 Found 113 hits with Last Name = 'lee' and Initial = 'wa'
Found 113 hits with Last Name = 'lee' and Initial = 'wa' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50021347
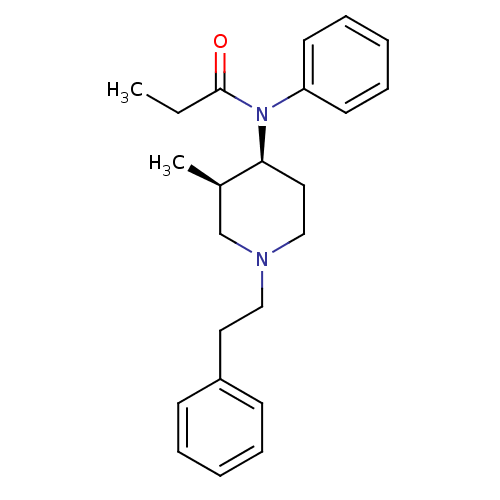
(CHEMBL12391 | N-(3-Methyl-1-phenethyl-piperidin-4-...)Show SMILES CCC(=O)N([C@H]1CCN(CCc2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O/c1-3-23(26)25(21-12-8-5-9-13-21)22-15-17-24(18-19(22)2)16-14-20-10-6-4-7-11-20/h4-13,19,22H,3,14-18H2,1-2H3/t19-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vivo binding affinity to opioid receptor preparations from rat brain |
J Med Chem 29: 1087-93 (1986)
BindingDB Entry DOI: 10.7270/Q2JQ1364 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50021347

(CHEMBL12391 | N-(3-Methyl-1-phenethyl-piperidin-4-...)Show SMILES CCC(=O)N([C@H]1CCN(CCc2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O/c1-3-23(26)25(21-12-8-5-9-13-21)22-15-17-24(18-19(22)2)16-14-20-10-6-4-7-11-20/h4-13,19,22H,3,14-18H2,1-2H3/t19-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vivo binding affinity to opioid receptor preparations from rat brain |
J Med Chem 29: 1087-93 (1986)
BindingDB Entry DOI: 10.7270/Q2JQ1364 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50017082
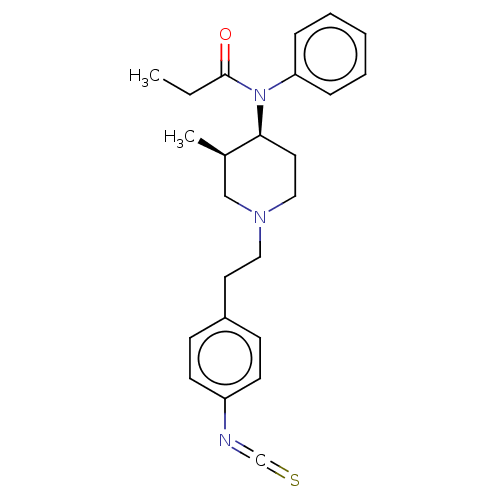
((+)-trans-N-{1-[2-(4-Isothiocyanato-phenyl)-ethyl]...)Show SMILES CCC(=O)N([C@H]1CCN(CCc2ccc(cc2)N=C=S)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C24H29N3OS/c1-3-24(28)27(22-7-5-4-6-8-22)23-14-16-26(17-19(23)2)15-13-20-9-11-21(12-10-20)25-18-29/h4-12,19,23H,3,13-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
The ability of comp[ound to inhibit [3H]-DADL binding to the delta receptor of the NG108-15 cells at 10 min and 37 degree C |
J Med Chem 32: 1392-8 (1989)
BindingDB Entry DOI: 10.7270/Q2JD4VSW |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM19264

