 Found 136 hits with Last Name = 'lemoine' and Initial = 'rc'
Found 136 hits with Last Name = 'lemoine' and Initial = 'rc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305799

(5-((3aR,6aS)-5-(2-(1-(3,3-difluorocyclobutanecarbo...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CC(F)(F)C3)c3ccccc3)C[C@@H]2C1)C#N |r| Show InChI InChI=1S/C31H35F2N5O2/c1-20-10-26(13-34)35-21(2)27(20)29(40)37-16-23-14-36(15-24(23)17-37)9-8-30(25-6-4-3-5-7-25)18-38(19-30)28(39)22-11-31(32,33)12-22/h3-7,10,22-24H,8-9,11-12,14-19H2,1-2H3/t23-,24+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 by cell-cell fusion assay |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50329256
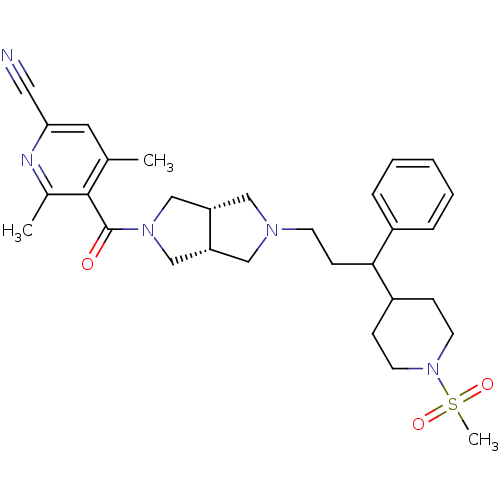
(4,6-dimethyl-5-((3aR,6aS)-5-(3-(1-(methylsulfonyl)...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3ccccc3)C[C@@H]2C1)C#N |r| Show InChI InChI=1S/C30H39N5O3S/c1-21-15-27(16-31)32-22(2)29(21)30(36)34-19-25-17-33(18-26(25)20-34)12-11-28(23-7-5-4-6-8-23)24-9-13-35(14-10-24)39(3,37)38/h4-8,15,24-26,28H,9-14,17-20H2,1-3H3/t25-,26+,28? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of CCR5 by cell-cell fusion inhibition assay |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50334986

(4,4-Difluoro-cyclohexanecarboxylic acid {(S)-3-[(1...)Show SMILES CC(C)c1nnc(C)n1[C@H]1C[C@@H]2CC[C@H](C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccccc1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C29H41F2N5O/c1-19(2)27-34-33-20(3)36(27)25-17-23-9-10-24(18-25)35(23)16-13-26(21-7-5-4-6-8-21)32-28(37)22-11-14-29(30,31)15-12-22/h4-8,19,22-26H,9-18H2,1-3H3,(H,32,37)/t23-,24+,25-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 20: 1674-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.080
BindingDB Entry DOI: 10.7270/Q23B6131 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305800
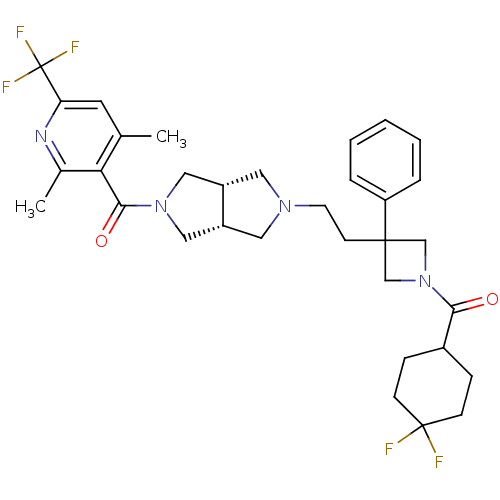
(((3aR,6aS)-5-(2-(1-(4,4-difluorocyclohexanecarbony...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CCC(F)(F)CC3)c3ccccc3)C[C@@H]2C1)C(F)(F)F |r| Show InChI InChI=1S/C33H39F5N4O2/c1-21-14-27(33(36,37)38)39-22(2)28(21)30(44)41-17-24-15-40(16-25(24)18-41)13-12-31(26-6-4-3-5-7-26)19-42(20-31)29(43)23-8-10-32(34,35)11-9-23/h3-7,14,23-25H,8-13,15-20H2,1-2H3/t24-,25+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 by cell-cell fusion assay |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305801
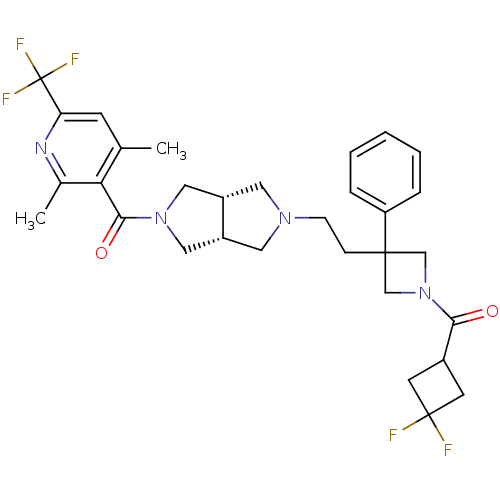
(((3aR,6aS)-5-(2-(1-(3,3-difluorocyclobutanecarbony...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CC(F)(F)C3)c3ccccc3)C[C@@H]2C1)C(F)(F)F |r| Show InChI InChI=1S/C31H35F5N4O2/c1-19-10-25(31(34,35)36)37-20(2)26(19)28(42)39-15-22-13-38(14-23(22)16-39)9-8-29(24-6-4-3-5-7-24)17-40(18-29)27(41)21-11-30(32,33)12-21/h3-7,10,21-23H,8-9,11-18H2,1-2H3/t22-,23+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 by cell-cell fusion assay |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310739
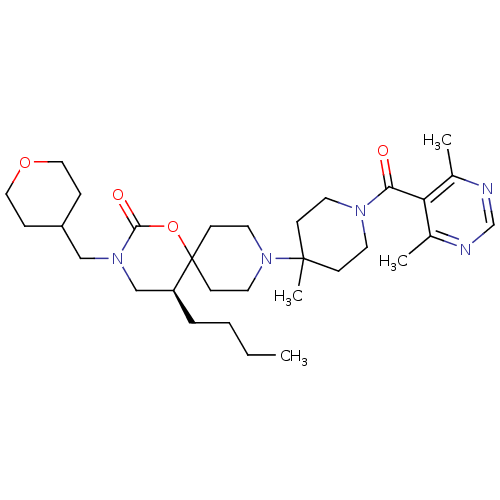
((S)-5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbony...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C31H49N5O4/c1-5-6-7-26-21-35(20-25-8-18-39-19-9-25)29(38)40-31(26)12-16-36(17-13-31)30(4)10-14-34(15-11-30)28(37)27-23(2)32-22-33-24(27)3/h22,25-26H,5-21H2,1-4H3/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]-RANTES from CCR5 receptor receptors expressed in CHO cells |
Bioorg Med Chem Lett 20: 1830-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.004
BindingDB Entry DOI: 10.7270/Q25Q4W7T |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305794

