 Found 1188 hits with Last Name = 'lee' and Initial = 'ma'
Found 1188 hits with Last Name = 'lee' and Initial = 'ma' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Insulin receptor
(Homo sapiens (Human)) | BDBM50256480
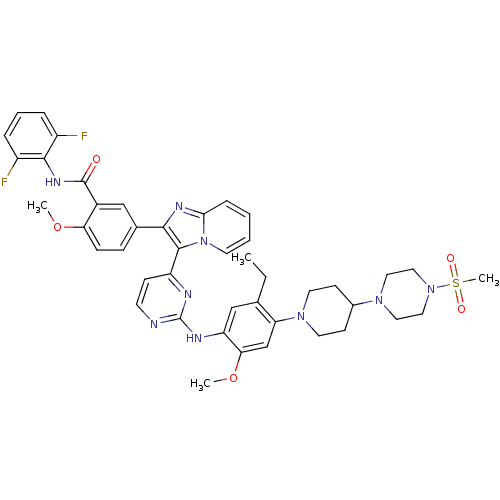
(CHEMBL466397 | N-(2,6-difluorophenyl)-5-(3-(2-(5-e...)Show SMILES CCc1cc(Nc2nccc(n2)-c2c(nc3ccccn23)-c2ccc(OC)c(c2)C(=O)Nc2c(F)cccc2F)c(OC)cc1N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C44H47F2N9O5S/c1-5-28-26-35(38(60-3)27-36(28)53-19-15-30(16-20-53)52-21-23-54(24-22-52)61(4,57)58)49-44-47-17-14-34(48-44)42-40(50-39-11-6-7-18-55(39)42)29-12-13-37(59-2)31(25-29)43(56)51-41-32(45)9-8-10-33(41)46/h6-14,17-18,25-27,30H,5,15-16,19-24H2,1-4H3,(H,51,56)(H,47,48,49) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to insulin receptor by liquid scintillation counting |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256480

(CHEMBL466397 | N-(2,6-difluorophenyl)-5-(3-(2-(5-e...)Show SMILES CCc1cc(Nc2nccc(n2)-c2c(nc3ccccn23)-c2ccc(OC)c(c2)C(=O)Nc2c(F)cccc2F)c(OC)cc1N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C44H47F2N9O5S/c1-5-28-26-35(38(60-3)27-36(28)53-19-15-30(16-20-53)52-21-23-54(24-22-52)61(4,57)58)49-44-47-17-14-34(48-44)42-40(50-39-11-6-7-18-55(39)42)29-12-13-37(59-2)31(25-29)43(56)51-41-32(45)9-8-10-33(41)46/h6-14,17-18,25-27,30H,5,15-16,19-24H2,1-4H3,(H,51,56)(H,47,48,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to IGF1R by liquid scintillation counting |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063920
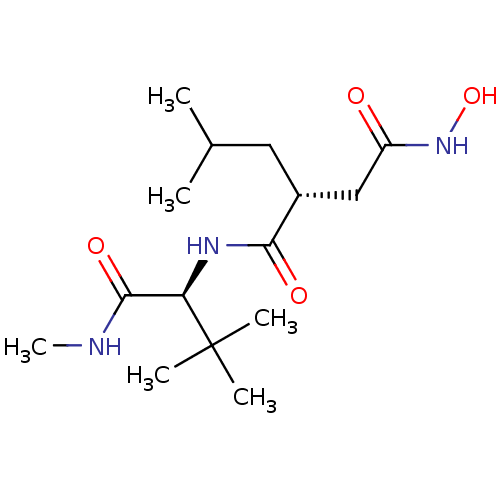
((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-9(2)7-10(8-11(19)18-22)13(20)17-12(14(21)16-6)15(3,4)5/h9-10,12,22H,7-8H2,1-6H3,(H,16,21)(H,17,20)(H,18,19)/t10-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063920

((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-9(2)7-10(8-11(19)18-22)13(20)17-12(14(21)16-6)15(3,4)5/h9-10,12,22H,7-8H2,1-6H3,(H,16,21)(H,17,20)(H,18,19)/t10-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50256478
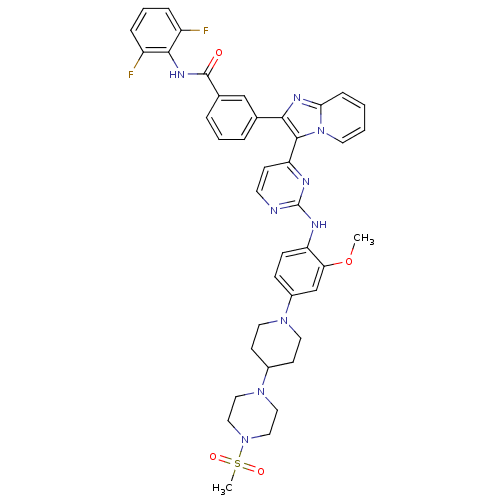
(CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c(nc2ccccn12)-c1cccc(c1)C(=O)Nc1c(F)cccc1F)N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C41H41F2N9O4S/c1-56-35-26-30(49-19-15-29(16-20-49)50-21-23-51(24-22-50)57(2,54)55)12-13-33(35)45-41-44-17-14-34(46-41)39-37(47-36-11-3-4-18-52(36)39)27-7-5-8-28(25-27)40(53)48-38-31(42)9-6-10-32(38)43/h3-14,17-18,25-26,29H,15-16,19-24H2,1-2H3,(H,48,53)(H,44,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to insulin receptor by liquid scintillation counting |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50112086
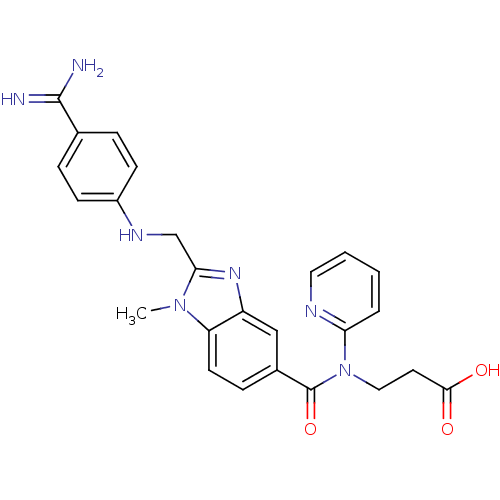
(3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C(=O)N(CCC(O)=O)c1ccccn1 Show InChI InChI=1S/C25H25N7O3/c1-31-20-10-7-17(25(35)32(13-11-23(33)34)21-4-2-3-12-28-21)14-19(20)30-22(31)15-29-18-8-5-16(6-9-18)24(26)27/h2-10,12,14,29H,11,13,15H2,1H3,(H3,26,27)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human coagulation factor alpha-thrombin using Boc-Val-Pro-Arg-AMC as fluorogenic substrate measured at 1 min interval for 1 hr by fluor... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01635
BindingDB Entry DOI: 10.7270/Q2JM2F72 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256478

(CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c(nc2ccccn12)-c1cccc(c1)C(=O)Nc1c(F)cccc1F)N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C41H41F2N9O4S/c1-56-35-26-30(49-19-15-29(16-20-49)50-21-23-51(24-22-50)57(2,54)55)12-13-33(35)45-41-44-17-14-34(46-41)39-37(47-36-11-3-4-18-52(36)39)27-7-5-8-28(25-27)40(53)48-38-31(42)9-6-10-32(38)43/h3-14,17-18,25-26,29H,15-16,19-24H2,1-2H3,(H,48,53)(H,44,45,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to IGF1R by liquid scintillation counting |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103099
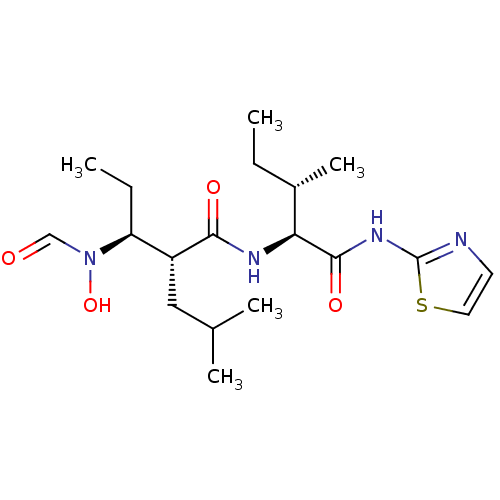
((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H32N4O4S/c1-6-13(5)16(18(26)22-19-20-8-9-28-19)21-17(25)14(10-12(3)4)15(7-2)23(27)11-24/h8-9,11-16,27H,6-7,10H2,1-5H3,(H,21,25)(H,20,22,26)/t13-,14+,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103097
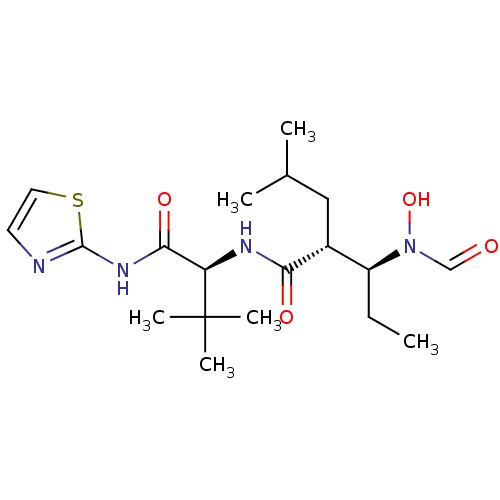
((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C)N(O)C=O Show InChI InChI=1S/C19H32N4O4S/c1-7-14(23(27)11-24)13(10-12(2)3)16(25)21-15(19(4,5)6)17(26)22-18-20-8-9-28-18/h8-9,11-15,27H,7,10H2,1-6H3,(H,21,25)(H,20,22,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103097