((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show InChI InChI=1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185589
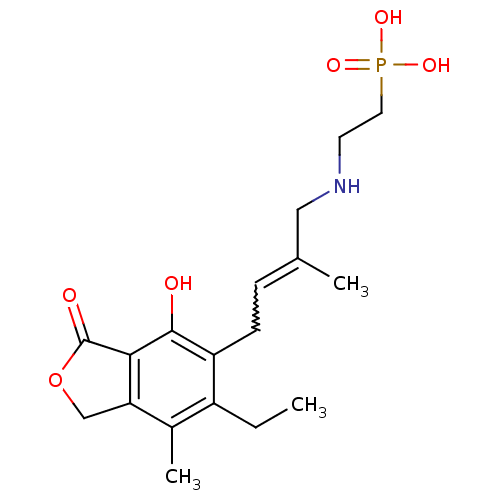
((E)-2-(4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-dih...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1CC=C(C)CNCCP(O)(O)=O |w:15.16| Show InChI InChI=1S/C18H26NO6P/c1-4-13-12(3)15-10-25-18(21)16(15)17(20)14(13)6-5-11(2)9-19-7-8-26(22,23)24/h5,19-20H,4,6-10H2,1-3H3,(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185586
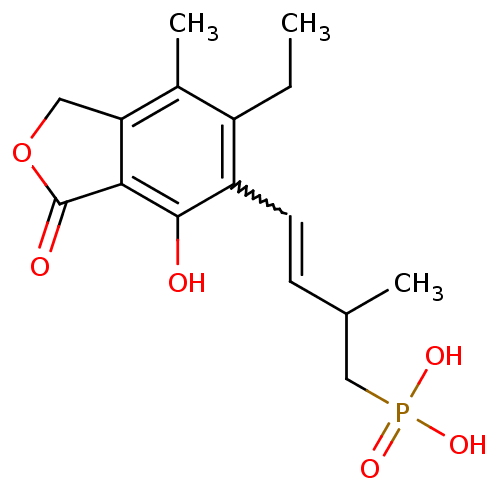
((E)-4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-dihydr...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1C=CC(C)CP(O)(O)=O |w:14.15| Show InChI InChI=1S/C16H21O6P/c1-4-11-10(3)13-7-22-16(18)14(13)15(17)12(11)6-5-9(2)8-23(19,20)21/h5-6,9,17H,4,7-8H2,1-3H3,(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50228233
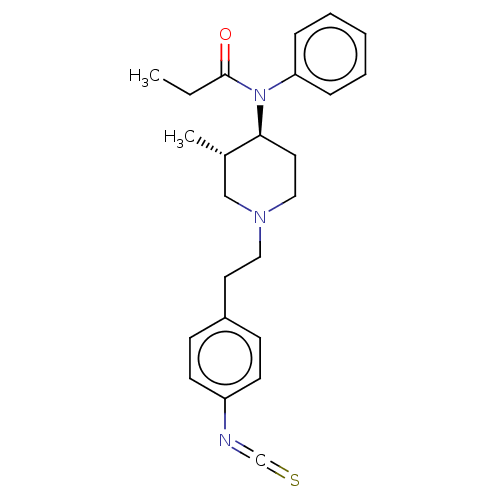
(CHEMBL3349043)Show SMILES CCC(=O)N([C@H]1CCN(CCc2ccc(cc2)N=C=S)C[C@@H]1C)c1ccccc1 |r| Show InChI InChI=1S/C24H29N3OS/c1-3-24(28)27(22-7-5-4-6-8-22)23-14-16-26(17-19(23)2)15-13-20-9-11-21(12-10-20)25-18-29/h4-12,19,23H,3,13-17H2,1-2H3/t19-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
The ability of compound to inhibit [3H]-DADL binding to the delta receptor of the NG108-15 cells at 10 min and 37 degree C |
J Med Chem 32: 1392-8 (1989)
BindingDB Entry DOI: 10.7270/Q2JD4VSW |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185592
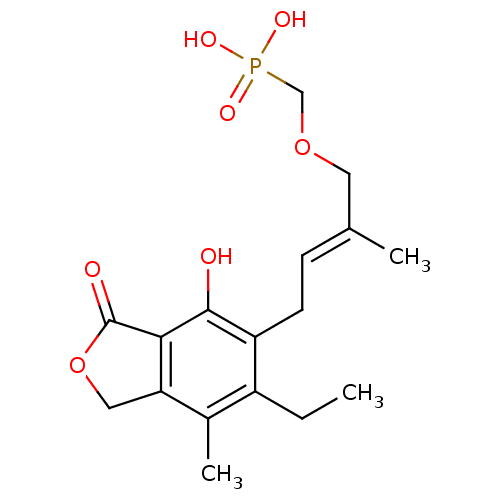
((E)-(4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-dihyd...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)COCP(O)(O)=O Show InChI InChI=1S/C17H23O7P/c1-4-12-11(3)14-8-24-17(19)15(14)16(18)13(12)6-5-10(2)7-23-9-25(20,21)22/h5,18H,4,6-9H2,1-3H3,(H2,20,21,22)/b10-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185601
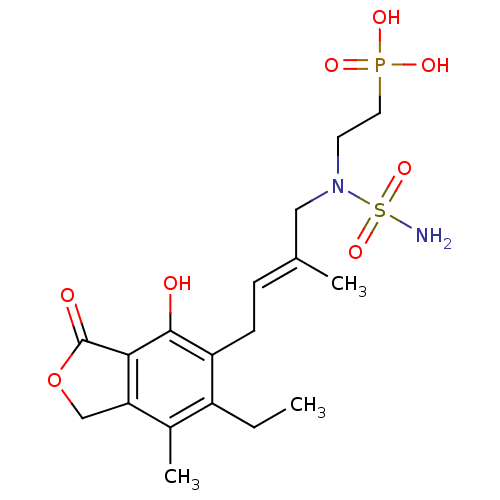
(CHEMBL380059)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CN(CCP(O)(O)=O)S(N)(=O)=O Show InChI InChI=1S/C18H27N2O8PS/c1-4-13-12(3)15-10-28-18(22)16(15)17(21)14(13)6-5-11(2)9-20(30(19,26)27)7-8-29(23,24)25/h5,21H,4,6-10H2,1-3H3,(H2,19,26,27)(H2,23,24,25)/b11-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50008984

(4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...)Show InChI InChI=1S/C22H28N2O/c1-2-22(25)24(20-11-7-4-8-12-20)21-14-17-23(18-15-21)16-13-19-9-5-3-6-10-19/h3-12,21H,2,13-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vivo binding affinity to opioid receptor preparations from rat brain |
J Med Chem 29: 1087-93 (1986)
BindingDB Entry DOI: 10.7270/Q2JQ1364 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM19264

((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show InChI InChI=1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH1 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50185589

((E)-2-(4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-dih...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1CC=C(C)CNCCP(O)(O)=O |w:15.16| Show InChI InChI=1S/C18H26NO6P/c1-4-13-12(3)15-10-25-18(21)16(15)17(20)14(13)6-5-11(2)9-19-7-8-26(22,23)24/h5,19-20H,4,6-10H2,1-3H3,(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH1 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50185592