(((3aR,6aS)-5-(2-(1-(4,4-difluorocyclohexanecarbony...)Show SMILES Cc1nc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CCC(F)(F)CC3)c3ccccc3)C[C@@H]2C1)C(F)(F)F |r| Show InChI InChI=1S/C32H38F5N5O2/c1-20-26(21(2)39-29(38-20)32(35,36)37)28(44)41-16-23-14-40(15-24(23)17-41)13-12-30(25-6-4-3-5-7-25)18-42(19-30)27(43)22-8-10-31(33,34)11-9-22/h3-7,22-24H,8-19H2,1-2H3/t23-,24+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 by cell-cell fusion assay |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50312846
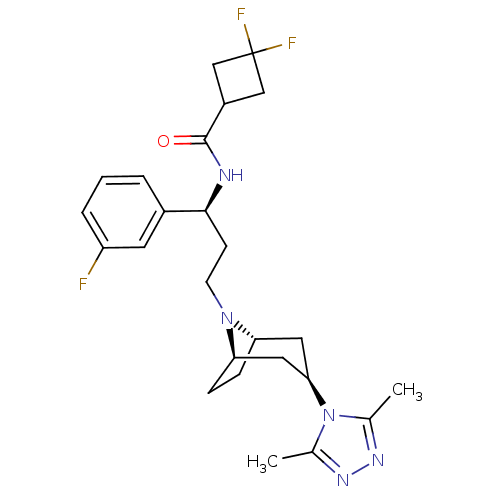
(CHEMBL1087535 | N-((S)-3-((1R,3S,5S)-3-(3,5-dimeth...)Show SMILES Cc1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CC[C@H](NC(=O)C1CC(F)(F)C1)c1cccc(F)c1 |r,THB:15:14:13.7.8:11.10| Show InChI InChI=1S/C25H32F3N5O/c1-15-30-31-16(2)33(15)22-11-20-6-7-21(12-22)32(20)9-8-23(17-4-3-5-19(26)10-17)29-24(34)18-13-25(27,28)14-18/h3-5,10,18,20-23H,6-9,11-14H2,1-2H3,(H,29,34)/t20-,21+,22+,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 20: 1674-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.080
BindingDB Entry DOI: 10.7270/Q23B6131 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50312848
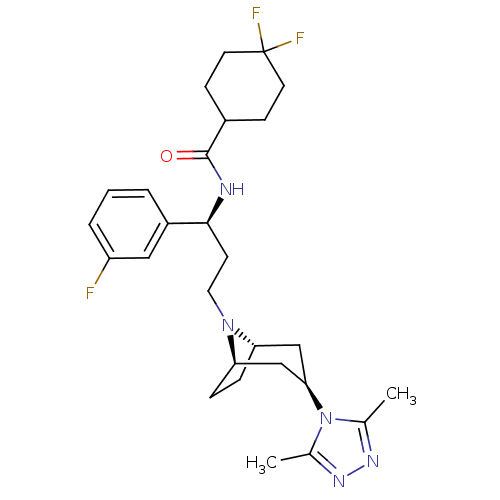
(CHEMBL1087153 | N-((S)-3-((1R,3S,5S)-3-(3,5-dimeth...)Show SMILES Cc1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1cccc(F)c1 |r,THB:15:14:13.7.8:11.10| Show InChI InChI=1S/C27H36F3N5O/c1-17-32-33-18(2)35(17)24-15-22-6-7-23(16-24)34(22)13-10-25(20-4-3-5-21(28)14-20)31-26(36)19-8-11-27(29,30)12-9-19/h3-5,14,19,22-25H,6-13,15-16H2,1-2H3,(H,31,36)/t22-,23+,24+,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 20: 1674-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.080
BindingDB Entry DOI: 10.7270/Q23B6131 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50312852
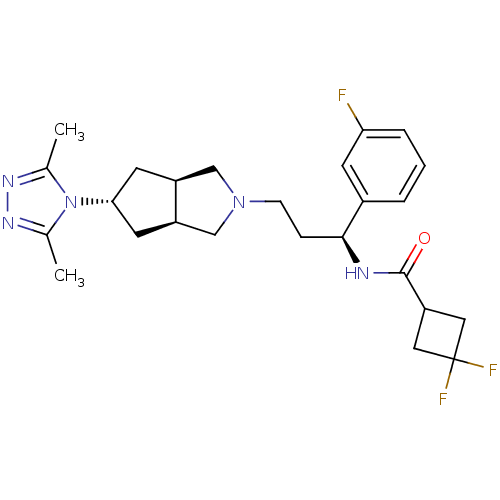
(CHEMBL1081476 | Exo-N-((S)-3-(5-(3,5-dimethyl-4H-1...)Show SMILES Cc1nnc(C)n1[C@@H]1C[C@H]2CN(CC[C@H](NC(=O)C3CC(F)(F)C3)c3cccc(F)c3)C[C@H]2C1 |r| Show InChI InChI=1S/C25H32F3N5O/c1-15-30-31-16(2)33(15)22-9-18-13-32(14-19(18)10-22)7-6-23(17-4-3-5-21(26)8-17)29-24(34)20-11-25(27,28)12-20/h3-5,8,18-20,22-23H,6-7,9-14H2,1-2H3,(H,29,34)/t18-,19+,22+,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 20: 1674-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.080
BindingDB Entry DOI: 10.7270/Q23B6131 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50312850
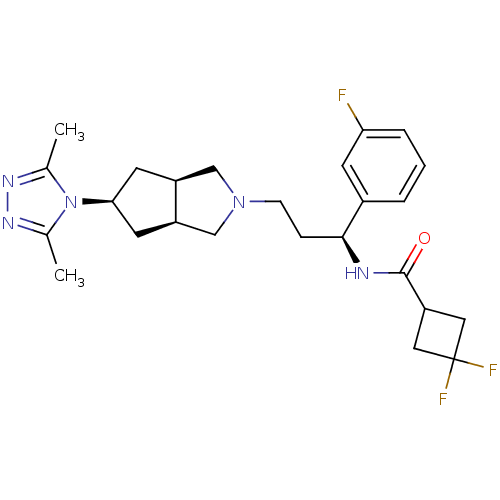
(CHEMBL1082191 | Endo-N-((S)-3-(5-(3,5-dimethyl-4H-...)Show SMILES Cc1nnc(C)n1[C@H]1C[C@H]2CN(CC[C@H](NC(=O)C3CC(F)(F)C3)c3cccc(F)c3)C[C@H]2C1 |r| Show InChI InChI=1S/C25H32F3N5O/c1-15-30-31-16(2)33(15)22-9-18-13-32(14-19(18)10-22)7-6-23(17-4-3-5-21(26)8-17)29-24(34)20-11-25(27,28)12-20/h3-5,8,18-20,22-23H,6-7,9-14H2,1-2H3,(H,29,34)/t18-,19+,22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 20: 1674-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.080
BindingDB Entry DOI: 10.7270/Q23B6131 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50312845
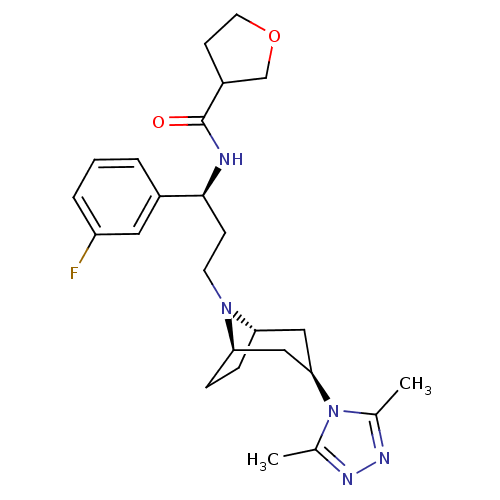
(CHEMBL1087534 | N-((S)-3-((1R,3S,5S)-3-(3,5-dimeth...)Show SMILES Cc1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CC[C@H](NC(=O)C1CCOC1)c1cccc(F)c1 |r,THB:15:14:13.7.8:11.10| Show InChI InChI=1S/C25H34FN5O2/c1-16-28-29-17(2)31(16)23-13-21-6-7-22(14-23)30(21)10-8-24(18-4-3-5-20(26)12-18)27-25(32)19-9-11-33-15-19/h3-5,12,19,21-24H,6-11,13-15H2,1-2H3,(H,27,32)/t19?,21-,22+,23+,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 20: 1674-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.080
BindingDB Entry DOI: 10.7270/Q23B6131 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50329255
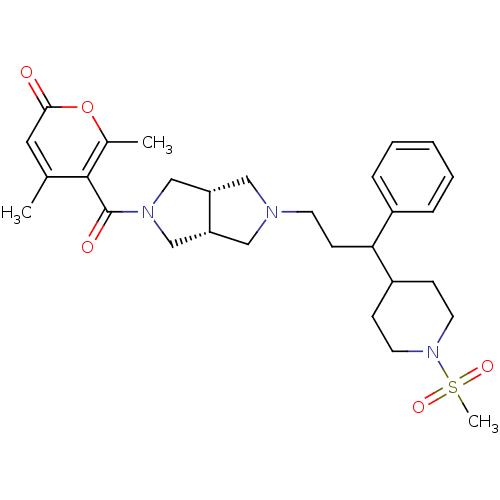
(4,6-dimethyl-5-((3aR,6aS)-5-(3-(1-(methylsulfonyl)...)Show SMILES Cc1cc(=O)oc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H39N3O5S/c1-20-15-27(33)37-21(2)28(20)29(34)31-18-24-16-30(17-25(24)19-31)12-11-26(22-7-5-4-6-8-22)23-9-13-32(14-10-23)38(3,35)36/h4-8,15,23-26H,9-14,16-19H2,1-3H3/t24-,25+,26? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of CCR5 by cell-cell fusion inhibition assay |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50312843
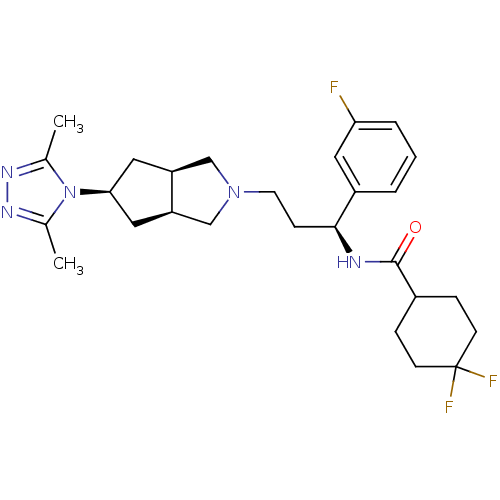
(CHEMBL1082202 | Endo-N-((S)-3-(5-(3,5-dimethyl-4H-...)Show SMILES Cc1nnc(C)n1[C@H]1C[C@H]2CN(CC[C@H](NC(=O)C3CCC(F)(F)CC3)c3cccc(F)c3)C[C@H]2C1 |r| Show InChI InChI=1S/C27H36F3N5O/c1-17-32-33-18(2)35(17)24-13-21-15-34(16-22(21)14-24)11-8-25(20-4-3-5-23(28)12-20)31-26(36)19-6-9-27(29,30)10-7-19/h3-5,12,19,21-22,24-25H,6-11,13-16H2,1-2H3,(H,31,36)/t21-,22+,24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 20: 1674-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.080
BindingDB Entry DOI: 10.7270/Q23B6131 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305791