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C)N(O)C=O Show InChI InChI=1S/C19H32N4O4S/c1-7-14(23(27)11-24)13(10-12(2)3)16(25)21-15(19(4,5)6)17(26)22-18-20-8-9-28-18/h8-9,11-15,27H,7,10H2,1-6H3,(H,21,25)(H,20,22,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103096
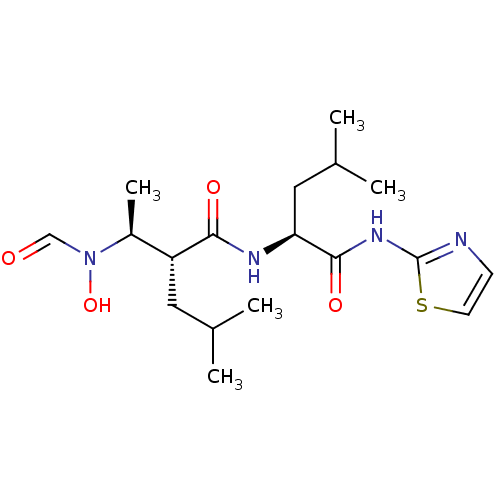
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H30N4O4S/c1-11(2)8-14(13(5)22(26)10-23)16(24)20-15(9-12(3)4)17(25)21-18-19-6-7-27-18/h6-7,10-15,26H,8-9H2,1-5H3,(H,20,24)(H,19,21,25)/t13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103102
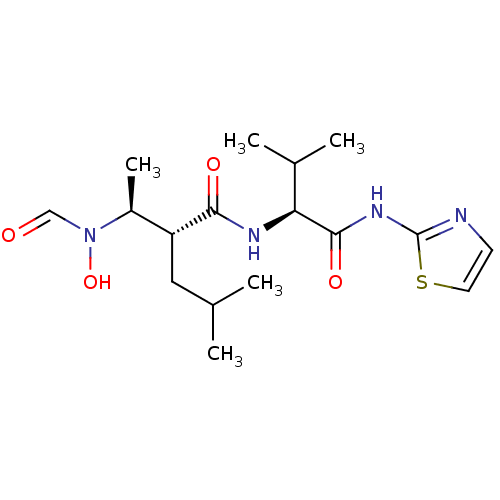
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@@H](C(C)C)C(=O)Nc1nccs1 Show InChI InChI=1S/C17H28N4O4S/c1-10(2)8-13(12(5)21(25)9-22)15(23)19-14(11(3)4)16(24)20-17-18-6-7-26-17/h6-7,9-14,25H,8H2,1-5H3,(H,19,23)(H,18,20,24)/t12-,13+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103092
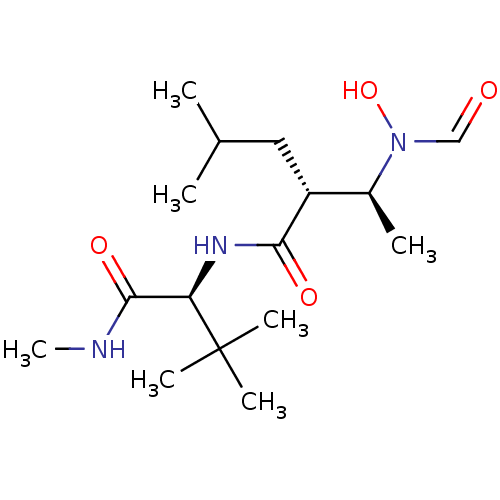
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-10(2)8-12(11(3)19(23)9-20)14(21)18-13(15(22)17-7)16(4,5)6/h9-13,23H,8H2,1-7H3,(H,17,22)(H,18,21)/t11-,12+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103093
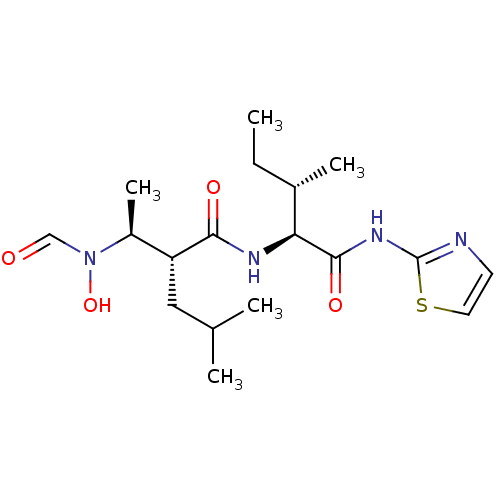
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H30N4O4S/c1-6-12(4)15(17(25)21-18-19-7-8-27-18)20-16(24)14(9-11(2)3)13(5)22(26)10-23/h7-8,10-15,26H,6,9H2,1-5H3,(H,20,24)(H,19,21,25)/t12-,13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103098
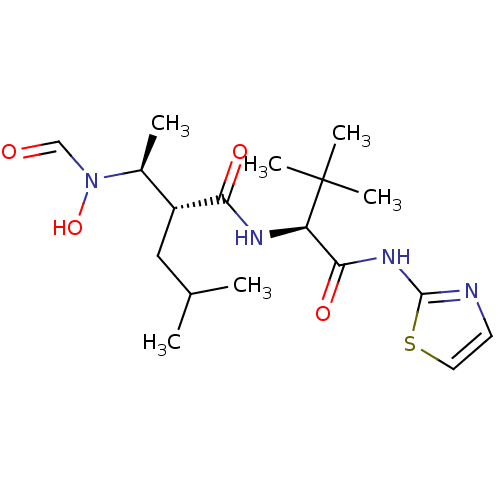
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C Show InChI InChI=1S/C18H30N4O4S/c1-11(2)9-13(12(3)22(26)10-23)15(24)20-14(18(4,5)6)16(25)21-17-19-7-8-27-17/h7-8,10-14,26H,9H2,1-6H3,(H,20,24)(H,19,21,25)/t12-,13+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103092

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-10(2)8-12(11(3)19(23)9-20)14(21)18-13(15(22)17-7)16(4,5)6/h9-13,23H,8H2,1-7H3,(H,17,22)(H,18,21)/t11-,12+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103099

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H32N4O4S/c1-6-13(5)16(18(26)22-19-20-8-9-28-19)21-17(25)14(10-12(3)4)15(7-2)23(27)11-24/h8-9,11-16,27H,6-7,10H2,1-5H3,(H,21,25)(H,20,22,26)/t13-,14+,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103102

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@@H](C(C)C)C(=O)Nc1nccs1 Show InChI InChI=1S/C17H28N4O4S/c1-10(2)8-13(12(5)21(25)9-22)15(23)19-14(11(3)4)16(24)20-17-18-6-7-26-17/h6-7,9-14,25H,8H2,1-5H3,(H,19,23)(H,18,20,24)/t12-,13+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103098

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C Show InChI InChI=1S/C18H30N4O4S/c1-11(2)9-13(12(3)22(26)10-23)15(24)20-14(18(4,5)6)16(25)21-17-19-7-8-27-17/h7-8,10-14,26H,9H2,1-6H3,(H,20,24)(H,19,21,25)/t12-,13+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103097

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C)N(O)C=O Show InChI InChI=1S/C19H32N4O4S/c1-7-14(23(27)11-24)13(10-12(2)3)16(25)21-15(19(4,5)6)17(26)22-18-20-8-9-28-18/h8-9,11-15,27H,7,10H2,1-6H3,(H,21,25)(H,20,22,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103095
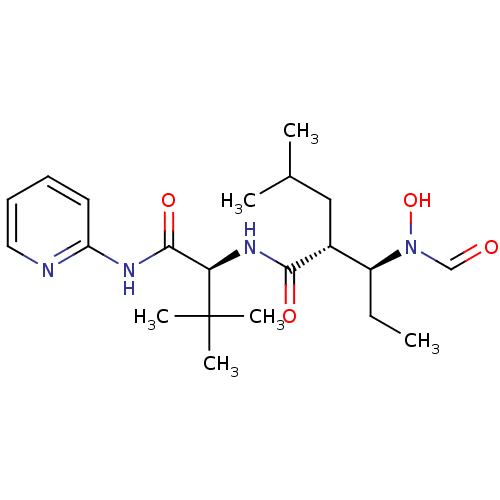
((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1ccccn1)C(C)(C)C)N(O)C=O Show InChI InChI=1S/C21H34N4O4/c1-7-16(25(29)13-26)15(12-14(2)3)19(27)24-18(21(4,5)6)20(28)23-17-10-8-9-11-22-17/h8-11,13-16,18,29H,7,12H2,1-6H3,(H,24,27)(H,22,23,28)/t15-,16+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103093

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H30N4O4S/c1-6-12(4)15(17(25)21-18-19-7-8-27-18)20-16(24)14(9-11(2)3)13(5)22(26)10-23/h7-8,10-15,26H,6,9H2,1-5H3,(H,20,24)(H,19,21,25)/t12-,13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470745

(CHEMBL286840)Show SMILES COc1ccc(cc1OC1CCCC1)C1CCN(C1)C(=O)OC(C)(C)C Show InChI InChI=1S/C21H31NO4/c1-21(2,3)26-20(23)22-12-11-16(14-22)15-9-10-18(24-4)19(13-15)25-17-7-5-6-8-17/h9-10,13,16-17H,5-8,11-12,14H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50285059
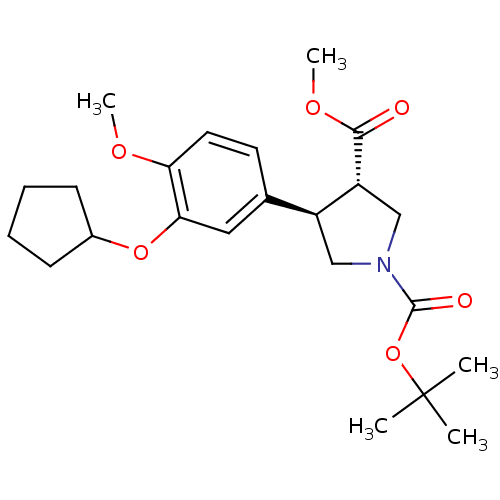
(1-(tert-butyl) 3-methyl 4-(3-cyclopentyloxy-4-meth...)Show SMILES COC(=O)[C@@H]1CN(C[C@H]1c1ccc(OC)c(OC2CCCC2)c1)C(=O)OC(C)(C)C Show InChI InChI=1S/C23H33NO6/c1-23(2,3)30-22(26)24-13-17(18(14-24)21(25)28-5)15-10-11-19(27-4)20(12-15)29-16-8-6-7-9-16/h10-12,16-18H,6-9,13-14H2,1-5H3/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit the catalytic activity of human Phosphodiesterase 4B (PDE IVB) |
Bioorg Med Chem Lett 5: 1977-1982 (1995)
Article DOI: 10.1016/0960-894X(95)00336-R
BindingDB Entry DOI: 10.7270/Q2PN95MX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50048248
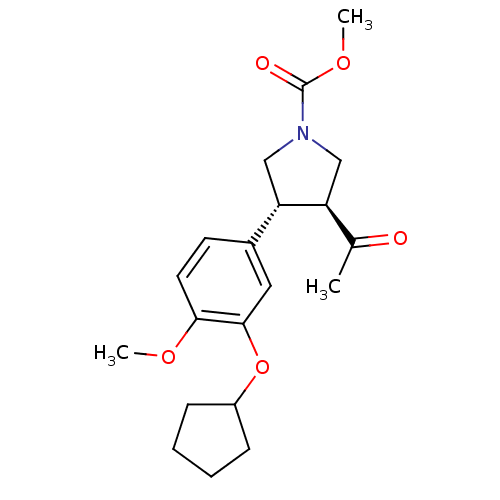
((3S,4R)-3-Acetyl-4-(3-cyclopentyloxy-4-methoxy-phe...)Show SMILES COC(=O)N1C[C@H]([C@@H](C1)c1ccc(OC)c(OC2CCCC2)c1)C(C)=O Show InChI InChI=1S/C20H27NO5/c1-13(22)16-11-21(20(23)25-3)12-17(16)14-8-9-18(24-2)19(10-14)26-15-6-4-5-7-15/h8-10,15-17H,4-7,11-12H2,1-3H3/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit the catalytic activity of human Phosphodiesterase 4B (PDE IVB) |
Bioorg Med Chem Lett 5: 1977-1982 (1995)
Article DOI: 10.1016/0960-894X(95)00336-R
BindingDB Entry DOI: 10.7270/Q2PN95MX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103101
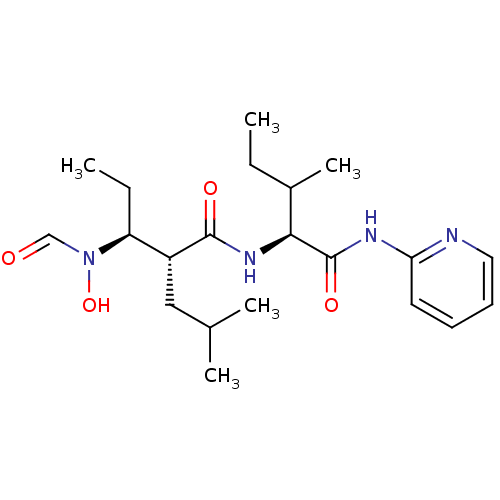
((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1ccccn1 Show InChI InChI=1S/C21H34N4O4/c1-6-15(5)19(21(28)23-18-10-8-9-11-22-18)24-20(27)16(12-14(3)4)17(7-2)25(29)13-26/h8-11,13-17,19,29H,6-7,12H2,1-5H3,(H,24,27)(H,22,23,28)/t15?,16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103100
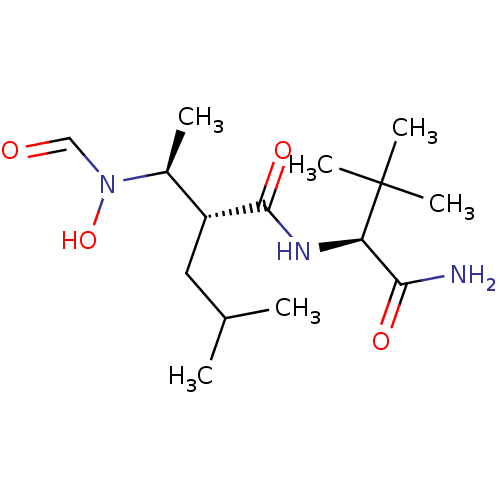
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@H](C(N)=O)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-9(2)7-11(10(3)18(22)8-19)14(21)17-12(13(16)20)15(4,5)6/h8-12,22H,7H2,1-6H3,(H2,16,20)(H,17,21)/t10-,11+,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103098

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C Show InChI InChI=1S/C18H30N4O4S/c1-11(2)9-13(12(3)22(26)10-23)15(24)20-14(18(4,5)6)16(25)21-17-19-7-8-27-17/h7-8,10-14,26H,9H2,1-6H3,(H,20,24)(H,19,21,25)/t12-,13+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470752
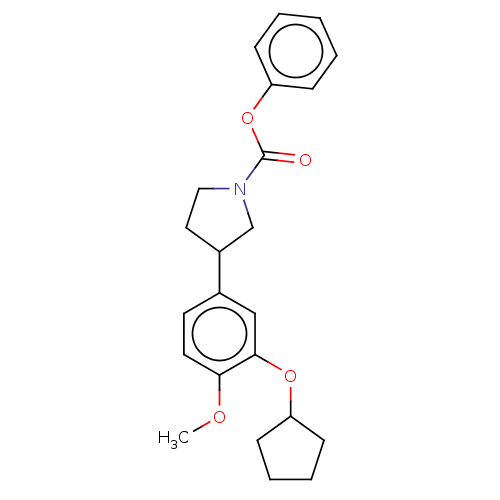
(CHEMBL38328)Show SMILES COc1ccc(cc1OC1CCCC1)C1CCN(C1)C(=O)Oc1ccccc1 Show InChI InChI=1S/C23H27NO4/c1-26-21-12-11-17(15-22(21)27-19-9-5-6-10-19)18-13-14-24(16-18)23(25)28-20-7-3-2-4-8-20/h2-4,7-8,11-12,15,18-19H,5-6,9-10,13-14,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103102

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@@H](C(C)C)C(=O)Nc1nccs1 Show InChI InChI=1S/C17H28N4O4S/c1-10(2)8-13(12(5)21(25)9-22)15(23)19-14(11(3)4)16(24)20-17-18-6-7-26-17/h6-7,9-14,25H,8H2,1-5H3,(H,19,23)(H,18,20,24)/t12-,13+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50103101

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1ccccn1 Show InChI InChI=1S/C21H34N4O4/c1-6-15(5)19(21(28)23-18-10-8-9-11-22-18)24-20(27)16(12-14(3)4)17(7-2)25(29)13-26/h8-11,13-17,19,29H,6-7,12H2,1-5H3,(H,24,27)(H,22,23,28)/t15?,16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-3 (MMP3) |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50285045
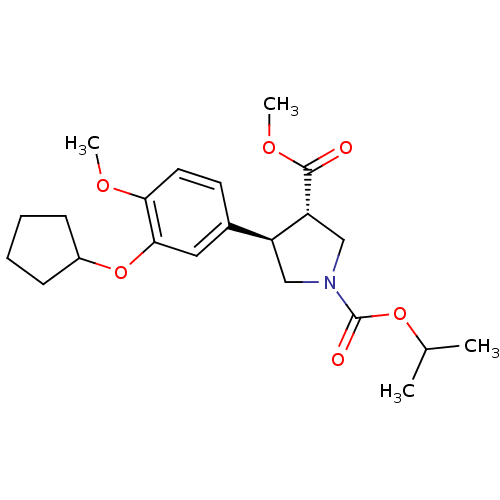
(1-isopropyl 3-methyl 4-(3-cyclopentyloxy-4-methoxy...)Show SMILES COC(=O)[C@@H]1CN(C[C@H]1c1ccc(OC)c(OC2CCCC2)c1)C(=O)OC(C)C Show InChI InChI=1S/C22H31NO6/c1-14(2)28-22(25)23-12-17(18(13-23)21(24)27-4)15-9-10-19(26-3)20(11-15)29-16-7-5-6-8-16/h9-11,14,16-18H,5-8,12-13H2,1-4H3/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit the catalytic activity of human Phosphodiesterase 4B (PDE IVB) |
Bioorg Med Chem Lett 5: 1977-1982 (1995)
Article DOI: 10.1016/0960-894X(95)00336-R
BindingDB Entry DOI: 10.7270/Q2PN95MX |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103093

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H30N4O4S/c1-6-12(4)15(17(25)21-18-19-7-8-27-18)20-16(24)14(9-11(2)3)13(5)22(26)10-23/h7-8,10-15,26H,6,9H2,1-5H3,(H,20,24)(H,19,21,25)/t12-,13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103099

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H32N4O4S/c1-6-13(5)16(18(26)22-19-20-8-9-28-19)21-17(25)14(10-12(3)4)15(7-2)23(27)11-24/h8-9,11-16,27H,6-7,10H2,1-5H3,(H,21,25)(H,20,22,26)/t13-,14+,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50103099

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H32N4O4S/c1-6-13(5)16(18(26)22-19-20-8-9-28-19)21-17(25)14(10-12(3)4)15(7-2)23(27)11-24/h8-9,11-16,27H,6-7,10H2,1-5H3,(H,21,25)(H,20,22,26)/t13-,14+,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-3 (MMP3) |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470756
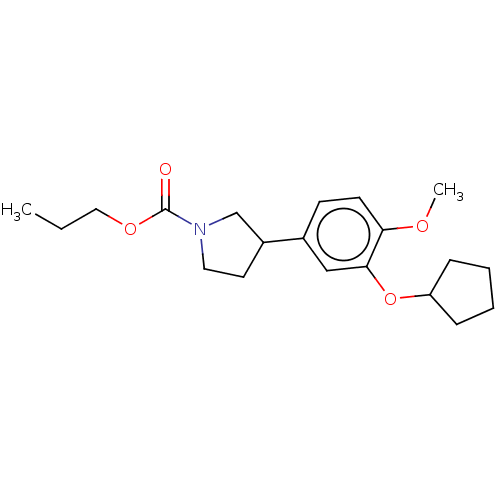
(CHEMBL37453)Show InChI InChI=1S/C20H29NO4/c1-3-12-24-20(22)21-11-10-16(14-21)15-8-9-18(23-2)19(13-15)25-17-6-4-5-7-17/h8-9,13,16-17H,3-7,10-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103095

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1ccccn1)C(C)(C)C)N(O)C=O Show InChI InChI=1S/C21H34N4O4/c1-7-16(25(29)13-26)15(12-14(2)3)19(27)24-18(21(4,5)6)20(28)23-17-10-8-9-11-22-17/h8-11,13-16,18,29H,7,12H2,1-6H3,(H,24,27)(H,22,23,28)/t15-,16+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50285038
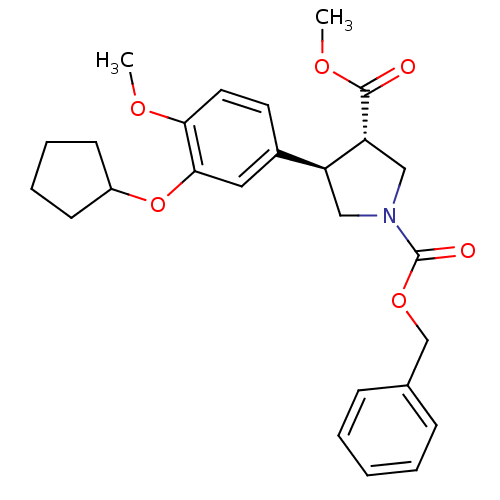
(1-benzyl 3-methyl 4-(3-cyclopentyloxy-4-methoxyphe...)Show SMILES COC(=O)[C@@H]1CN(C[C@H]1c1ccc(OC)c(OC2CCCC2)c1)C(=O)OCc1ccccc1 Show InChI InChI=1S/C26H31NO6/c1-30-23-13-12-19(14-24(23)33-20-10-6-7-11-20)21-15-27(16-22(21)25(28)31-2)26(29)32-17-18-8-4-3-5-9-18/h3-5,8-9,12-14,20-22H,6-7,10-11,15-17H2,1-2H3/t21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit the catalytic activity of human Phosphodiesterase 4B (PDE IVB) |
Bioorg Med Chem Lett 5: 1977-1982 (1995)
Article DOI: 10.1016/0960-894X(95)00336-R
BindingDB Entry DOI: 10.7270/Q2PN95MX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50285042
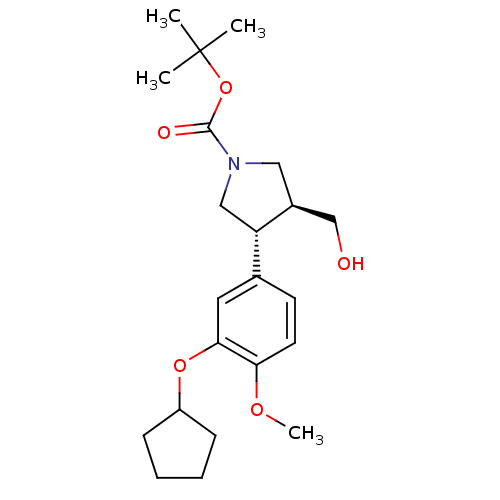
(CHEMBL302632 | tert-butyl 3-(3-cyclopentyloxy-4-me...)Show SMILES COc1ccc(cc1OC1CCCC1)[C@@H]1CN(C[C@H]1CO)C(=O)OC(C)(C)C Show InChI InChI=1S/C22H33NO5/c1-22(2,3)28-21(25)23-12-16(14-24)18(13-23)15-9-10-19(26-4)20(11-15)27-17-7-5-6-8-17/h9-11,16-18,24H,5-8,12-14H2,1-4H3/t16-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit the catalytic activity of human Phosphodiesterase 4B (PDE IVB) |
Bioorg Med Chem Lett 5: 1977-1982 (1995)
Article DOI: 10.1016/0960-894X(95)00336-R
BindingDB Entry DOI: 10.7270/Q2PN95MX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103096

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H30N4O4S/c1-11(2)8-14(13(5)22(26)10-23)16(24)20-15(9-12(3)4)17(25)21-18-19-6-7-27-18/h6-7,10-15,26H,8-9H2,1-5H3,(H,20,24)(H,19,21,25)/t13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103101

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1ccccn1 Show InChI InChI=1S/C21H34N4O4/c1-6-15(5)19(21(28)23-18-10-8-9-11-22-18)24-20(27)16(12-14(3)4)17(7-2)25(29)13-26/h8-11,13-17,19,29H,6-7,12H2,1-5H3,(H,24,27)(H,22,23,28)/t15?,16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470750
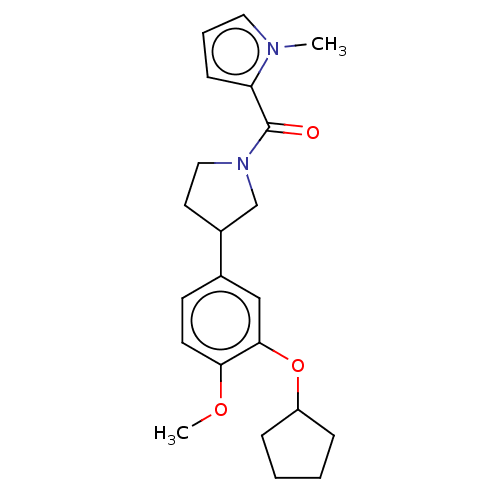
(CHEMBL286291)Show InChI InChI=1S/C22H28N2O3/c1-23-12-5-8-19(23)22(25)24-13-11-17(15-24)16-9-10-20(26-2)21(14-16)27-18-6-3-4-7-18/h5,8-10,12,14,17-18H,3-4,6-7,11,13,15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470731
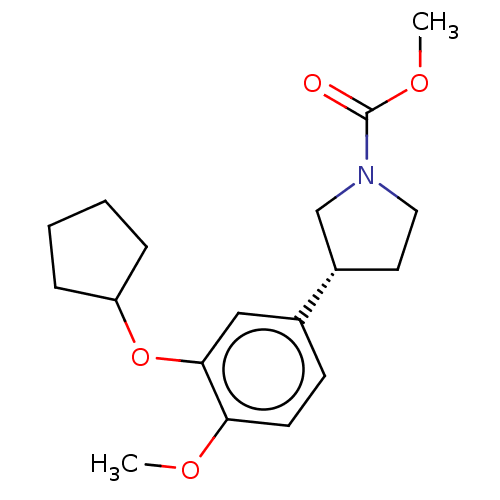
(CHEMBL146946)Show SMILES COC(=O)N1CC[C@@H](C1)c1ccc(OC)c(OC2CCCC2)c1 Show InChI InChI=1S/C18H25NO4/c1-21-16-8-7-13(11-17(16)23-15-5-3-4-6-15)14-9-10-19(12-14)18(20)22-2/h7-8,11,14-15H,3-6,9-10,12H2,1-2H3/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50285050
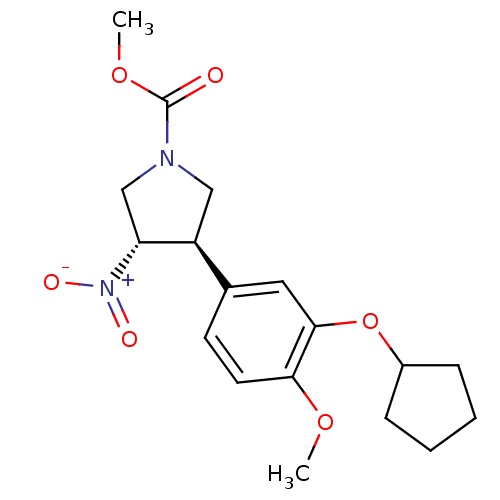
(CHEMBL59386 | methyl 3-(3-cyclopentyloxy-4-methoxy...)Show SMILES COC(=O)N1C[C@H]([C@@H](C1)[N+]([O-])=O)c1ccc(OC)c(OC2CCCC2)c1 Show InChI InChI=1S/C18H24N2O6/c1-24-16-8-7-12(9-17(16)26-13-5-3-4-6-13)14-10-19(18(21)25-2)11-15(14)20(22)23/h7-9,13-15H,3-6,10-11H2,1-2H3/t14-,15+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit the catalytic activity of human Phosphodiesterase 4B (PDE IVB) |
Bioorg Med Chem Lett 5: 1977-1982 (1995)
Article DOI: 10.1016/0960-894X(95)00336-R
BindingDB Entry DOI: 10.7270/Q2PN95MX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470736
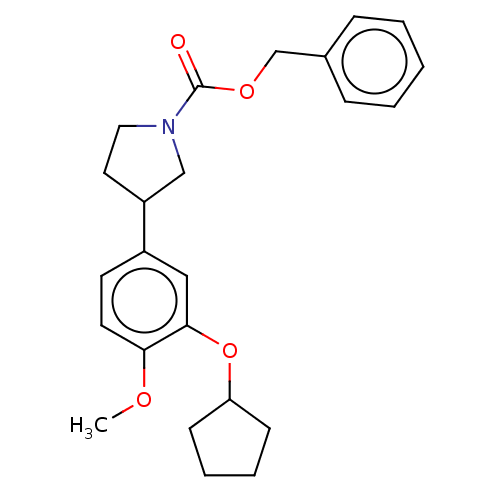
(CHEMBL37029)Show SMILES COc1ccc(cc1OC1CCCC1)C1CCN(C1)C(=O)OCc1ccccc1 Show InChI InChI=1S/C24H29NO4/c1-27-22-12-11-19(15-23(22)29-21-9-5-6-10-21)20-13-14-25(16-20)24(26)28-17-18-7-3-2-4-8-18/h2-4,7-8,11-12,15,20-21H,5-6,9-10,13-14,16-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103094
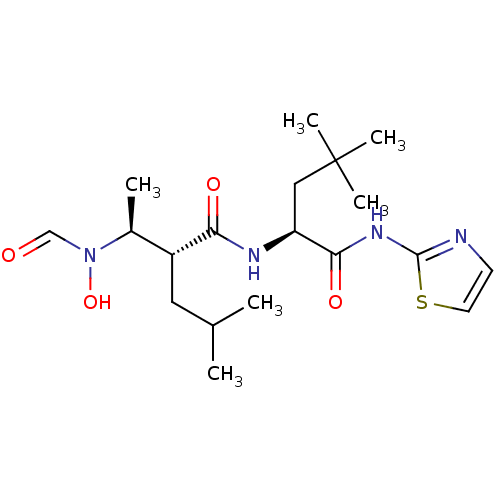
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@@H](CC(C)(C)C)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H32N4O4S/c1-12(2)9-14(13(3)23(27)11-24)16(25)21-15(10-19(4,5)6)17(26)22-18-20-7-8-28-18/h7-8,11-15,27H,9-10H2,1-6H3,(H,21,25)(H,20,22,26)/t13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470740

(CHEMBL264851)Show InChI InChI=1S/C20H29NO4/c1-14(2)24-20(22)21-11-10-16(13-21)15-8-9-18(23-3)19(12-15)25-17-6-4-5-7-17/h8-9,12,14,16-17H,4-7,10-11,13H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470754
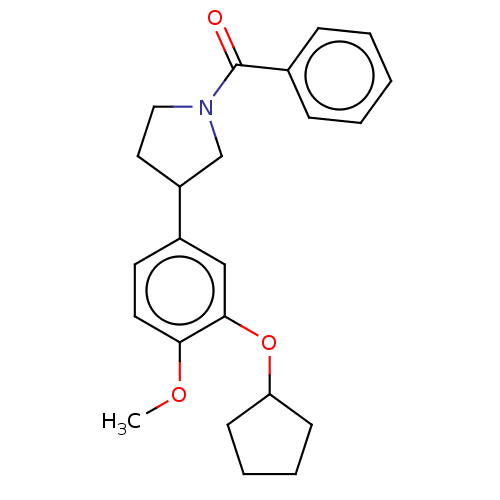
(CHEMBL288928)Show InChI InChI=1S/C23H27NO3/c1-26-21-12-11-18(15-22(21)27-20-9-5-6-10-20)19-13-14-24(16-19)23(25)17-7-3-2-4-8-17/h2-4,7-8,11-12,15,19-20H,5-6,9-10,13-14,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470725
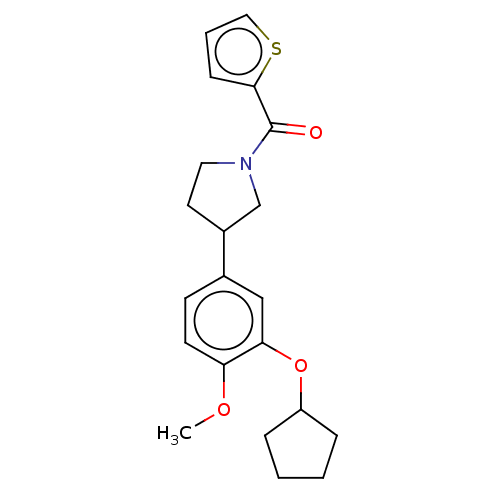
(CHEMBL36318)Show InChI InChI=1S/C21H25NO3S/c1-24-18-9-8-15(13-19(18)25-17-5-2-3-6-17)16-10-11-22(14-16)21(23)20-7-4-12-26-20/h4,7-9,12-13,16-17H,2-3,5-6,10-11,14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470743
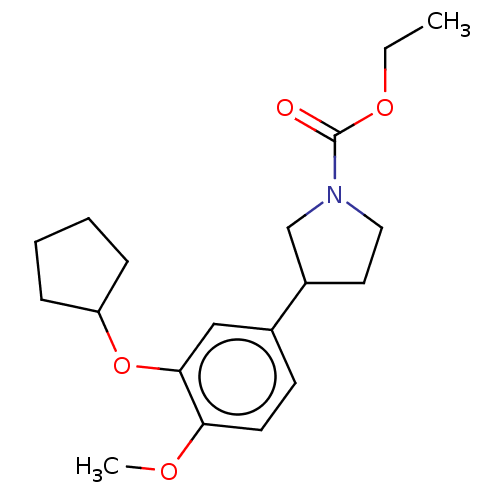
(CHEMBL422578)Show InChI InChI=1S/C19H27NO4/c1-3-23-19(21)20-11-10-15(13-20)14-8-9-17(22-2)18(12-14)24-16-6-4-5-7-16/h8-9,12,15-16H,3-7,10-11,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data