((E)-(4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-dihyd...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)COCP(O)(O)=O Show InChI InChI=1S/C17H23O7P/c1-4-12-11(3)14-8-24-17(19)15(14)16(18)13(12)6-5-10(2)7-23-9-25(20,21)22/h5,18H,4,6-9H2,1-3H3,(H2,20,21,22)/b10-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH1 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50228234
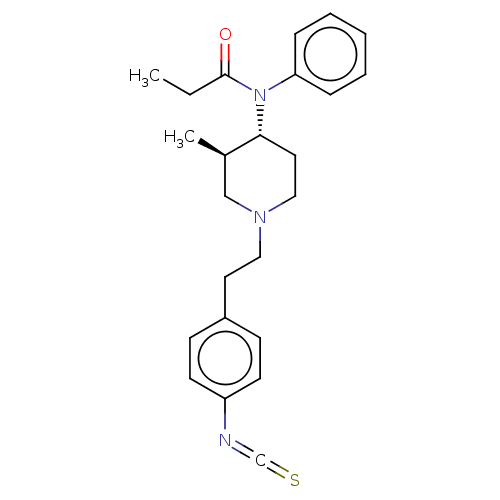
(CHEMBL273222)Show SMILES CCC(=O)N([C@@H]1CCN(CCc2ccc(cc2)N=C=S)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C24H29N3OS/c1-3-24(28)27(22-7-5-4-6-8-22)23-14-16-26(17-19(23)2)15-13-20-9-11-21(12-10-20)25-18-29/h4-12,19,23H,3,13-17H2,1-2H3/t19-,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
The ability of comp[ound to inhibit [3H]DADL binding to the delta receptor of the NG108-15 cells at 10 min and 37 degree C |
J Med Chem 32: 1392-8 (1989)
BindingDB Entry DOI: 10.7270/Q2JD4VSW |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185595
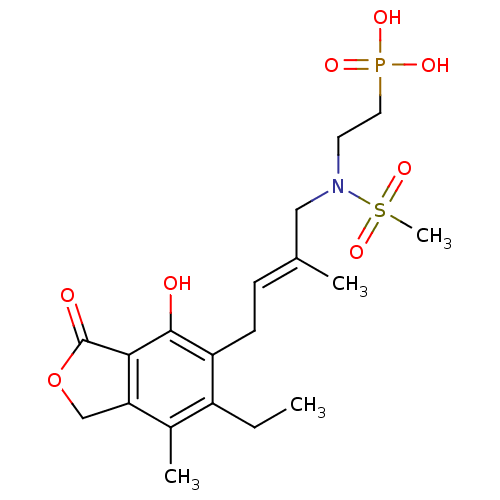
((E)-2-(N-(4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CN(CCP(O)(O)=O)S(C)(=O)=O Show InChI InChI=1S/C19H28NO8PS/c1-5-14-13(3)16-11-28-19(22)17(16)18(21)15(14)7-6-12(2)10-20(30(4,26)27)8-9-29(23,24)25/h6,21H,5,7-11H2,1-4H3,(H2,23,24,25)/b12-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50185586

((E)-4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-dihydr...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1C=CC(C)CP(O)(O)=O |w:14.15| Show InChI InChI=1S/C16H21O6P/c1-4-11-10(3)13-7-22-16(18)14(13)15(17)12(11)6-5-9(2)8-23(19,20)21/h5-6,9,17H,4,7-8H2,1-3H3,(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH1 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185585

((E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihy...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1CC=C(C)CCCP(O)(O)=O |w:15.16| Show InChI InChI=1S/C17H23O7P/c1-10(5-4-8-25(20,21)22)6-7-12-15(18)14-13(9-24-17(14)19)11(2)16(12)23-3/h6,18H,4-5,7-9H2,1-3H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185590

((2-{[(2E)-4-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CN(CCP(O)(O)=O)S(N)(=O)=O Show InChI InChI=1S/C17H25N2O9PS/c1-10(8-19(30(18,25)26)6-7-29(22,23)24)4-5-12-15(20)14-13(9-28-17(14)21)11(2)16(12)27-3/h4,20H,5-9H2,1-3H3,(H2,18,25,26)(H2,22,23,24)/b10-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185596
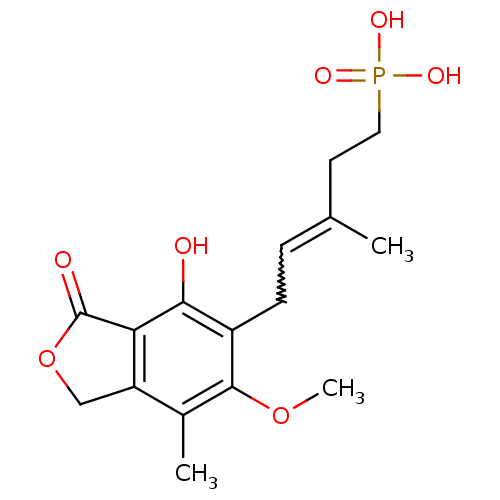
((E)-5-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihy...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1CC=C(C)CCP(O)(O)=O |w:15.16| Show InChI InChI=1S/C16H21O7P/c1-9(6-7-24(19,20)21)4-5-11-14(17)13-12(8-23-16(13)18)10(2)15(11)22-3/h4,17H,5-8H2,1-3H3,(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185604

((E)-2-((4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-di...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CN(C)CCP(O)(O)=O Show InChI InChI=1S/C19H28NO6P/c1-5-14-13(3)16-11-26-19(22)17(16)18(21)15(14)7-6-12(2)10-20(4)8-9-27(23,24)25/h6,21H,5,7-11H2,1-4H3,(H2,23,24,25)/b12-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185603

((E)-4-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihy...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C=CC(C)CP(O)(O)=O |w:14.15| Show InChI InChI=1S/C15H19O7P/c1-8(7-23(18,19)20)4-5-10-13(16)12-11(6-22-15(12)17)9(2)14(10)21-3/h4-5,8,16H,6-7H2,1-3H3,(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185599
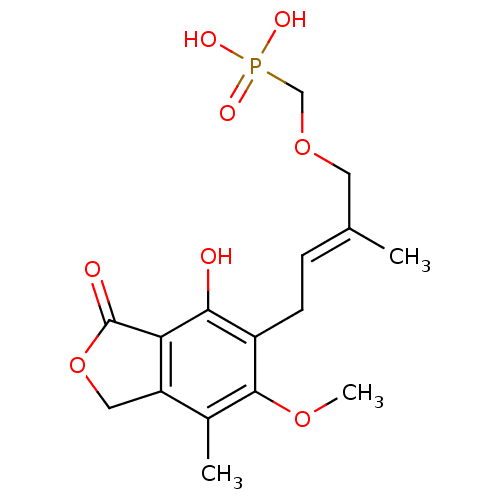
((E)-(4-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)COCP(O)(O)=O Show InChI InChI=1S/C16H21O8P/c1-9(6-23-8-25(19,20)21)4-5-11-14(17)13-12(7-24-16(13)18)10(2)15(11)22-3/h4,17H,5-8H2,1-3H3,(H2,19,20,21)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185587

(7-hydroxy-5-methoxy-4-methyl-6-(3-methylbut-2-enyl...)Show SMILES [#6]-[#8]-c1c(-[#6])c2-[#6]-[#8]-[#6](=O)-c2c(-[#8])c1-[#6]\[#6]=[#6](/[#6])-[#6] Show InChI InChI=1S/C15H18O4/c1-8(2)5-6-10-13(16)12-11(7-19-15(12)17)9(3)14(10)18-4/h5,16H,6-7H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185594

((E)-7-hydroxy-5-methoxy-6-(4-methoxy-3-methylbut-2...)Show InChI InChI=1S/C16H20O5/c1-9(7-19-3)5-6-11-14(17)13-12(8-21-16(13)18)10(2)15(11)20-4/h5,17H,6-8H2,1-4H3/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 273 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185588
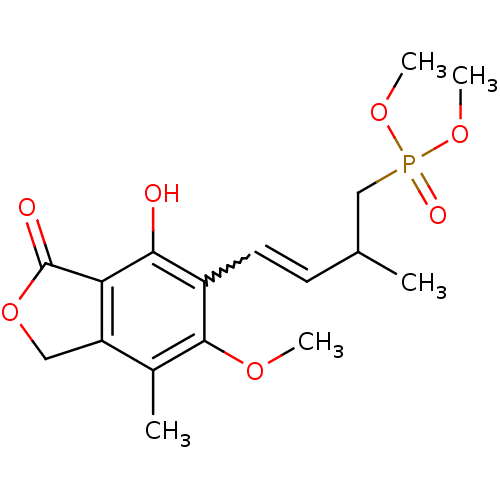
((E)-dimethyl 4-(4-hydroxy-6-methoxy-7-methyl-3-oxo...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C=CC(C)CP(=O)(OC)OC |w:14.15| Show InChI InChI=1S/C17H23O7P/c1-10(9-25(20,22-4)23-5)6-7-12-15(18)14-13(8-24-17(14)19)11(2)16(12)21-3/h6-7,10,18H,8-9H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 289 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50228233

(CHEMBL3349043)Show SMILES CCC(=O)N([C@H]1CCN(CCc2ccc(cc2)N=C=S)C[C@@H]1C)c1ccccc1 |r| Show InChI InChI=1S/C24H29N3OS/c1-3-24(28)27(22-7-5-4-6-8-22)23-14-16-26(17-19(23)2)15-13-20-9-11-21(12-10-20)25-18-29/h4-12,19,23H,3,13-17H2,1-2H3/t19-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
The ability of comp[ound to inhibit [3H]-DADL binding to the delta receptor of the NG108-15 cells at 10 min and 37 degree C |
J Med Chem 32: 1392-8 (1989)
BindingDB Entry DOI: 10.7270/Q2JD4VSW |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185598

((2-{[(E)-4-(4-Hydroxy-6-methoxy-7-methyl-3-oxo-1,3...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CN(CCP(O)(O)=O)S(C)(=O)=O Show InChI InChI=1S/C18H26NO9PS/c1-11(9-19(30(4,25)26)7-8-29(22,23)24)5-6-13-16(20)15-14(10-28-18(15)21)12(2)17(13)27-3/h5,20H,6-10H2,1-4H3,(H2,22,23,24)/b11-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 498 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185602
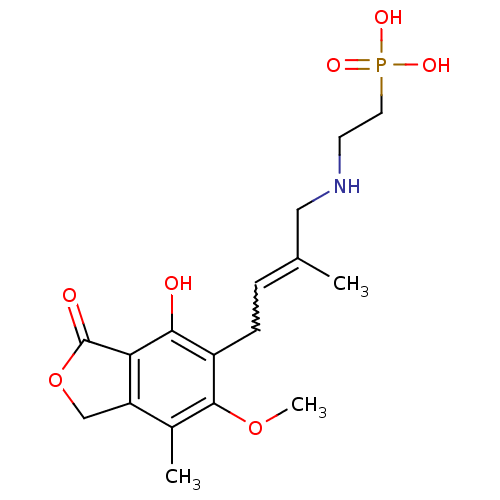
((E)-2-(4-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-d...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1CC=C(C)CNCCP(O)(O)=O |w:15.16| Show InChI InChI=1S/C17H24NO7P/c1-10(8-18-6-7-26(21,22)23)4-5-12-15(19)14-13(9-25-17(14)20)11(2)16(12)24-3/h4,18-19H,5-9H2,1-3H3,(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 499 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185600

