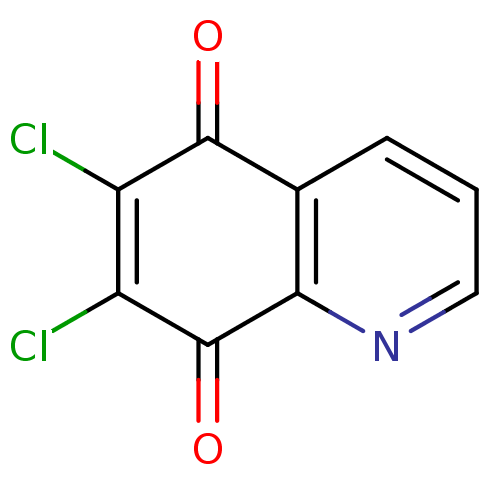 (6,7-Dichloro-5,8-quinolinequinone | 6,7-Dichloro-q...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462022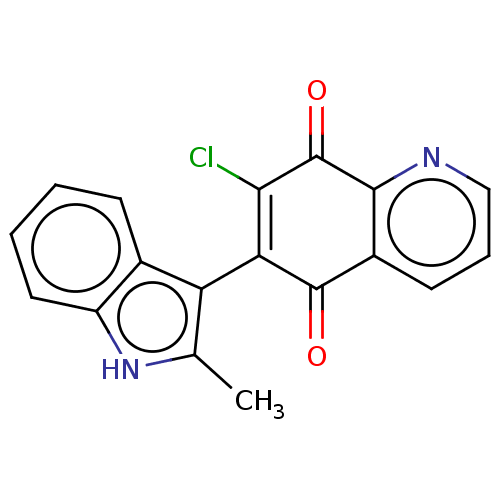 (CHEMBL4227412) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462021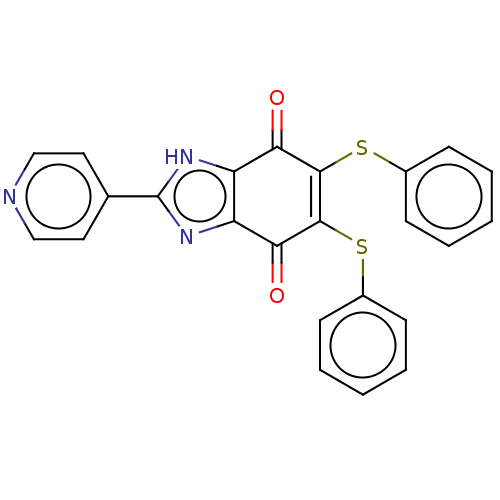 (CHEMBL4227020) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462025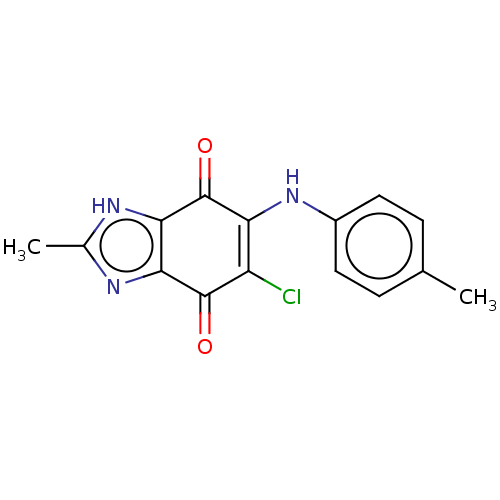 (CHEMBL331941) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462019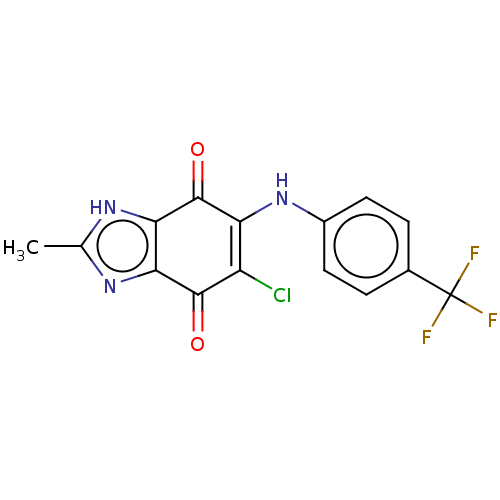 (CHEMBL259499) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50462014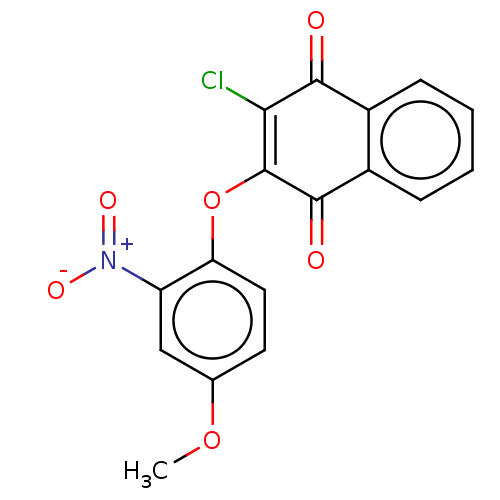 (CHEMBL4224853) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human rhinovirus protease 3C using fluorogenic-Dabcyl-KTSAVLQSGFRKME-Edan as substrate after 5 mins by FRET assay | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462026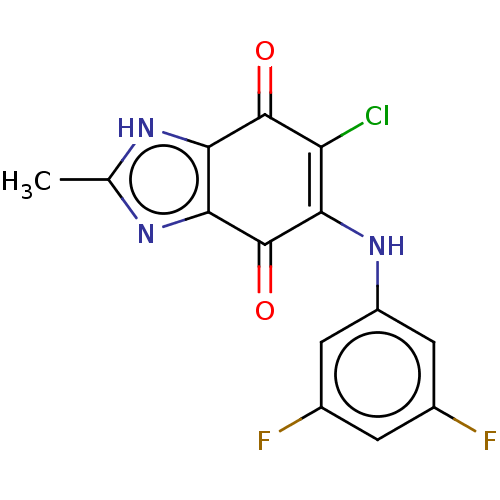 (CHEMBL4229177) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462023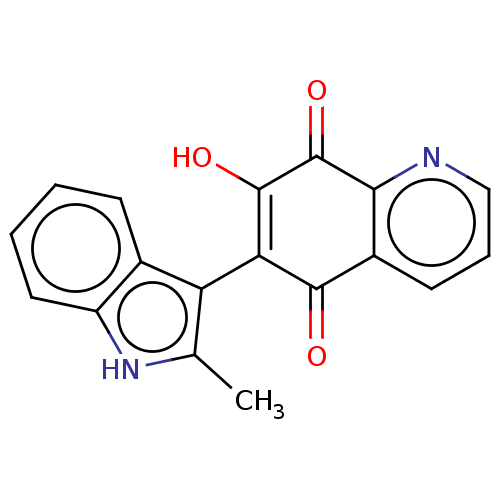 (CHEMBL4226298) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462018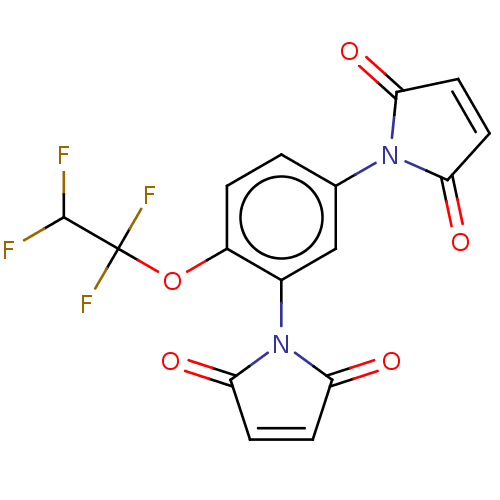 (CHEMBL4225972) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462015 (CHEMBL4227149) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462014 (CHEMBL4224853) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462017 (CHEMBL4229199) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50157911 (13a-hydroxy-10-(4-methoxyphenyl)-5,5,5a,7a,13b-pen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory concentration against Acetylcholinesterase activity | Bioorg Med Chem Lett 15: 353-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.067 BindingDB Entry DOI: 10.7270/Q2CN73CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090486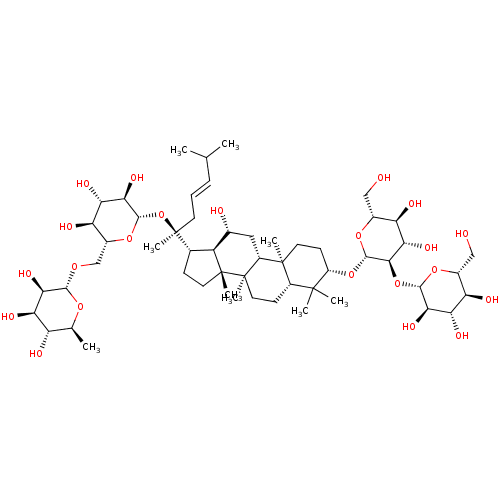 (CHEMBL3581713) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462016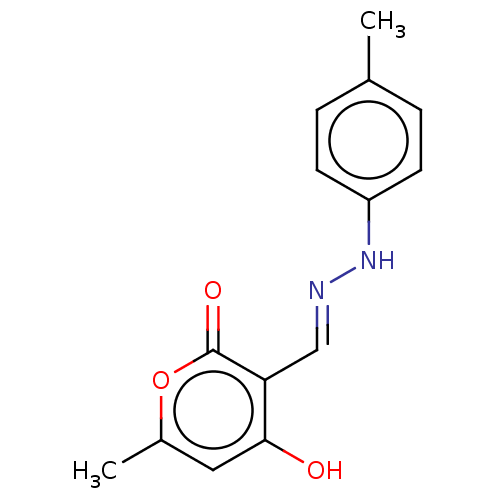 (CHEMBL3213811) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462024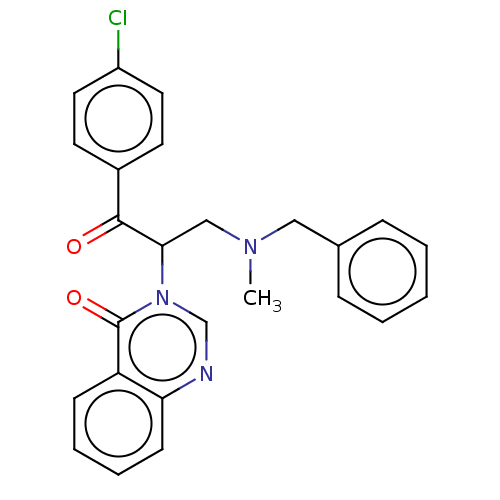 (CHEMBL4225121) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462027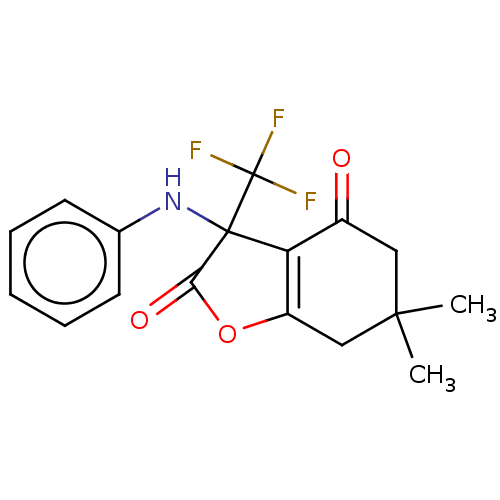 (CHEMBL4227809) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50462026 (CHEMBL4229177) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human rhinovirus protease 3C using fluorogenic-Dabcyl-KTSAVLQSGFRKME-Edan as substrate after 5 mins by FRET assay | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50476598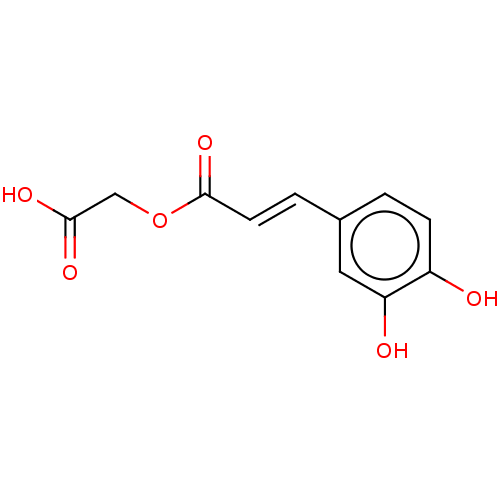 (CHEMBL396065) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science & Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 integrase expressed in Escherichia coli | Eur J Med Chem 42: 1309-15 (2007) Article DOI: 10.1016/j.ejmech.2007.02.016 BindingDB Entry DOI: 10.7270/Q2JD50K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM36895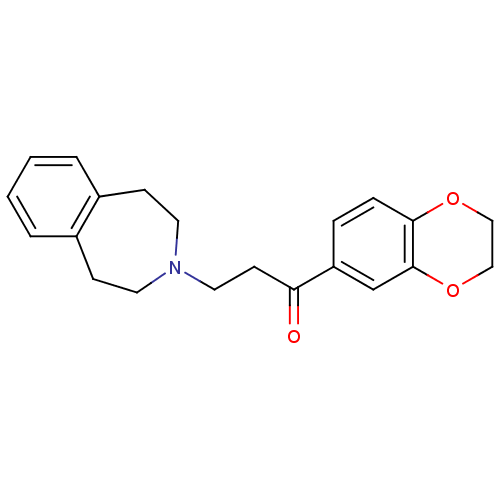 (1-(2,3-dihydro-1,4-benzodioxin-6-yl)-3-(1,2,4,5-te...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50476600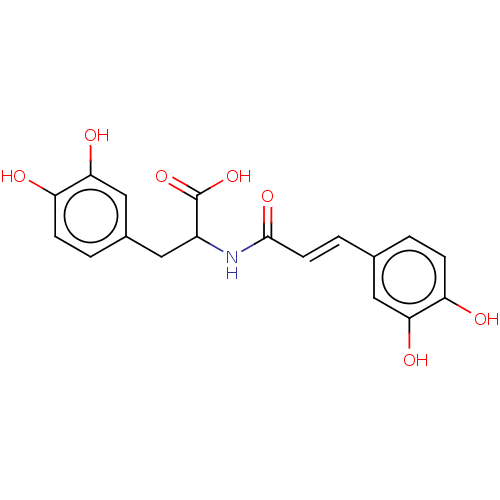 (CHEMBL442795) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science & Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 integrase expressed in Escherichia coli | Eur