

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasmepsin X (Plasmodium falciparum (isolate 3D7)) | BDBM50595797 (CHEMBL5171401) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00355 BindingDB Entry DOI: 10.7270/Q2F47T4D | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin X (Plasmodium falciparum (isolate 3D7)) | BDBM50595792 (CHEMBL5182418) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00355 BindingDB Entry DOI: 10.7270/Q2F47T4D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin X (Plasmodium falciparum (isolate 3D7)) | BDBM50595793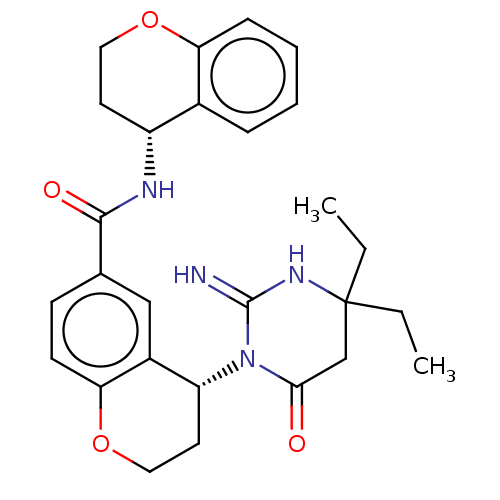 (CHEMBL5187792) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00355 BindingDB Entry DOI: 10.7270/Q2F47T4D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin X (Plasmodium falciparum (isolate 3D7)) | BDBM50595794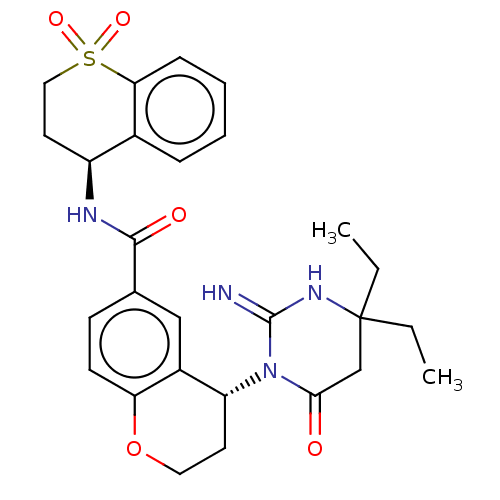 (CHEMBL5193119) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00355 BindingDB Entry DOI: 10.7270/Q2F47T4D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin X (Plasmodium falciparum (isolate 3D7)) | BDBM50595796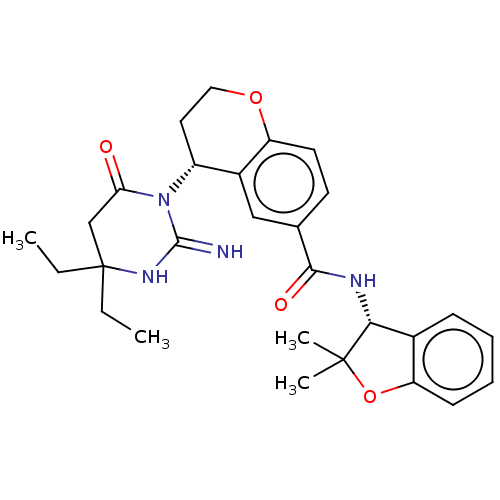 (CHEMBL5199100) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00355 BindingDB Entry DOI: 10.7270/Q2F47T4D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50322823 ((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
St. John's University Curated by ChEMBL | Assay Description Inhibition of EGFR L858R mutant (unknown origin) | Bioorg Med Chem Lett 27: 4832-4837 (2017) Article DOI: 10.1016/j.bmcl.2017.09.048 BindingDB Entry DOI: 10.7270/Q22Z183X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50322823 ((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
St. John's University Curated by ChEMBL | Assay Description Inhibition of wild-type EGFR (unknown origin) | Bioorg Med Chem Lett 27: 4832-4837 (2017) Article DOI: 10.1016/j.bmcl.2017.09.048 BindingDB Entry DOI: 10.7270/Q22Z183X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin X (Plasmodium falciparum (isolate 3D7)) | BDBM50595795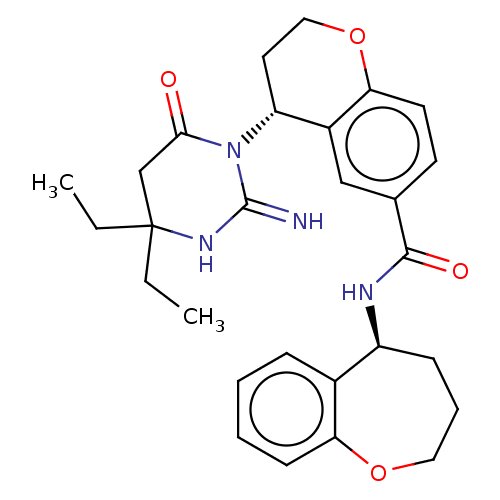 (CHEMBL5207556) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00355 BindingDB Entry DOI: 10.7270/Q2F47T4D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50331031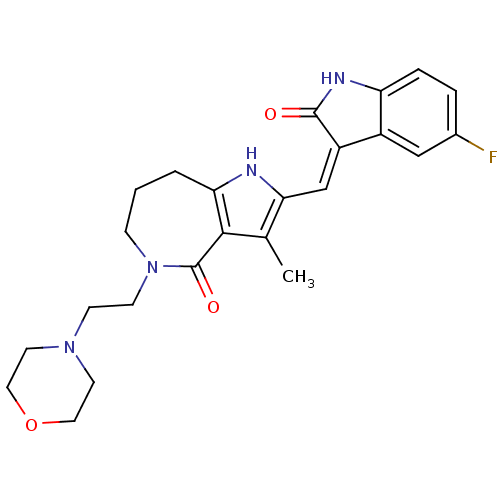 ((Z)-2-(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenem...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human VEGFR2 after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50331028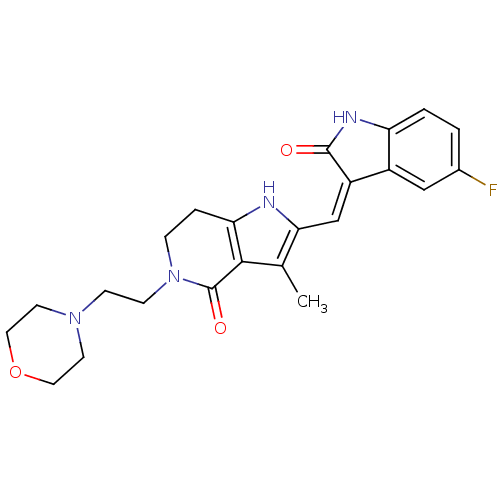 ((Z)-2-(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenem...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of c-Kit after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50331029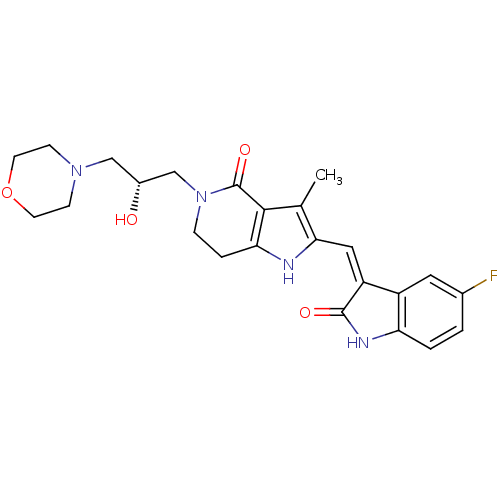 ((R,Z)-2-(5-Fluoro-2-oxo-1,2-dihydro-indol-3-yliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human VEGFR2 after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50331029 ((R,Z)-2-(5-Fluoro-2-oxo-1,2-dihydro-indol-3-yliden...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of c-Kit after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50331026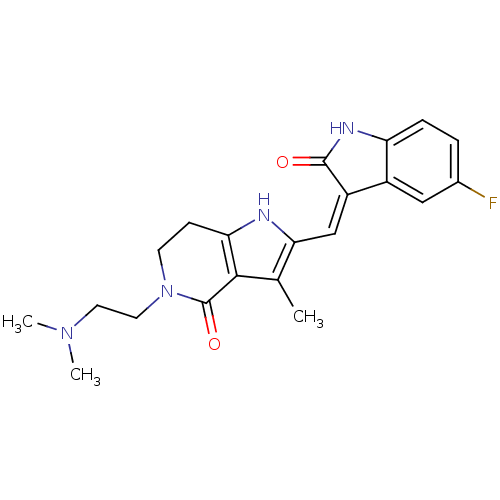 ((Z)-2-(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenem...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of c-Kit after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin IX (Plasmodium falciparum (isolate 3D7)) | BDBM50595797 (CHEMBL5171401) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00355 BindingDB Entry DOI: 10.7270/Q2F47T4D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin X (Plasmodium falciparum (isolate 3D7)) | BDBM50595790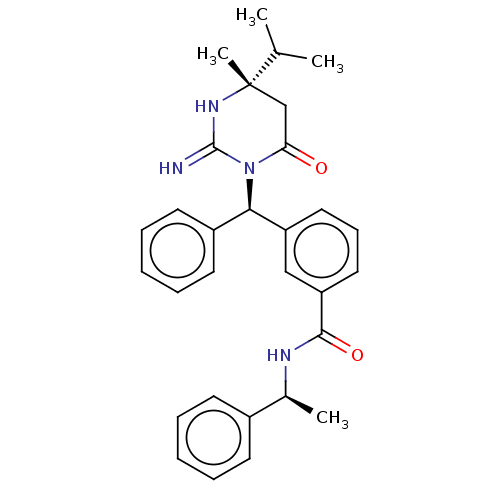 (CHEMBL5197877) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00355 BindingDB Entry DOI: 10.7270/Q2F47T4D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
St. John's University Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) | Bioorg Med Chem Lett 27: 4832-4837 (2017) Article DOI: 10.1016/j.bmcl.2017.09.048 BindingDB Entry DOI: 10.7270/Q22Z183X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50331026 ((Z)-2-(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenem...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human VEGFR2 after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50331023 ((Z)-2-(5-Fluoro-2-oxo-1,2-dihydroindol-3-ylideneme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of c-Kit after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50331023 ((Z)-2-(5-Fluoro-2-oxo-1,2-dihydroindol-3-ylideneme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of VEGFR2-induced cell proliferation in HUVEC after 72 hrs by sulforhodamine B method | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50331024 ((Z)-2-(5-Chloro-2-oxo-1,2-dihydro-indol-3-ylidenem...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human PDGFRbeta after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin IX (Plasmodium falciparum (isolate 3D7)) | BDBM50595793 (CHEMBL5187792) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00355 BindingDB Entry DOI: 10.7270/Q2F47T4D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50028525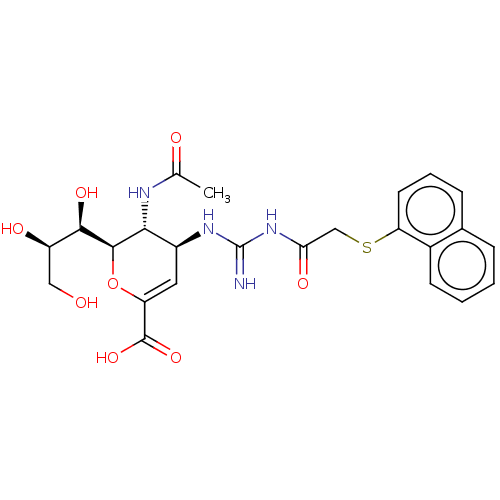 (CHEMBL3355596) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuyi University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/WSN/1933(H1N1) Neuraminidase using MU-NANA as substrate preincubated for 30 mins followed by substrate addition and... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111841 BindingDB Entry DOI: 10.7270/Q2930XD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267389 (2-fluoro-3-((1-((5-(4-fluorophenyl)-6-oxo-1,6-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267390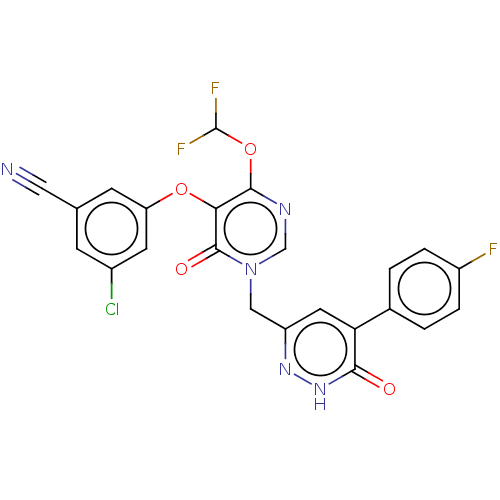 (3-chloro-5-((1-((5-(4- fluorophenyl)-6-oxo- 1,6-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267395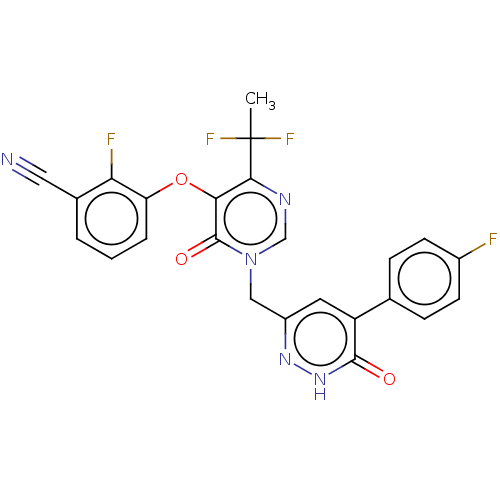 (3-((4-(1,1-difluoroethyl)-1-((5-(4-fluorophenyl)-6...