((1E,3E)-5-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)\C=C\P(O)(O)=O Show InChI InChI=1S/C16H19O7P/c1-9(6-7-24(19,20)21)4-5-11-14(17)13-12(8-23-16(13)18)10(2)15(11)22-3/h4,6-7,17H,5,8H2,1-3H3,(H2,19,20,21)/b7-6+,9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 506 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185597

((E)-2-(N-(4-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CN(CCP(O)(O)=O)C=O Show InChI InChI=1S/C18H24NO8P/c1-11(8-19(10-20)6-7-28(23,24)25)4-5-13-16(21)15-14(9-27-18(15)22)12(2)17(13)26-3/h4,10,21H,5-9H2,1-3H3,(H2,23,24,25)/b11-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 749 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185583

((E)-2-(N-(4-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CN(CCP(O)(O)=O)C(C)=O Show InChI InChI=1S/C19H26NO8P/c1-11(9-20(13(3)21)7-8-29(24,25)26)5-6-14-17(22)16-15(10-28-19(16)23)12(2)18(14)27-4/h5,22H,6-10H2,1-4H3,(H2,24,25,26)/b11-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 785 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185584

((E)-(4-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1CC=C(C)CNCP(O)(O)=O |w:15.16| Show InChI InChI=1S/C16H22NO7P/c1-9(6-17-8-25(20,21)22)4-5-11-14(18)13-12(7-24-16(13)19)10(2)15(11)23-3/h4,17-18H,5-8H2,1-3H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185591

((E)-2-((4-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CN(C)CCP(O)(O)=O Show InChI InChI=1S/C18H26NO7P/c1-11(9-19(3)7-8-27(22,23)24)5-6-13-16(20)15-14(10-26-18(15)21)12(2)17(13)25-4/h5,20H,6-10H2,1-4H3,(H2,22,23,24)/b11-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185593
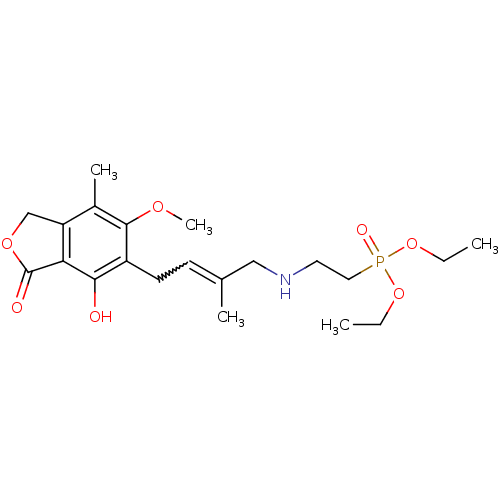
((E)-diethyl 2-(4-(4-hydroxy-6-methoxy-7-methyl-3-o...)Show SMILES CCOP(=O)(CCNCC(C)=CCc1c(O)c2C(=O)OCc2c(C)c1OC)OCC |w:11.11| Show InChI InChI=1S/C21H32NO7P/c1-6-28-30(25,29-7-2)11-10-22-12-14(3)8-9-16-19(23)18-17(13-27-21(18)24)15(4)20(16)26-5/h8,22-23H,6-7,9-13H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50335554
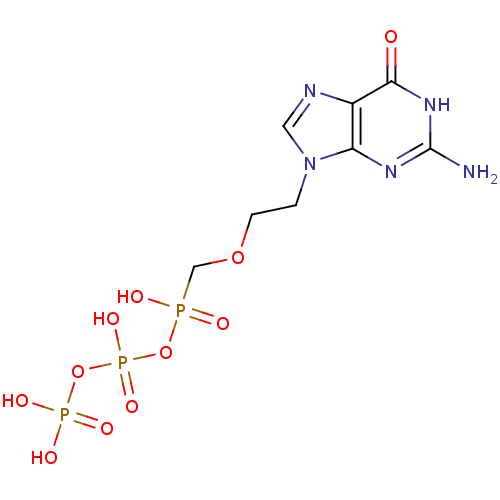
(({[({[2-(2-amino-6-oxo-6,9-dihydro-3H-purin-9-yl)e...)Show SMILES Nc1nc2n(CCOCP(O)(=O)OP(O)(=O)OP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C8H14N5O11P3/c9-8-11-6-5(7(14)12-8)10-3-13(6)1-2-22-4-25(15,16)23-27(20,21)24-26(17,18)19/h3H,1-2,4H2,(H,15,16)(H,20,21)(H2,17,18,19)(H3,9,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase beta by microplate reader analysis |
Antimicrob Agents Chemother 53: 2777-84 (2009)
Article DOI: 10.1128/AAC.00103-09
BindingDB Entry DOI: 10.7270/Q2M32W2N |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50335554