J Med Chem 42: 1309-15 (2007) Article DOI: 10.1016/j.ejmech.2007.02.016 BindingDB Entry DOI: 10.7270/Q2JD50K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50476592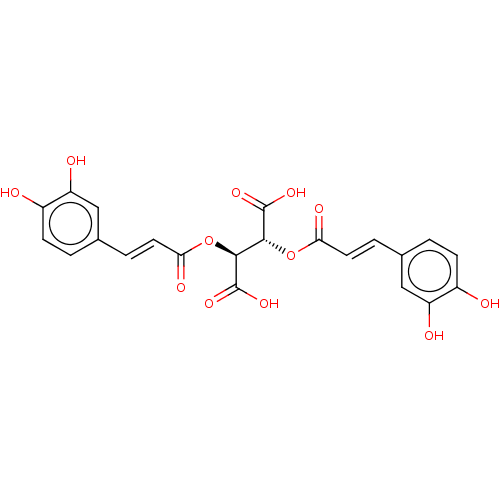 (CHEMBL397730) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science & Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 integrase expressed in Escherichia coli | Eur J Med Chem 42: 1309-15 (2007) Article DOI: 10.1016/j.ejmech.2007.02.016 BindingDB Entry DOI: 10.7270/Q2JD50K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090480 (CHEMBL3581705) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090481 (CHEMBL3581711) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090475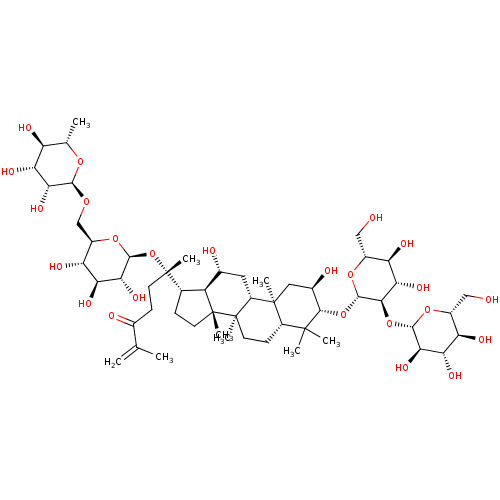 (CHEMBL3581709) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090477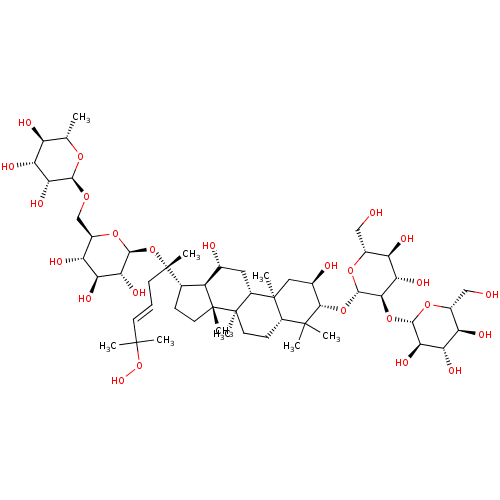 (CHEMBL3581707) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090478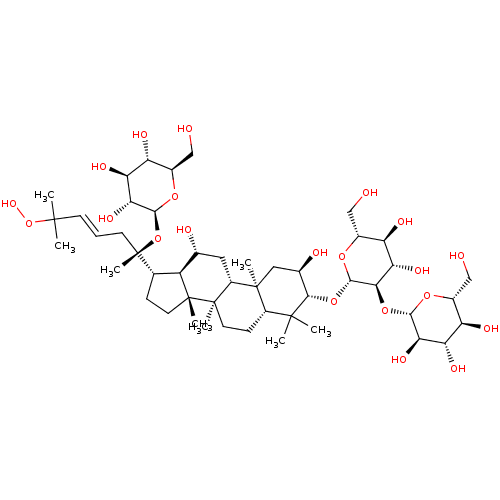 (CHEMBL3581706) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090479 (CHEMBL3581704) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50090476 (CHEMBL3581708) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 activation in human Hep3B cells after 12 hrs by luciferase assay | J Nat Prod 78: 971-6 (2015) Article DOI: 10.1021/np500803e BindingDB Entry DOI: 10.7270/Q22B90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50476591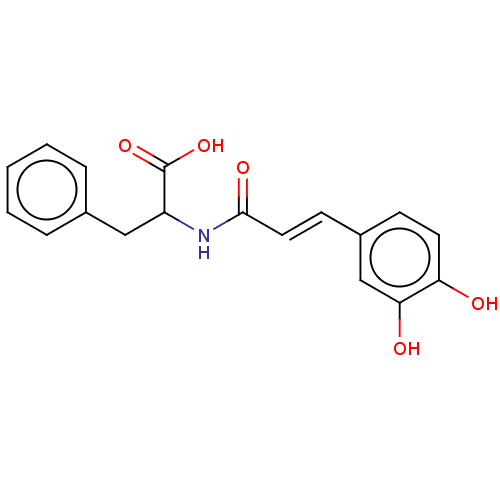 (CHEMBL243183) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science & Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 integrase expressed in Escherichia coli | Eur J Med Chem 42: 1309-15 (2007) Article DOI: 10.1016/j.ejmech.2007.02.016 BindingDB Entry DOI: 10.7270/Q2JD50K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50476599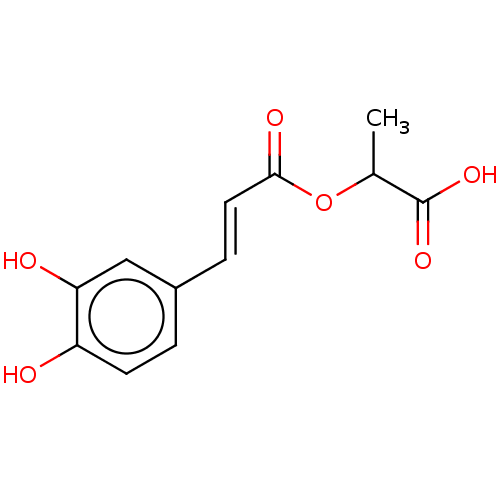 (CHEMBL244868) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science & Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 integrase expressed in Escherichia coli | Eur J Med Chem 42: 1309-15 (2007) Article DOI: 10.1016/j.ejmech.2007.02.016 BindingDB Entry DOI: 10.7270/Q2JD50K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |