

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462020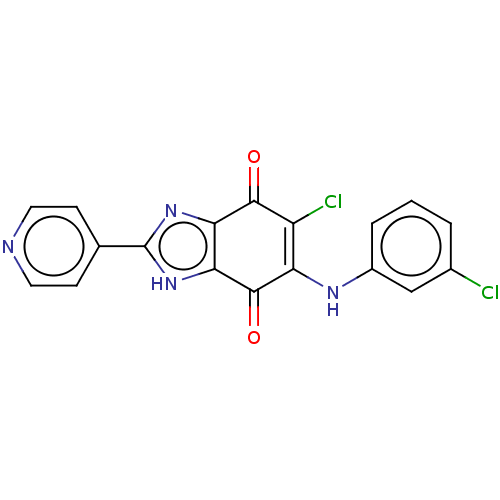 (CHEMBL515513) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462028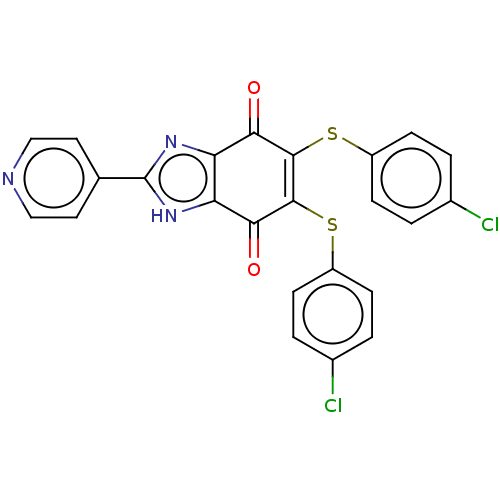 (CHEMBL4226173) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50106501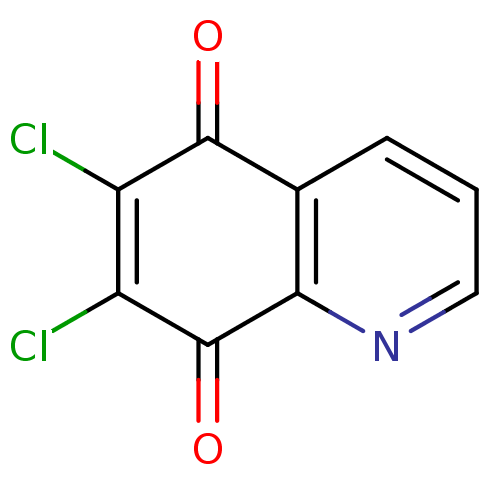 (6,7-Dichloro-5,8-quinolinequinone | 6,7-Dichloro-q...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462022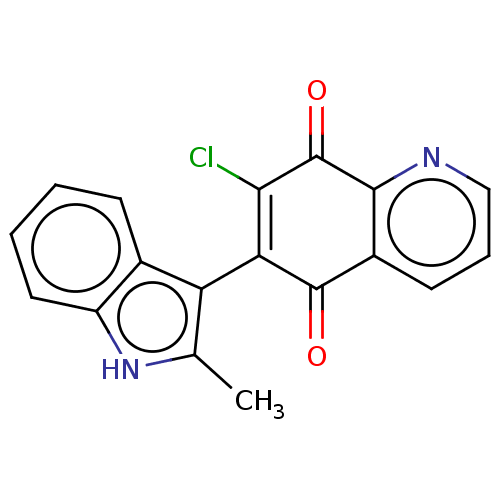 (CHEMBL4227412) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462021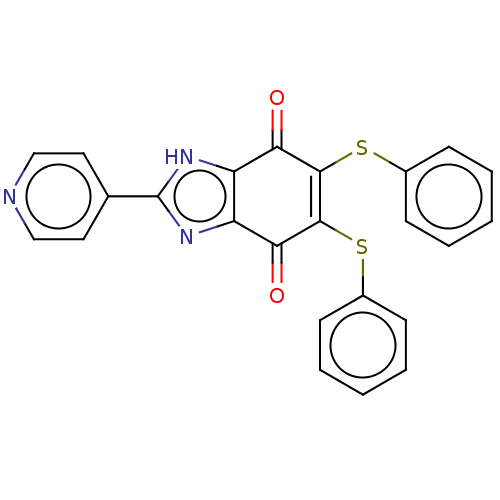 (CHEMBL4227020) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462025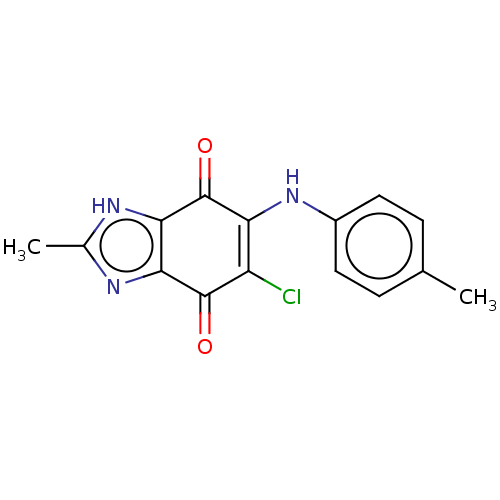 (CHEMBL331941) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462019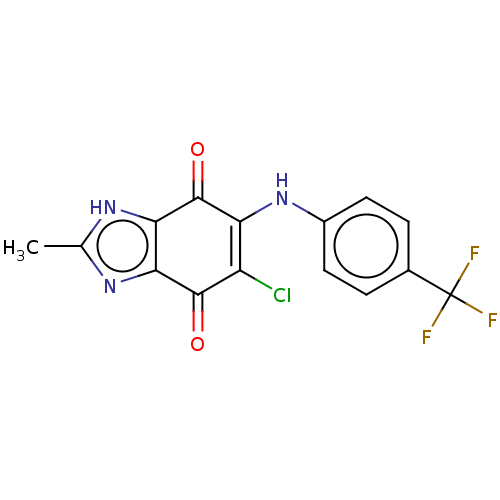 (CHEMBL259499) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50462014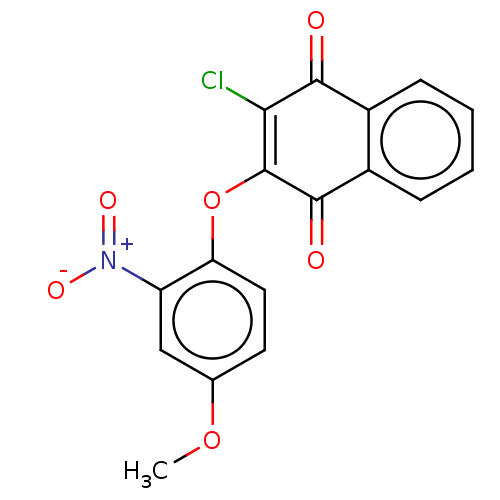 (CHEMBL4224853) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human rhinovirus protease 3C using fluorogenic-Dabcyl-KTSAVLQSGFRKME-Edan as substrate after 5 mins by FRET assay | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462026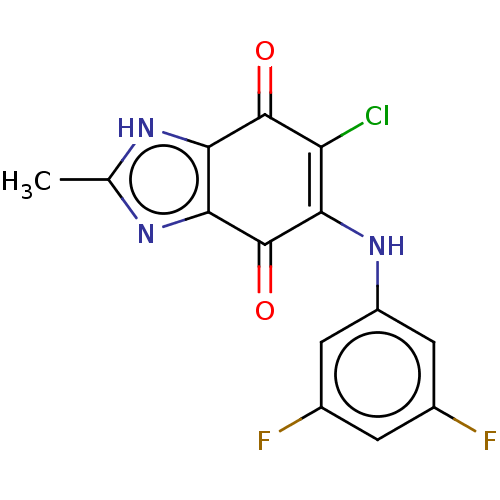 (CHEMBL4229177) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462023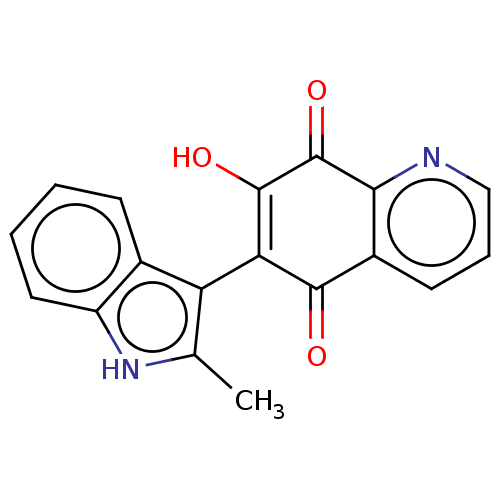 (CHEMBL4226298) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462018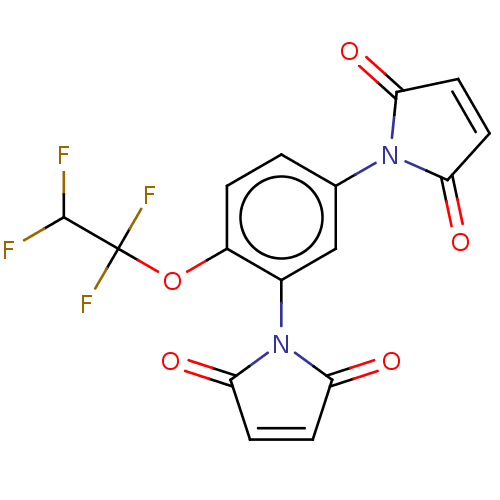 (CHEMBL4225972) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462015 (CHEMBL4227149) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462014 (CHEMBL4224853) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462017 (CHEMBL4229199) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50178490 (7,12-dioxo-5-(2-piperidin-1-yl-ethoxy)-7,12-dihydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against EGFR kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4367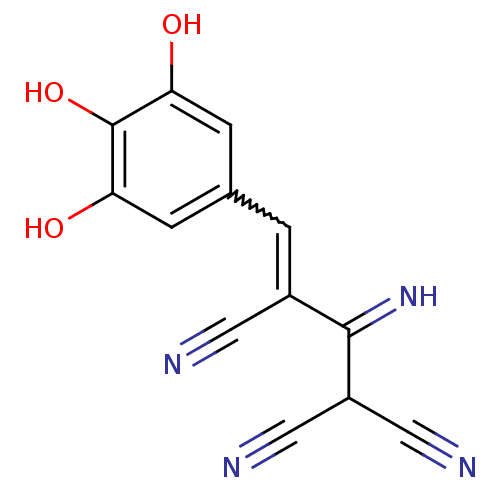 ((3Z)-2-amino-3-[(3,4,5-trihydroxyphenyl)methyliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4367 ((3Z)-2-amino-3-[(3,4,5-trihydroxyphenyl)methyliden...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against EGFR kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4367 ((3Z)-2-amino-3-[(3,4,5-trihydroxyphenyl)methyliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against VEGFR2 kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50178490 (7,12-dioxo-5-(2-piperidin-1-yl-ethoxy)-7,12-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against VEGFR2 kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4367 ((3Z)-2-amino-3-[(3,4,5-trihydroxyphenyl)methyliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against FGFR1 kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50178494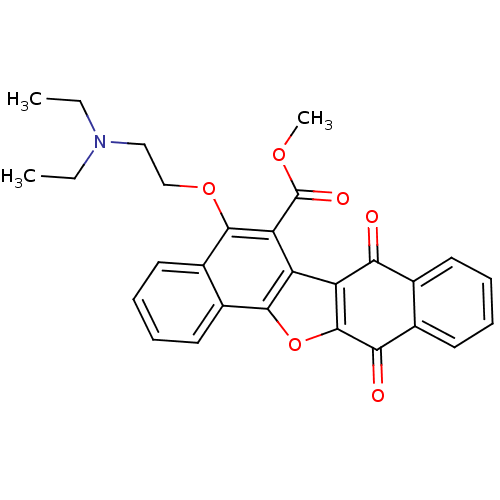 (5-(2-diethylamino-ethoxy)-7,12-dioxo-7,12-dihydro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against EGFR kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462016 (CHEMBL3213811) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50178490 (7,12-dioxo-5-(2-piperidin-1-yl-ethoxy)-7,12-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50178492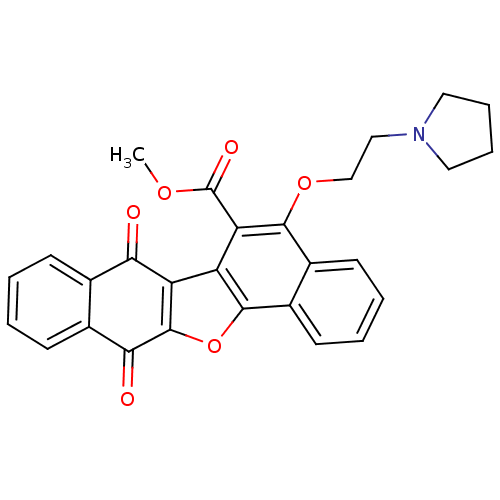 (7,12-dioxo-5-(2-pyrrolidin-1-yl-ethoxy)-7,12-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against VEGFR2 kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462024 (CHEMBL4225121) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50178490 (7,12-dioxo-5-(2-piperidin-1-yl-ethoxy)-7,12-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against FGFR1 kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50178495 (5-(2-dimethylamino-ethoxy)-7,12-dioxo-7,12-dihydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50462027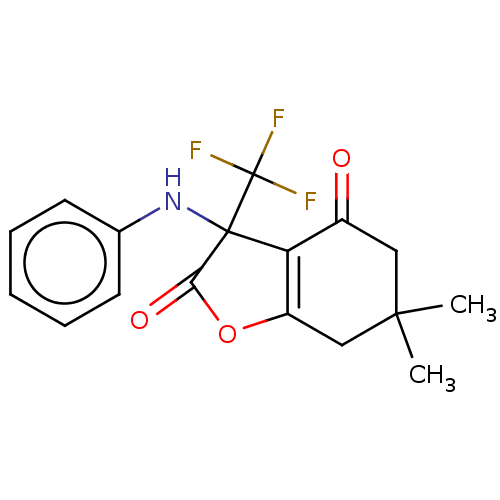 (CHEMBL4227809) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50462026 (CHEMBL4229177) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human rhinovirus protease 3C using fluorogenic-Dabcyl-KTSAVLQSGFRKME-Edan as substrate after 5 mins by FRET assay | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50178492 (7,12-dioxo-5-(2-pyrrolidin-1-yl-ethoxy)-7,12-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50178494 (5-(2-diethylamino-ethoxy)-7,12-dioxo-7,12-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against FGFR1 kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50178492 (7,12-dioxo-5-(2-pyrrolidin-1-yl-ethoxy)-7,12-dihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against EGFR kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50178491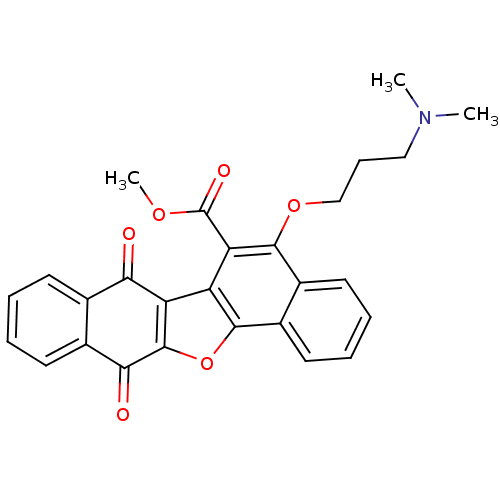 (5-(3-dimethylamino-propoxy)-7,12-dioxo-7,12-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM36895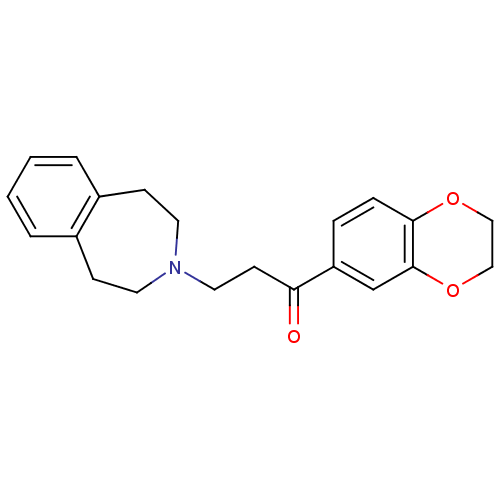 (1-(2,3-dihydro-1,4-benzodioxin-6-yl)-3-(1,2,4,5-te...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human coxsackievirus B3 protease 3C expressed in Escherichia coli (BL21) using fluorogenic-Dabcyl-KEALFQGPPQFE-Edans as substrate after... | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50178494 (5-(2-diethylamino-ethoxy)-7,12-dioxo-7,12-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against VEGFR2 kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50178493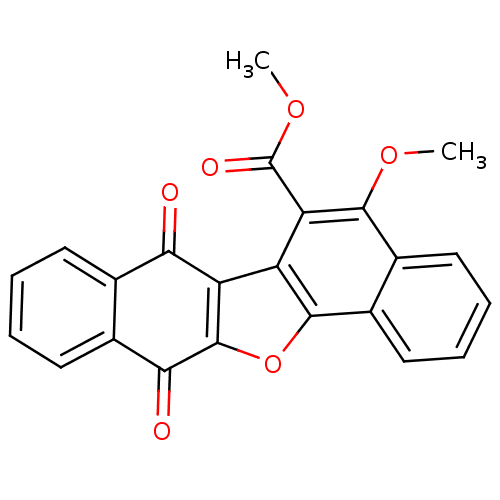 (5-methoxy-7,12-dioxo-7,12-dihydro-dinaphtho[1,2-b;...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against EGFR kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50178495 (5-(2-dimethylamino-ethoxy)-7,12-dioxo-7,12-dihydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against VEGFR2 kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50178491 (5-(3-dimethylamino-propoxy)-7,12-dioxo-7,12-dihydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against EGFR kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50178491 (5-(3-dimethylamino-propoxy)-7,12-dioxo-7,12-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against VEGFR2 kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50178494 (5-(2-diethylamino-ethoxy)-7,12-dioxo-7,12-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50178495 (5-(2-dimethylamino-ethoxy)-7,12-dioxo-7,12-dihydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against FGFR1 kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50178492 (7,12-dioxo-5-(2-pyrrolidin-1-yl-ethoxy)-7,12-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against FGFR1 kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50178495 (5-(2-dimethylamino-ethoxy)-7,12-dioxo-7,12-dihydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against EGFR kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50178491 (5-(3-dimethylamino-propoxy)-7,12-dioxo-7,12-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against FGFR1 kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50178493 (5-methoxy-7,12-dioxo-7,12-dihydro-dinaphtho[1,2-b;...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against FGFR1 kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50178496 (7,12-dioxo-5-propoxy-7,12-dihydro-dinaphtho[1,2-b;...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50178496 (7,12-dioxo-5-propoxy-7,12-dihydro-dinaphtho[1,2-b;...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against EGFR kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50178493 (5-methoxy-7,12-dioxo-7,12-dihydro-dinaphtho[1,2-b;...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50178496 (7,12-dioxo-5-propoxy-7,12-dihydro-dinaphtho[1,2-b;...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against FGFR1 kinase | Bioorg Med Chem Lett 16: 737-42 (2005) Article DOI: 10.1016/j.bmcl.2005.08.115 BindingDB Entry DOI: 10.7270/Q2SF2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||