(({[({[2-(2-amino-6-oxo-6,9-dihydro-3H-purin-9-yl)e...)Show SMILES Nc1nc2n(CCOCP(O)(=O)OP(O)(=O)OP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C8H14N5O11P3/c9-8-11-6-5(7(14)12-8)10-3-13(6)1-2-22-4-25(15,16)23-27(20,21)24-26(17,18)19/h3H,1-2,4H2,(H,15,16)(H,20,21)(H2,17,18,19)(H3,9,11,12,14) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha by microplate reader analysis |
Antimicrob Agents Chemother 53: 2777-84 (2009)
Article DOI: 10.1128/AAC.00103-09
BindingDB Entry DOI: 10.7270/Q2M32W2N |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50335553
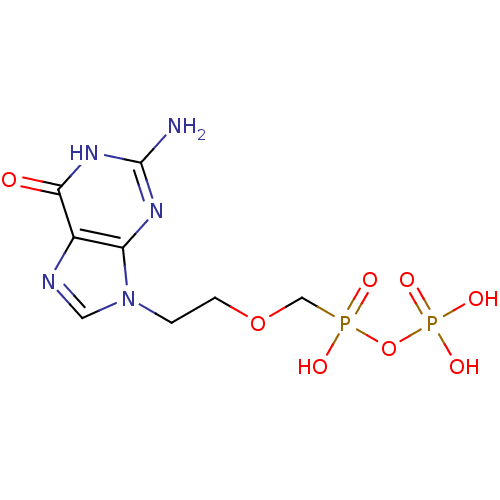
(CHEMBL1652466 | [({[2-(2-amino-6-oxo-6,9-dihydro-3...)Show SMILES Nc1nc2n(CCOCP(O)(=O)OP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C8H13N5O8P2/c9-8-11-6-5(7(14)12-8)10-3-13(6)1-2-20-4-22(15,16)21-23(17,18)19/h3H,1-2,4H2,(H,15,16)(H2,17,18,19)(H3,9,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase beta by microplate reader analysis |
Antimicrob Agents Chemother 53: 2777-84 (2009)
Article DOI: 10.1128/AAC.00103-09
BindingDB Entry DOI: 10.7270/Q2M32W2N |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50335556
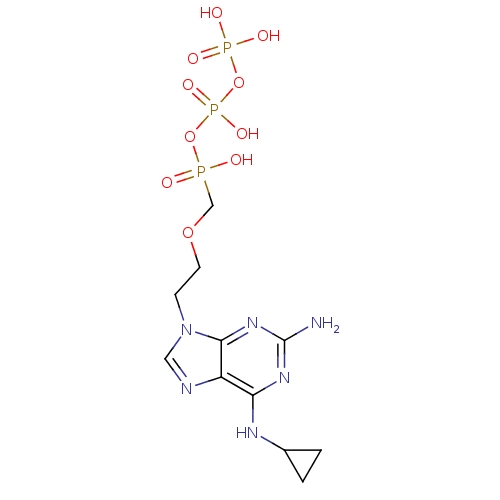
(CHEMBL1652469 | [({[({2-[2-amino-6-(cyclopropylami...)Show SMILES Nc1nc(NC2CC2)c2ncn(CCOCP(O)(=O)OP(O)(=O)OP(O)(O)=O)c2n1 Show InChI InChI=1S/C11H19N6O10P3/c12-11-15-9(14-7-1-2-7)8-10(16-11)17(5-13-8)3-4-25-6-28(18,19)26-30(23,24)27-29(20,21)22/h5,7H,1-4,6H2,(H,18,19)(H,23,24)(H2,20,21,22)(H3,12,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase beta by microplate reader analysis |
Antimicrob Agents Chemother 53: 2777-84 (2009)
Article DOI: 10.1128/AAC.00103-09
BindingDB Entry DOI: 10.7270/Q2M32W2N |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50335555
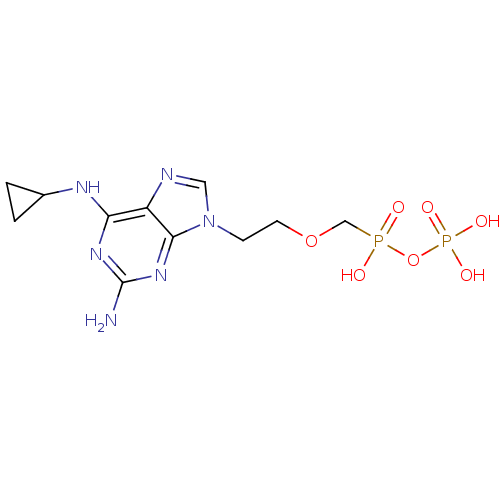
(CHEMBL1652468 | {[({2-[2-amino-6-(cyclopropylamino...)Show SMILES Nc1nc(NC2CC2)c2ncn(CCOCP(O)(=O)OP(O)(O)=O)c2n1 Show InChI InChI=1S/C11H18N6O7P2/c12-11-15-9(14-7-1-2-7)8-10(16-11)17(5-13-8)3-4-23-6-25(18,19)24-26(20,21)22/h5,7H,1-4,6H2,(H,18,19)(H2,20,21,22)(H3,12,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase beta by microplate reader analysis |
Antimicrob Agents Chemother 53: 2777-84 (2009)
Article DOI: 10.1128/AAC.00103-09
BindingDB Entry DOI: 10.7270/Q2M32W2N |
More data for this
Ligand-Target Pair | |
DNA polymerase subunit gamma-1
(Homo sapiens (Human)) | BDBM50335554

(({[({[2-(2-amino-6-oxo-6,9-dihydro-3H-purin-9-yl)e...)Show SMILES Nc1nc2n(CCOCP(O)(=O)OP(O)(=O)OP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C8H14N5O11P3/c9-8-11-6-5(7(14)12-8)10-3-13(6)1-2-22-4-25(15,16)23-27(20,21)24-26(17,18)19/h3H,1-2,4H2,(H,15,16)(H,20,21)(H2,17,18,19)(H3,9,11,12,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase gamma by microplate reader analysis |
Antimicrob Agents Chemother 53: 2777-84 (2009)
Article DOI: 10.1128/AAC.00103-09
BindingDB Entry DOI: 10.7270/Q2M32W2N |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50335553

(CHEMBL1652466 | [({[2-(2-amino-6-oxo-6,9-dihydro-3...)Show SMILES Nc1nc2n(CCOCP(O)(=O)OP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C8H13N5O8P2/c9-8-11-6-5(7(14)12-8)10-3-13(6)1-2-20-4-22(15,16)21-23(17,18)19/h3H,1-2,4H2,(H,15,16)(H2,17,18,19)(H3,9,11,12,14) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha by microplate reader analysis |
Antimicrob Agents Chemother 53: 2777-84 (2009)
Article DOI: 10.1128/AAC.00103-09
BindingDB Entry DOI: 10.7270/Q2M32W2N |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50335555