(((3aR,6aS)-5-(2-(1-(4,4-difluorocyclohexanecarbony...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CCC(F)(F)CC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C31H39F2N5O2/c1-21-27(22(2)35-20-34-21)29(40)37-16-24-14-36(15-25(24)17-37)13-12-30(26-6-4-3-5-7-26)18-38(19-30)28(39)23-8-10-31(32,33)11-9-23/h3-7,20,23-25H,8-19H2,1-2H3/t24-,25+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 by cell-cell fusion assay |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50329231
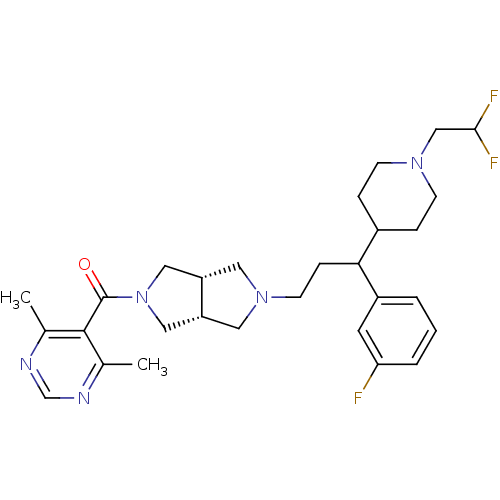
(((3aR,6aS)-5-(3-(1-(2,2-difluoroethyl)piperidin-4-...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC(F)F)CC3)c3cccc(F)c3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H38F3N5O/c1-19-28(20(2)34-18-33-19)29(38)37-15-23-13-36(14-24(23)16-37)11-8-26(22-4-3-5-25(30)12-22)21-6-9-35(10-7-21)17-27(31)32/h3-5,12,18,21,23-24,26-27H,6-11,13-17H2,1-2H3/t23-,24+,26? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of CCR5 by cell-cell fusion inhibition assay |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50312847
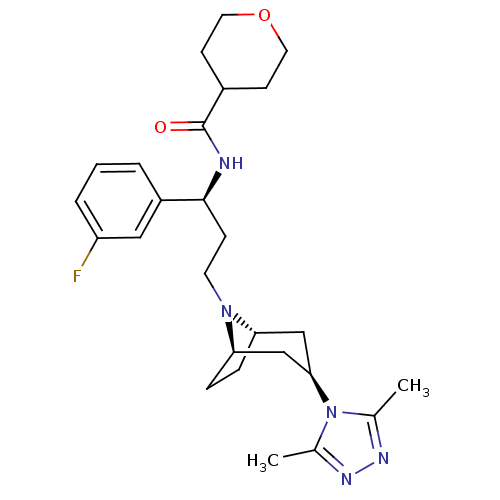
(CHEMBL1087028 | N-((S)-3-((1R,3S,5S)-3-(3,5-dimeth...)Show SMILES Cc1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CC[C@H](NC(=O)C1CCOCC1)c1cccc(F)c1 |r,THB:15:14:13.7.8:11.10| Show InChI InChI=1S/C26H36FN5O2/c1-17-29-30-18(2)32(17)24-15-22-6-7-23(16-24)31(22)11-8-25(20-4-3-5-21(27)14-20)28-26(33)19-9-12-34-13-10-19/h3-5,14,19,22-25H,6-13,15-16H2,1-2H3,(H,28,33)/t22-,23+,24+,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 20: 1674-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.080
BindingDB Entry DOI: 10.7270/Q23B6131 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50313879
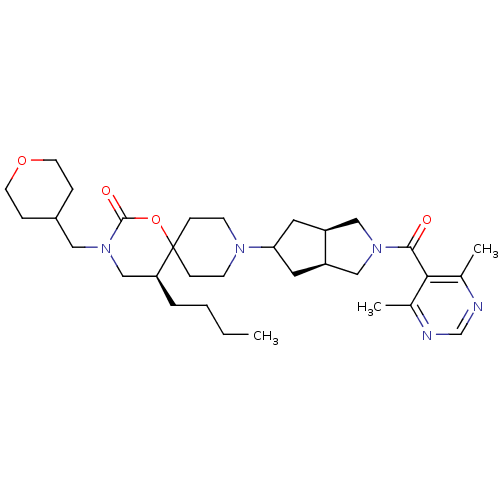
((5S)-5-butyl-9-((3aR,6aS)-2-(4,6-dimethylpyrimidin...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C32H49N5O4/c1-4-5-6-27-20-37(17-24-7-13-40-14-8-24)31(39)41-32(27)9-11-35(12-10-32)28-15-25-18-36(19-26(25)16-28)30(38)29-22(2)33-21-34-23(29)3/h21,24-28H,4-20H2,1-3H3/t25-,26+,27-,28?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]-RANTES from CCR5 receptor receptors expressed in CHO cells |
Bioorg Med Chem Lett 20: 1830-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.004
BindingDB Entry DOI: 10.7270/Q25Q4W7T |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305780

(5-((3aR,6aS)-5-(2-(4-(3-fluorophenyl)-1-pivaloylpi...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CCN(CC3)C(=O)C(C)(C)C)c3cccc(F)c3)C[C@@H]2C1)C#N |r| Show InChI InChI=1S/C33H42FN5O2/c1-22-15-28(17-35)36-23(2)29(22)30(40)39-20-24-18-37(19-25(24)21-39)12-9-33(26-7-6-8-27(34)16-26)10-13-38(14-11-33)31(41)32(3,4)5/h6-8,15-16,24-25H,9-14,18-21H2,1-5H3/t24-,25+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305796
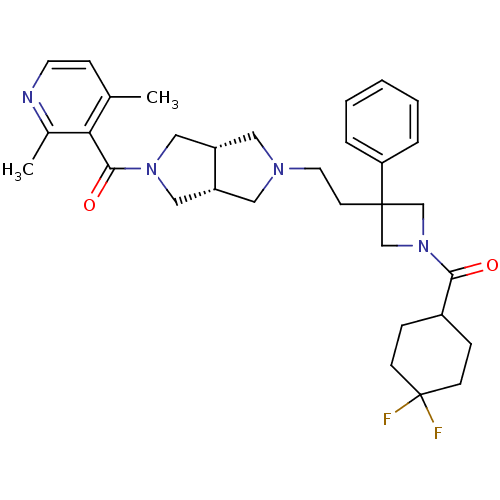
(((3aR,6aS)-5-(2-(1-(4,4-difluorocyclohexanecarbony...)Show SMILES Cc1ccnc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CCC(F)(F)CC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C32H40F2N4O2/c1-22-10-14-35-23(2)28(22)30(40)37-18-25-16-36(17-26(25)19-37)15-13-31(27-6-4-3-5-7-27)20-38(21-31)29(39)24-8-11-32(33,34)12-9-24/h3-7,10,14,24-26H,8-9,11-13,15-21H2,1-2H3/t25-,26+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 by cell-cell fusion assay |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50313880
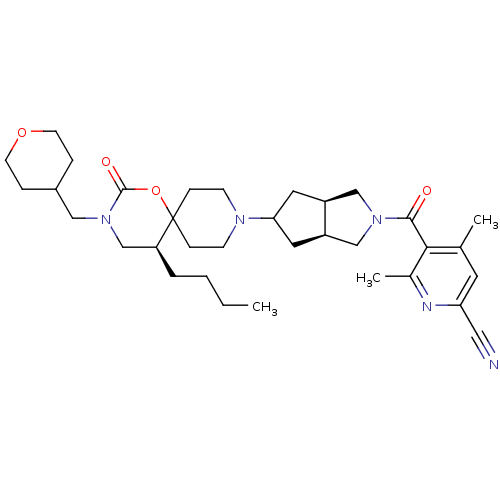
(5-((3aR,6aS)-5-((S)-5-butyl-2-oxo-3-((tetrahydro-2...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)cc(nc1C)C#N |r| Show InChI InChI=1S/C34H49N5O4/c1-4-5-6-28-22-39(19-25-7-13-42-14-8-25)33(41)43-34(28)9-11-37(12-10-34)30-16-26-20-38(21-27(26)17-30)32(40)31-23(2)15-29(18-35)36-24(31)3/h15,25-28,30H,4-14,16-17,19-22H2,1-3H3/t26-,27+,28-,30?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]-RANTES from CCR5 receptor receptors expressed in CHO cells |
Bioorg Med Chem Lett 20: 1830-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.004
BindingDB Entry DOI: 10.7270/Q25Q4W7T |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305798

(5-((3aR,6aS)-5-(2-(1-(4,4-difluorocyclohexanecarbo...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CCC(F)(F)CC3)c3ccccc3)C[C@@H]2C1)C#N |r| Show InChI InChI=1S/C33H39F2N5O2/c1-22-14-28(15-36)37-23(2)29(22)31(42)39-18-25-16-38(17-26(25)19-39)13-12-32(27-6-4-3-5-7-27)20-40(21-32)30(41)24-8-10-33(34,35)11-9-24/h3-7,14,24-26H,8-13,16-21H2,1-2H3/t25-,26+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329229

((4,6-dimethylpyrimidin-5-yl)((3aR,6aS)-5-(3-phenyl...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC(F)(F)F)CC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H38F3N5O/c1-20-27(21(2)34-19-33-20)28(38)37-16-24-14-36(15-25(24)17-37)13-10-26(22-6-4-3-5-7-22)23-8-11-35(12-9-23)18-29(30,31)32/h3-7,19,23-26H,8-18H2,1-2H3/t24-,25+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50312849
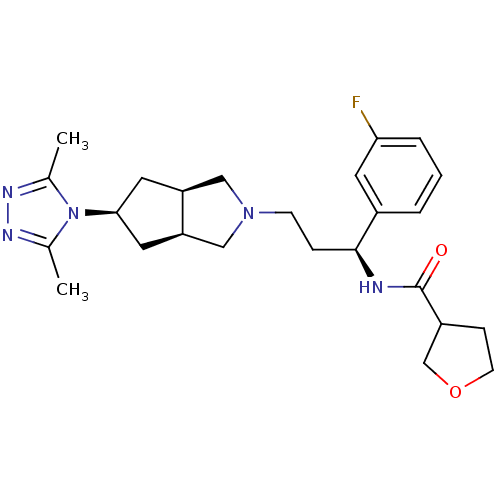
(CHEMBL1081486 | Endo-N-((S)-3-(5-(3,5-dimethyl-4H-...)Show SMILES Cc1nnc(C)n1[C@H]1C[C@H]2CN(CC[C@H](NC(=O)C3CCOC3)c3cccc(F)c3)C[C@H]2C1 |r| Show InChI InChI=1S/C25H34FN5O2/c1-16-28-29-17(2)31(16)23-11-20-13-30(14-21(20)12-23)8-6-24(18-4-3-5-22(26)10-18)27-25(32)19-7-9-33-15-19/h3-5,10,19-21,23-24H,6-9,11-15H2,1-2H3,(H,27,32)/t19?,20-,21+,23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 20: 1674-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.080
BindingDB Entry DOI: 10.7270/Q23B6131 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50329232

(((3aR,6aS)-5-(3-(1-(2,2-difluoroethyl)piperidin-4-...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC(F)F)CC3)c3cc(F)cc(F)c3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H37F4N5O/c1-18-28(19(2)35-17-34-18)29(39)38-14-22-12-37(13-23(22)15-38)8-5-26(21-9-24(30)11-25(31)10-21)20-3-6-36(7-4-20)16-27(32)33/h9-11,17,20,22-23,26-27H,3-8,12-16H2,1-2H3/t22-,23+,26? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of CCR5 by cell-cell fusion inhibition assay |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305800

(((3aR,6aS)-5-(2-(1-(4,4-difluorocyclohexanecarbony...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CCC(F)(F)CC3)c3ccccc3)C[C@@H]2C1)C(F)(F)F |r| Show InChI InChI=1S/C33H39F5N4O2/c1-21-14-27(33(36,37)38)39-22(2)28(21)30(44)41-17-24-15-40(16-25(24)18-41)13-12-31(26-6-4-3-5-7-26)19-42(20-31)29(43)23-8-10-32(34,35)11-9-23/h3-7,14,23-25H,8-13,15-20H2,1-2H3/t24-,25+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305801

(((3aR,6aS)-5-(2-(1-(3,3-difluorocyclobutanecarbony...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CC(F)(F)C3)c3ccccc3)C[C@@H]2C1)C(F)(F)F |r| Show InChI InChI=1S/C31H35F5N4O2/c1-19-10-25(31(34,35)36)37-20(2)26(19)28(42)39-15-22-13-38(14-23(22)16-39)9-8-29(24-6-4-3-5-7-24)17-40(18-29)27(41)21-11-30(32,33)12-21/h3-7,10,21-23H,8-9,11-18H2,1-2H3/t22-,23+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329246
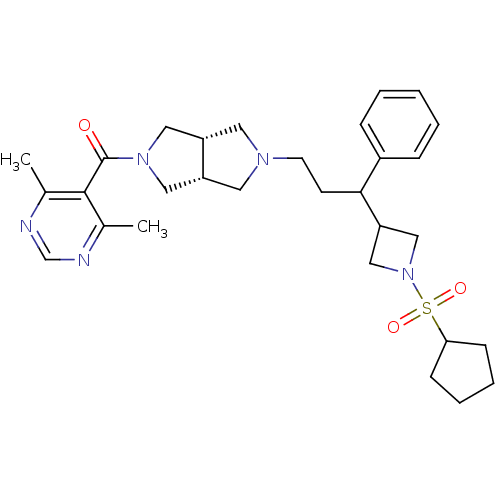
(((3aR,6aS)-5-(3-(1-(cyclopentylsulfonyl)azetidin-3...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CN(C3)S(=O)(=O)C3CCCC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C30H41N5O3S/c1-21-29(22(2)32-20-31-21)30(36)34-16-24-14-33(15-25(24)17-34)13-12-28(23-8-4-3-5-9-23)26-18-35(19-26)39(37,38)27-10-6-7-11-27/h3-5,8-9,20,24-28H,6-7,10-19H2,1-2H3/t24-,25+,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50313885
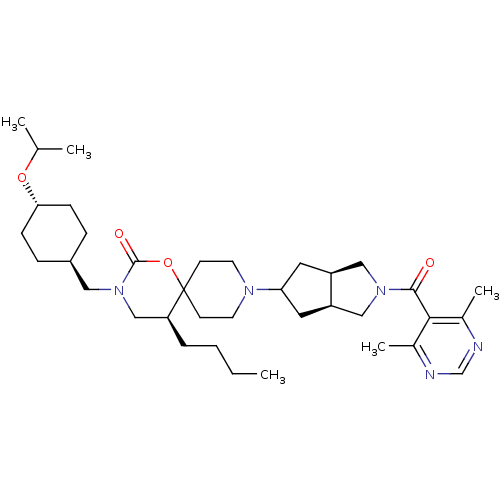
(CHEMBL1077364 | trans-(5S)-5-butyl-9-((3aR,6aS)-2-...)Show SMILES CCCC[C@H]1CN(C[C@H]2CC[C@@H](CC2)OC(C)C)C(=O)OC11CCN(CC1)C1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ncnc1C |r,wU:8.7,wD:29.32,33.35,4.3,11.14,(.35,-43.61,;-1.19,-43.62,;-1.96,-44.95,;-3.5,-44.96,;-4.26,-46.29,;-5.8,-46.3,;-6.57,-47.64,;-8.11,-47.64,;-8.89,-46.31,;-10.43,-46.32,;-11.2,-44.98,;-10.43,-43.64,;-8.89,-43.65,;-8.12,-44.98,;-11.19,-42.31,;-10.42,-40.98,;-8.88,-40.98,;-11.19,-39.64,;-5.8,-48.98,;-6.56,-50.31,;-4.25,-48.97,;-3.48,-47.63,;-2.7,-46.31,;-1.17,-46.32,;-.4,-47.66,;-1.18,-48.99,;-2.72,-48.98,;1.14,-47.67,;2.05,-48.91,;3.51,-48.45,;4.97,-48.93,;5.89,-47.69,;4.99,-46.44,;3.52,-46.91,;2.06,-46.42,;7.43,-47.7,;8.2,-46.37,;8.19,-49.04,;7.41,-50.36,;5.87,-50.35,;8.17,-51.7,;9.71,-51.71,;10.49,-50.37,;9.72,-49.04,;10.5,-47.7,)| Show InChI InChI=1S/C36H57N5O4/c1-6-7-8-30-22-41(19-27-9-11-32(12-10-27)44-24(2)3)35(43)45-36(30)13-15-39(16-14-36)31-17-28-20-40(21-29(28)18-31)34(42)33-25(4)37-23-38-26(33)5/h23-24,27-32H,6-22H2,1-5H3/t27-,28-,29+,30-,31?,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]-RANTES from CCR5 receptor receptors expressed in CHO cells |
Bioorg Med Chem Lett 20: 1830-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.004
BindingDB Entry DOI: 10.7270/Q25Q4W7T |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329250

(((3aR,6aS)-5-(3-(1-(cyclopentylsulfonyl)piperidin-...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(=O)(=O)C3CCCC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C32H45N5O3S/c1-23-31(24(2)34-22-33-23)32(38)36-20-27-18-35(19-28(27)21-36)15-14-30(25-8-4-3-5-9-25)26-12-16-37(17-13-26)41(39,40)29-10-6-7-11-29/h3-5,8-9,22,26-30H,6-7,10-21H2,1-2H3/t27-,28+,30? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329241
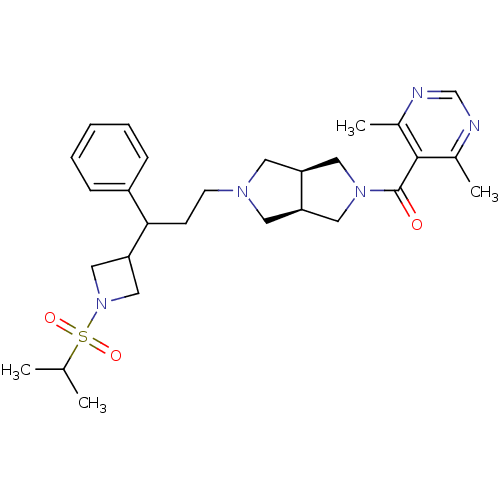
((4,6-dimethylpyrimidin-5-yl)((3aR,6aS)-5-(3-(1-(is...)Show SMILES CC(C)S(=O)(=O)N1CC(C1)C(CCN1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ncnc1C)c1ccccc1 |r| Show InChI InChI=1S/C28H39N5O3S/c1-19(2)37(35,36)33-16-25(17-33)26(22-8-6-5-7-9-22)10-11-31-12-23-14-32(15-24(23)13-31)28(34)27-20(3)29-18-30-21(27)4/h5-9,18-19,23-26H,10-17H2,1-4H3/t23-,24+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305799

(5-((3aR,6aS)-5-(2-(1-(3,3-difluorocyclobutanecarbo...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CC(F)(F)C3)c3ccccc3)C[C@@H]2C1)C#N |r| Show InChI InChI=1S/C31H35F2N5O2/c1-20-10-26(13-34)35-21(2)27(20)29(40)37-16-23-14-36(15-24(23)17-37)9-8-30(25-6-4-3-5-7-25)18-38(19-30)28(39)22-11-31(32,33)12-22/h3-7,10,22-24H,8-9,11-12,14-19H2,1-2H3/t23-,24+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50313881
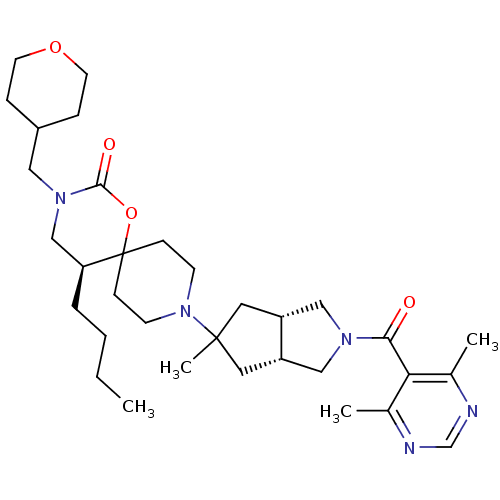
((5S)-5-butyl-9-((3aR,6aS)-2-(4,6-dimethylpyrimidin...)Show SMILES CCCC[C@H]1CN(CC2CCOCC2)C(=O)OC11CCN(CC1)C1(C)C[C@@H]2CN(C[C@@H]2C1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C33H51N5O4/c1-5-6-7-28-21-37(18-25-8-14-41-15-9-25)31(40)42-33(28)10-12-38(13-11-33)32(4)16-26-19-36(20-27(26)17-32)30(39)29-23(2)34-22-35-24(29)3/h22,25-28H,5-21H2,1-4H3/t26-,27+,28-,32?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]-RANTES from CCR5 receptor receptors expressed in CHO cells |
Bioorg Med Chem Lett 20: 1830-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.004
BindingDB Entry DOI: 10.7270/Q25Q4W7T |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329232

(((3aR,6aS)-5-(3-(1-(2,2-difluoroethyl)piperidin-4-...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC(F)F)CC3)c3cc(F)cc(F)c3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H37F4N5O/c1-18-28(19(2)35-17-34-18)29(39)38-14-22-12-37(13-23(22)15-38)8-5-26(21-9-24(30)11-25(31)10-21)20-3-6-36(7-4-20)16-27(32)33/h9-11,17,20,22-23,26-27H,3-8,12-16H2,1-2H3/t22-,23+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329249
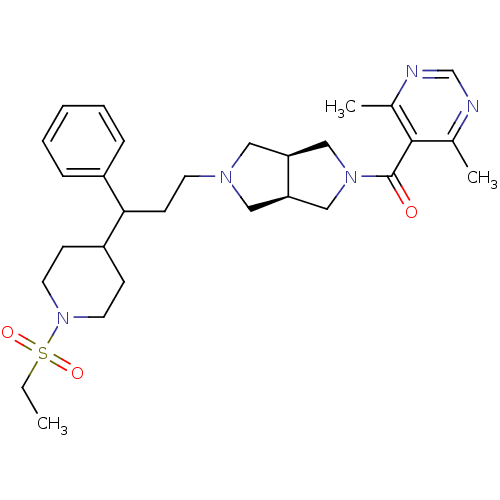
((4,6-dimethylpyrimidin-5-yl)((3aR,6aS)-5-(3-(1-(et...)Show SMILES CCS(=O)(=O)N1CCC(CC1)C(CCN1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ncnc1C)c1ccccc1 |r| Show InChI InChI=1S/C29H41N5O3S/c1-4-38(36,37)34-14-10-24(11-15-34)27(23-8-6-5-7-9-23)12-13-32-16-25-18-33(19-26(25)17-32)29(35)28-21(2)30-20-31-22(28)3/h5-9,20,24-27H,4,10-19H2,1-3H3/t25-,26+,27? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305788
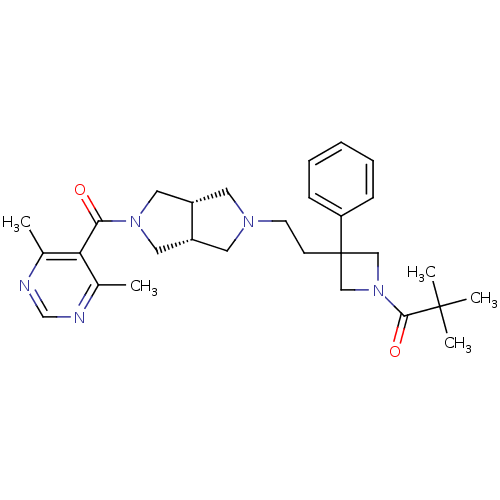
(1-(3-(2-((3aR,6aS)-5-(4,6-dimethylpyrimidine-5-car...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C(C)(C)C)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H39N5O2/c1-20-25(21(2)31-19-30-20)26(35)33-15-22-13-32(14-23(22)16-33)12-11-29(24-9-7-6-8-10-24)17-34(18-29)27(36)28(3,4)5/h6-10,19,22-23H,11-18H2,1-5H3/t22-,23+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305790
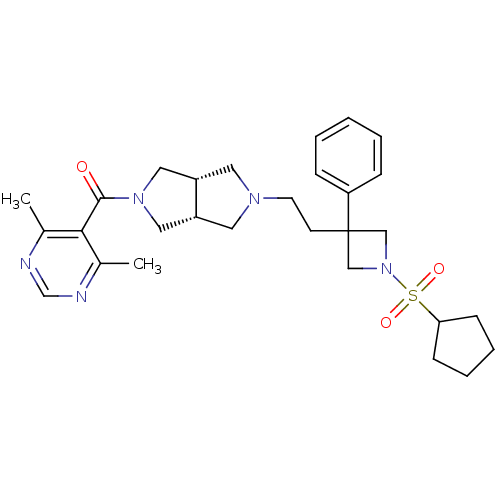
(((3aR,6aS)-5-(2-(1-(cyclopentylsulfonyl)-3-phenyla...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)S(=O)(=O)C3CCCC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H39N5O3S/c1-21-27(22(2)31-20-30-21)28(35)33-16-23-14-32(15-24(23)17-33)13-12-29(25-8-4-3-5-9-25)18-34(19-29)38(36,37)26-10-6-7-11-26/h3-5,8-9,20,23-24,26H,6-7,10-19H2,1-2H3/t23-,24+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50313886
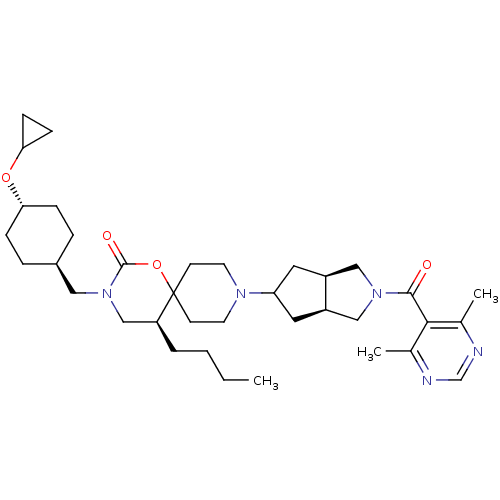
(CHEMBL1077365 | trans-(5S)-5-butyl-3-((4-cycloprop...)Show SMILES CCCC[C@H]1CN(C[C@H]2CC[C@@H](CC2)OC2CC2)C(=O)OC11CCN(CC1)C1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ncnc1C |r,wU:8.7,wD:29.33,33.36,4.3,11.14,(22.73,-44.79,;21.19,-44.79,;20.43,-46.13,;18.89,-46.13,;18.12,-47.47,;16.58,-47.47,;15.81,-48.81,;14.27,-48.81,;13.5,-47.48,;11.95,-47.49,;11.18,-46.15,;11.96,-44.82,;13.5,-44.82,;14.26,-46.15,;11.19,-43.48,;11.96,-42.15,;13.3,-41.38,;11.96,-40.61,;16.59,-50.15,;15.82,-51.48,;18.14,-50.15,;18.9,-48.81,;19.68,-47.49,;21.22,-47.5,;21.99,-48.83,;21.21,-50.17,;19.66,-50.15,;23.53,-48.84,;24.43,-50.09,;25.9,-49.62,;27.36,-50.11,;28.27,-48.87,;27.37,-47.62,;25.9,-48.08,;24.44,-47.6,;29.81,-48.87,;30.59,-47.54,;30.57,-50.21,;29.79,-51.54,;28.25,-51.53,;30.56,-52.87,;32.1,-52.88,;32.87,-51.54,;32.11,-50.21,;32.88,-48.88,)| Show InChI InChI=1S/C36H55N5O4/c1-4-5-6-29-22-41(19-26-7-9-31(10-8-26)44-32-11-12-32)35(43)45-36(29)13-15-39(16-14-36)30-17-27-20-40(21-28(27)18-30)34(42)33-24(2)37-23-38-25(33)3/h23,26-32H,4-22H2,1-3H3/t26-,27-,28+,29-,30?,31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]-RANTES from CCR5 receptor receptors expressed in CHO cells |
Bioorg Med Chem Lett 20: 1830-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.004
BindingDB Entry DOI: 10.7270/Q25Q4W7T |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329230
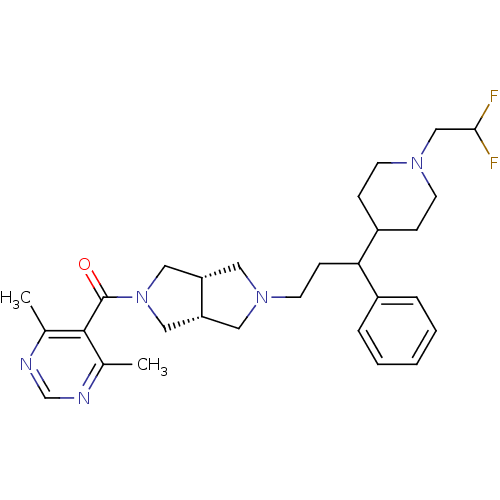
(((3aR,6aS)-5-(3-(1-(2,2-difluoroethyl)piperidin-4-...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC(F)F)CC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H39F2N5O/c1-20-28(21(2)33-19-32-20)29(37)36-16-24-14-35(15-25(24)17-36)13-10-26(22-6-4-3-5-7-22)23-8-11-34(12-9-23)18-27(30)31/h3-7,19,23-27H,8-18H2,1-2H3/t24-,25+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329248
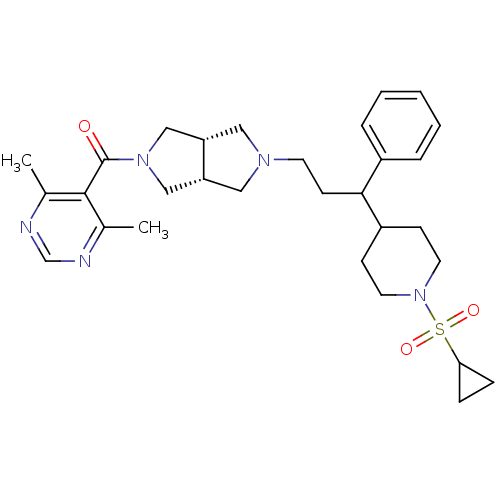
(((3aR,6aS)-5-(3-(1-(cyclopropylsulfonyl)piperidin-...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(=O)(=O)C3CC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C30H41N5O3S/c1-21-29(22(2)32-20-31-21)30(36)34-18-25-16-33(17-26(25)19-34)13-12-28(23-6-4-3-5-7-23)24-10-14-35(15-11-24)39(37,38)27-8-9-27/h3-7,20,24-28H,8-19H2,1-2H3/t25-,26+,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329255