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267399 (3-chloro-5-((4-(1,1- difluoroethyl)-1-((6- (difluo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267334 (3-chloro-5-((6-oxo-1-((3-oxo-6-phenyl-2,3-dihydrop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50331027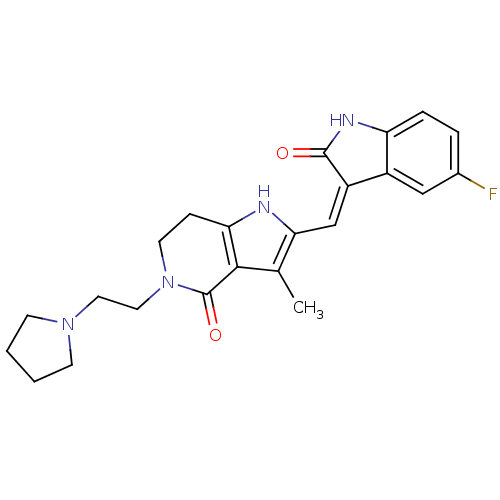 ((Z)-2-(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenem...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of c-Kit after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267245 (3-chloro-5-((6-oxo-1-((6-oxo-5-phenyl-1,6-dihydrop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267252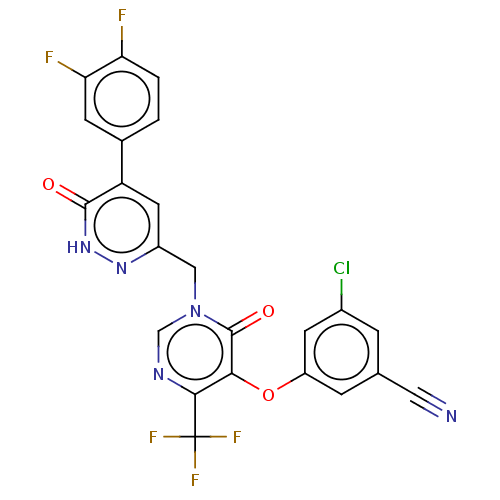 (3-chloro-5-((1-((5- (3,4-difluorophenyl)- 6-oxo-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267287 (3-chloro-5-((1-((5- (2,3- dihydrobenzofuran-5- yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50331025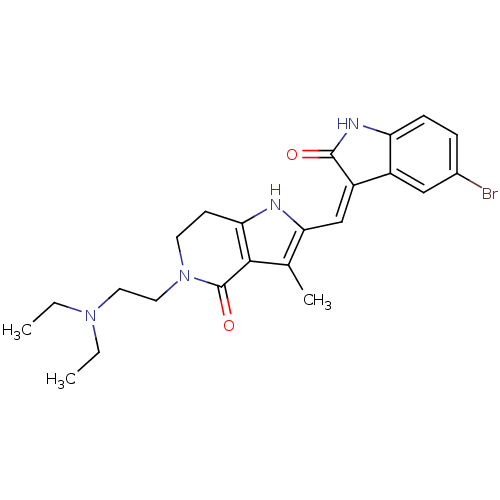 ((Z)-2-(5-Bromo-2-oxo-1,2-dihydro-indol-3-ylideneme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human PDGFRbeta after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50331026 ((Z)-2-(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenem...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human PDGFRbeta after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267344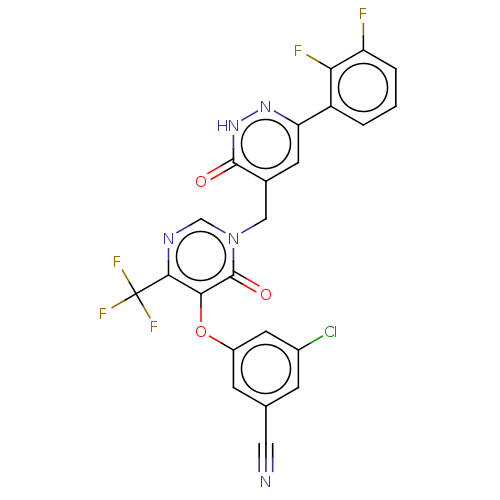 (3-chloro-5-((1-((6- (2,3- difluorophenyl)-3- oxo-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267393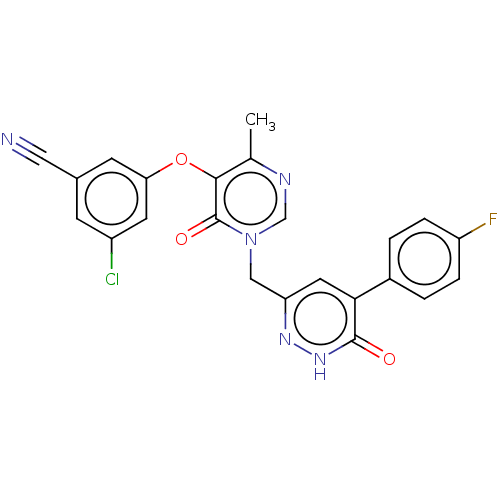 (3-chloro-5-((1-((5-(4-fluorophenyl)-6-oxo-1,6-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267275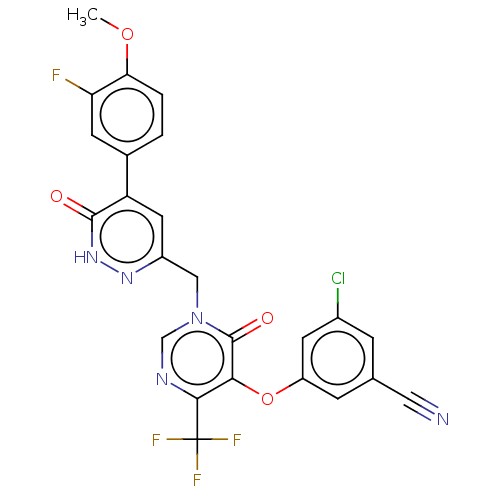 (3-chloro-5-((1-((5-(3- fluoro-4- methoxyphenyl)-6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50331031 ((Z)-2-(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenem...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of c-Kit after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267350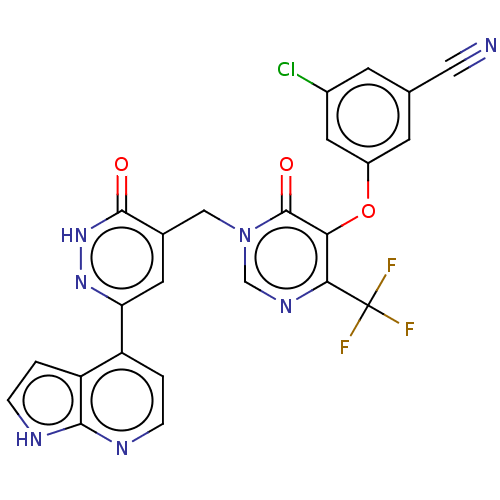 (3-chloro-5-((6-oxo- 1-((3-oxo-6-(1H- pyrrolo[2,3- ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267352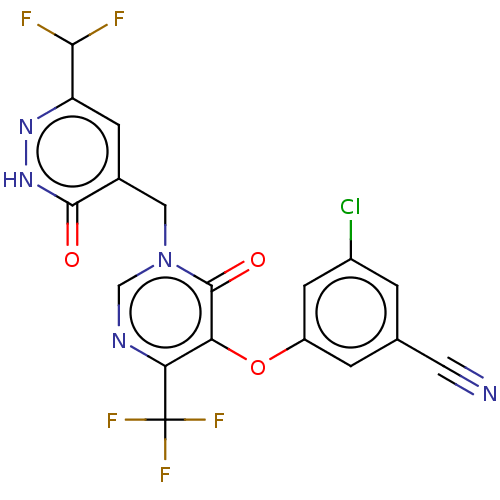 (3-chloro-5-((1-((6-(difluoromethyl)-3-oxo-2,3-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267253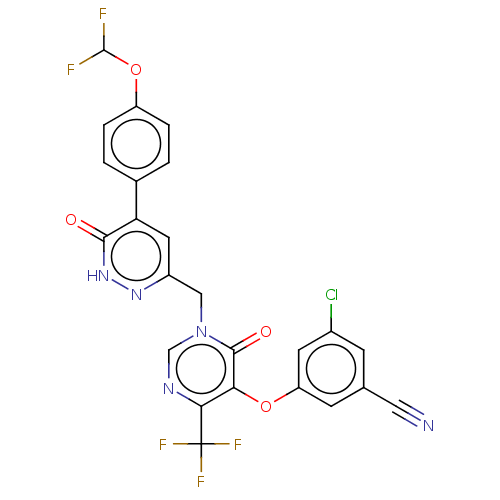 (3-chloro-5-((1-((5-(4- (difluoromethoxy) phenyl)-6...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267259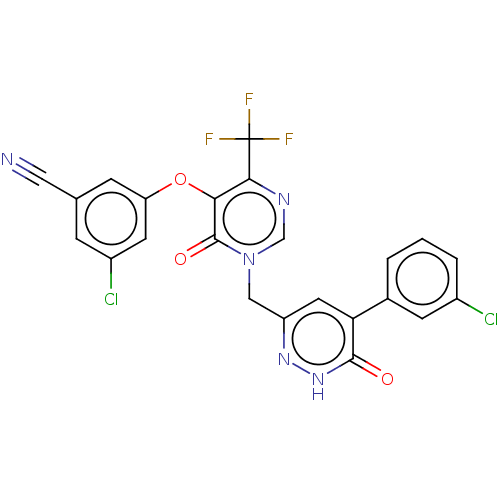 (3-chloro-5-((1-((5-(3- chlorophenyl)-6-oxo- 1,6-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267267 (3-chloro-5-((1-((5-(2- fluoro-3- methoxyphenyl)-6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50331027 ((Z)-2-(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenem...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human PDGFRbeta after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267281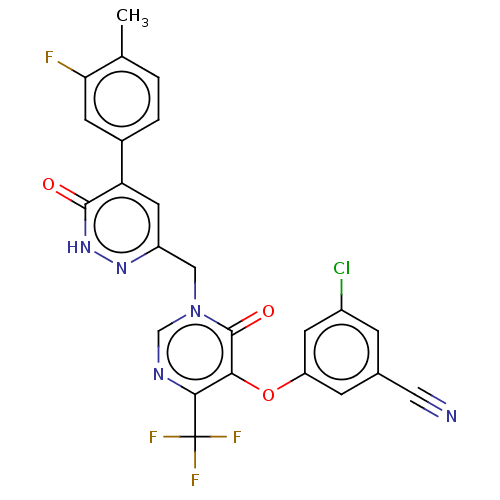 (3-chloro-5-((1-((5-(3- fluoro-4- methylphenyl)-6-o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM267300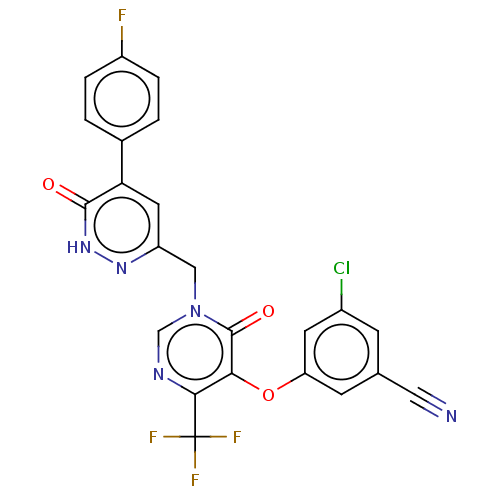 (3-chloro-5-((1-((5-(4-fluorophenyl)-6-oxo-1,6-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The heterodimeric nucleic acid substrate used in the HIV-1 RT polymerase reactions was generated by annealing the DNA primer, biotinylated pD500 (Sig... | US Patent US9718819 (2017) BindingDB Entry DOI: 10.7270/Q2WM1GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50331023 ((Z)-2-(5-Fluoro-2-oxo-1,2-dihydroindol-3-ylideneme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human PDGFRbeta after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50331024 ((Z)-2-(5-Chloro-2-oxo-1,2-dihydro-indol-3-ylidenem...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of c-Kit after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50331025 ((Z)-2-(5-Bromo-2-oxo-1,2-dihydro-indol-3-ylideneme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of c-Kit after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50331030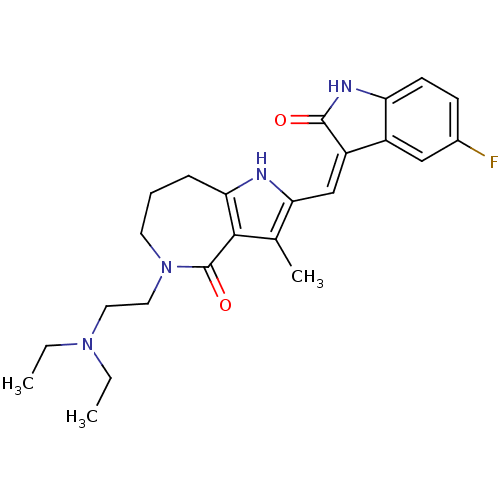 ((Z)-5-(2-Diethylamino-ethyl)-2-(5-fluoro-2-oxo-1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of c-Kit after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin IX (Plasmodium falciparum (isolate 3D7)) | BDBM50595792 (CHEMBL5182418) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00355 BindingDB Entry DOI: 10.7270/Q2F47T4D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 708 total ) | Next | Last >> |