(CHEMBL1652468 | {[({2-[2-amino-6-(cyclopropylamino...)Show SMILES Nc1nc(NC2CC2)c2ncn(CCOCP(O)(=O)OP(O)(O)=O)c2n1 Show InChI InChI=1S/C11H18N6O7P2/c12-11-15-9(14-7-1-2-7)8-10(16-11)17(5-13-8)3-4-23-6-25(18,19)24-26(20,21)22/h5,7H,1-4,6H2,(H,18,19)(H2,20,21,22)(H3,12,14,15,16) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha by microplate reader analysis |
Antimicrob Agents Chemother 53: 2777-84 (2009)
Article DOI: 10.1128/AAC.00103-09
BindingDB Entry DOI: 10.7270/Q2M32W2N |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50335556

(CHEMBL1652469 | [({[({2-[2-amino-6-(cyclopropylami...)Show SMILES Nc1nc(NC2CC2)c2ncn(CCOCP(O)(=O)OP(O)(=O)OP(O)(O)=O)c2n1 Show InChI InChI=1S/C11H19N6O10P3/c12-11-15-9(14-7-1-2-7)8-10(16-11)17(5-13-8)3-4-25-6-28(18,19)26-30(23,24)27-29(20,21)22/h5,7H,1-4,6H2,(H,18,19)(H,23,24)(H2,20,21,22)(H3,12,14,15,16) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha by microplate reader analysis |
Antimicrob Agents Chemother 53: 2777-84 (2009)
Article DOI: 10.1128/AAC.00103-09
BindingDB Entry DOI: 10.7270/Q2M32W2N |
More data for this
Ligand-Target Pair | |
DNA polymerase subunit gamma-1
(Homo sapiens (Human)) | BDBM50335556

(CHEMBL1652469 | [({[({2-[2-amino-6-(cyclopropylami...)Show SMILES Nc1nc(NC2CC2)c2ncn(CCOCP(O)(=O)OP(O)(=O)OP(O)(O)=O)c2n1 Show InChI InChI=1S/C11H19N6O10P3/c12-11-15-9(14-7-1-2-7)8-10(16-11)17(5-13-8)3-4-25-6-28(18,19)26-30(23,24)27-29(20,21)22/h5,7H,1-4,6H2,(H,18,19)(H,23,24)(H2,20,21,22)(H3,12,14,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase gamma by microplate reader analysis |
Antimicrob Agents Chemother 53: 2777-84 (2009)
Article DOI: 10.1128/AAC.00103-09
BindingDB Entry DOI: 10.7270/Q2M32W2N |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50236025
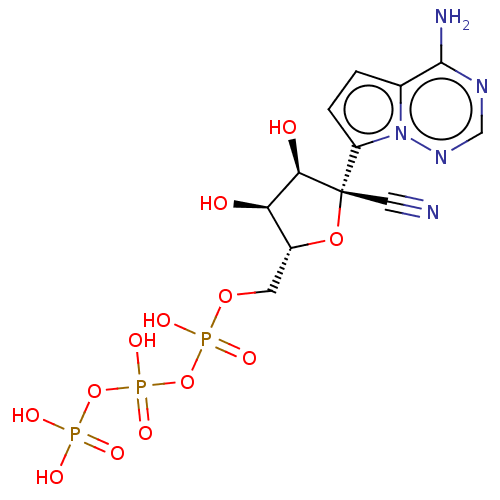
(CHEMBL2016761 | US11149049, No. C2)Show SMILES Nc1ncnn2c(ccc12)[C@@]1(O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O)C#N |r| Show InChI InChI=1S/C12H16N5O13P3/c13-4-12(8-2-1-6-11(14)15-5-16-17(6)8)10(19)9(18)7(28-12)3-27-32(23,24)30-33(25,26)29-31(20,21)22/h1-2,5,7,9-10,18-19H,3H2,(H,23,24)(H,25,26)(H2,14,15,16)(H2,20,21,22)/t7-,9-,10-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DNA polymerase beta preincubated with enzyme followed by addition of dATP/dGTP/TTP as substrate in presence of [gamma... |
J Med Chem 60: 1648-1661 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01594
BindingDB Entry DOI: 10.7270/Q2QZ2D7N |
More data for this
Ligand-Target Pair | |
DNA-directed RNA polymerase, mitochondrial
(Homo sapiens (Human)) | BDBM50236025

(CHEMBL2016761 | US11149049, No. C2)Show SMILES Nc1ncnn2c(ccc12)[C@@]1(O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O)C#N |r| Show InChI InChI=1S/C12H16N5O13P3/c13-4-12(8-2-1-6-11(14)15-5-16-17(6)8)10(19)9(18)7(28-12)3-27-32(23,24)30-33(25,26)29-31(20,21)22/h1-2,5,7,9-10,18-19H,3H2,(H,23,24)(H,25,26)(H2,14,15,16)(H2,20,21,22)/t7-,9-,10-,12+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human mitochondrial RNA polymerase after 30 mins in presence of [33P]GTP |
J Med Chem 60: 1648-1661 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01594
BindingDB Entry DOI: 10.7270/Q2QZ2D7N |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50236025

(CHEMBL2016761 | US11149049, No. C2)Show SMILES Nc1ncnn2c(ccc12)[C@@]1(O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O)C#N |r| Show InChI InChI=1S/C12H16N5O13P3/c13-4-12(8-2-1-6-11(14)15-5-16-17(6)8)10(19)9(18)7(28-12)3-27-32(23,24)30-33(25,26)29-31(20,21)22/h1-2,5,7,9-10,18-19H,3H2,(H,23,24)(H,25,26)(H2,14,15,16)(H2,20,21,22)/t7-,9-,10-,12+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DNA polymerase alpha preincubated with enzyme followed by addition of dATP/dGTP/TTP as substrate in presence of [gamm... |
J Med Chem 60: 1648-1661 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01594
BindingDB Entry DOI: 10.7270/Q2QZ2D7N |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50027089
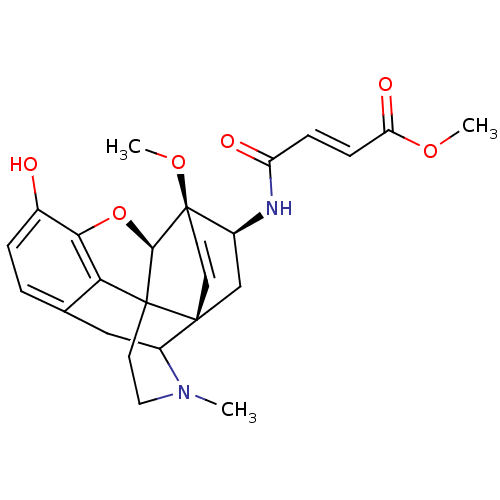
(methyl 3-(11-hydroxy-15-methoxy-3-methyl-13-oxa-3-...)Show SMILES COC(=O)\C=C\C(=O)N[C@H]1C[C@]23C=C[C@]1(OC)[C@@H]1Oc4c5c(CC2N(C)CCC315)ccc4O |wD:17.18,11.11,9.8,14.15,c:12,TLB:19:20:11:24.27.26,12:11:20.21.22:24.27.26,25:24:11:20.21.22,(25.56,-19.2,;25.95,-17.71,;24.85,-16.61,;25.24,-15.13,;23.37,-17.03,;22.98,-18.54,;21.48,-18.96,;21.09,-20.46,;20.36,-17.87,;18.81,-17.87,;18.06,-16.6,;16.52,-16.57,;17.86,-17.35,;16.71,-18.44,;18.06,-19.22,;18.83,-20.51,;18.81,-22.06,;16.49,-19.19,;14.87,-20.06,;13.45,-19.12,;14.22,-17.81,;13.48,-16.51,;14.28,-15.18,;15.8,-15.21,;15.77,-13.66,;17.12,-12.87,;14.84,-15.74,;15.55,-16.47,;15.74,-17.84,;11.95,-16.47,;11.16,-17.73,;11.9,-19.09,;11.09,-20.39,)| Show InChI InChI=1S/C25H28N2O6/c1-27-11-10-24-20-14-4-5-15(28)21(20)33-22(24)25(32-3)9-8-23(24,17(27)12-14)13-16(25)26-18(29)6-7-19(30)31-2/h4-9,16-17,22,28H,10-13H2,1-3H3,(H,26,29)/b7-6+/t16-,17?,22+,23+,24?,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The binding affinity was evaluated against [3H]dalamid binding to rat brain membranes (Opioid receptor delta 1) |
J Med Chem 27: 1570-4 (1985)
BindingDB Entry DOI: 10.7270/Q2S18328 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50027086
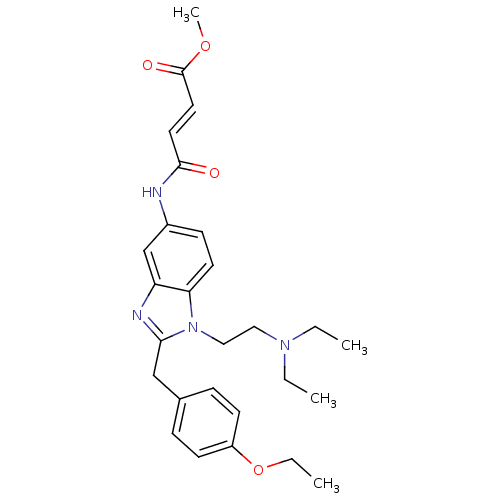
(3-[1-(2-Diethylamino-ethyl)-2-(4-ethoxy-benzyl)-1H...)Show SMILES CCOc1ccc(Cc2nc3cc(NC(=O)\C=C\C(=O)OC)ccc3n2CCN(CC)CC)cc1 Show InChI InChI=1S/C27H34N4O4/c1-5-30(6-2)16-17-31-24-13-10-21(28-26(32)14-15-27(33)34-4)19-23(24)29-25(31)18-20-8-11-22(12-9-20)35-7-3/h8-15,19H,5-7,16-18H2,1-4H3,(H,28,32)/b15-14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The binding affinity was evaluated against [3H]dalamid binding to rat brain membranes (Opioid receptor delta 1) |
J Med Chem 27: 1570-4 (1985)
BindingDB Entry DOI: 10.7270/Q2S18328 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50027087

(2-Bromo-N-[1-(2-diethylamino-ethyl)-2-(4-ethoxy-be...)Show SMILES CCOc1ccc(Cc2nc3cc(NC(=O)CBr)ccc3n2CCN(CC)CC)cc1 Show InChI InChI=1S/C24H31BrN4O2/c1-4-28(5-2)13-14-29-22-12-9-19(26-24(30)17-25)16-21(22)27-23(29)15-18-7-10-20(11-8-18)31-6-3/h7-12,16H,4-6,13-15,17H2,1-3H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The binding affinity was evaluated against [3H]dalamid binding to neuroblastoma X glioma hybrid cell NG108-15 (Opioid receptor delta 1)membranes |
J Med Chem 27: 1570-4 (1985)
BindingDB Entry DOI: 10.7270/Q2S18328 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data