(4,6-dimethyl-5-((3aR,6aS)-5-(3-(1-(methylsulfonyl)...)Show SMILES Cc1cc(=O)oc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H39N3O5S/c1-20-15-27(33)37-21(2)28(20)29(34)31-18-24-16-30(17-25(24)19-31)12-11-26(22-7-5-4-6-8-22)23-9-13-32(14-10-23)38(3,35)36/h4-8,15,23-26H,9-14,16-19H2,1-3H3/t24-,25+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305786

(2-cyclopentyl-1-(3-(2-((3aR,6aS)-5-(4,6-dimethylpy...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)CC3CCCC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C31H41N5O2/c1-22-29(23(2)33-21-32-22)30(38)35-17-25-15-34(16-26(25)18-35)13-12-31(27-10-4-3-5-11-27)19-36(20-31)28(37)14-24-8-6-7-9-24/h3-5,10-11,21,24-26H,6-9,12-20H2,1-2H3/t25-,26+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305791

(((3aR,6aS)-5-(2-(1-(4,4-difluorocyclohexanecarbony...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CCC(F)(F)CC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C31H39F2N5O2/c1-21-27(22(2)35-20-34-21)29(40)37-16-24-14-36(15-25(24)17-37)13-12-30(26-6-4-3-5-7-26)18-38(19-30)28(39)23-8-10-31(32,33)11-9-23/h3-7,20,23-25H,8-19H2,1-2H3/t24-,25+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305792
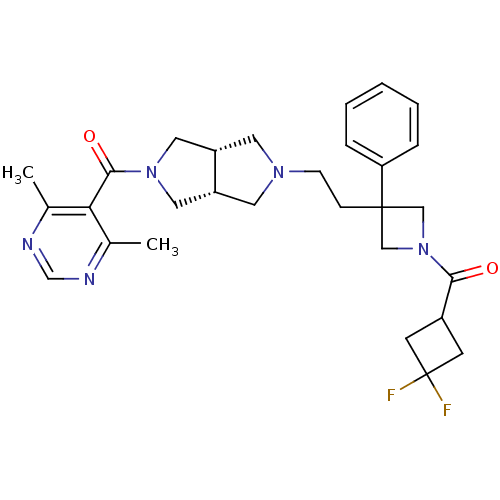
(((3aR,6aS)-5-(2-(1-(3,3-difluorocyclobutanecarbony...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CC(F)(F)C3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H35F2N5O2/c1-19-25(20(2)33-18-32-19)27(38)35-14-22-12-34(13-23(22)15-35)9-8-28(24-6-4-3-5-7-24)16-36(17-28)26(37)21-10-29(30,31)11-21/h3-7,18,21-23H,8-17H2,1-2H3/t22-,23+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305794

(((3aR,6aS)-5-(2-(1-(4,4-difluorocyclohexanecarbony...)Show SMILES Cc1nc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CCC(F)(F)CC3)c3ccccc3)C[C@@H]2C1)C(F)(F)F |r| Show InChI InChI=1S/C32H38F5N5O2/c1-20-26(21(2)39-29(38-20)32(35,36)37)28(44)41-16-23-14-40(15-24(23)17-41)13-12-30(25-6-4-3-5-7-25)18-42(19-30)27(43)22-8-10-31(33,34)11-9-22/h3-7,22-24H,8-19H2,1-2H3/t23-,24+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR5 |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50329243

(((3aR,6aS)-5-(3-(1-(cyclopropylsulfonyl)azetidin-3...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CN(C3)S(=O)(=O)C3CC3)c3cccc(F)c3)C[C@@H]2C1 |r| Show InChI InChI=1S/C28H36FN5O3S/c1-18-27(19(2)31-17-30-18)28(35)33-13-21-11-32(12-22(21)14-33)9-8-26(20-4-3-5-24(29)10-20)23-15-34(16-23)38(36,37)25-6-7-25/h3-5,10,17,21-23,25-26H,6-9,11-16H2,1-2H3/t21-,22+,26? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of CCR5 by cell-cell fusion inhibition assay |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50312842
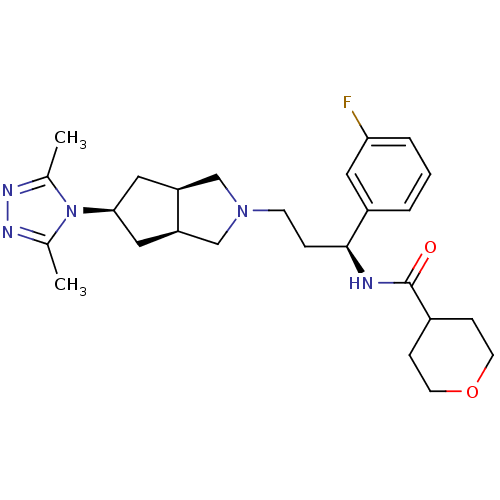
(CHEMBL1082201 | Endo-N-((S)-3-(5-(3,5-dimethyl-4H-...)Show SMILES Cc1nnc(C)n1[C@H]1C[C@H]2CN(CC[C@H](NC(=O)C3CCOCC3)c3cccc(F)c3)C[C@H]2C1 |r| Show InChI InChI=1S/C26H36FN5O2/c1-17-29-30-18(2)32(17)24-13-21-15-31(16-22(21)14-24)9-6-25(20-4-3-5-23(27)12-20)28-26(33)19-7-10-34-11-8-19/h3-5,12,19,21-22,24-25H,6-11,13-16H2,1-2H3,(H,28,33)/t21-,22+,24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 20: 1674-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.080
BindingDB Entry DOI: 10.7270/Q23B6131 |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329256

(4,6-dimethyl-5-((3aR,6aS)-5-(3-(1-(methylsulfonyl)...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3ccccc3)C[C@@H]2C1)C#N |r| Show InChI InChI=1S/C30H39N5O3S/c1-21-15-27(16-31)32-22(2)29(21)30(36)34-19-25-17-33(18-26(25)20-34)12-11-28(23-7-5-4-6-8-23)24-9-13-35(14-10-24)39(3,37)38/h4-8,15,24-26,28H,9-14,17-20H2,1-3H3/t25-,26+,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329235

(1-(4-(3-((3aR,6aS)-5-(4,6-dimethylpyrimidine-5-car...)Show SMILES CCC(=O)N1CCC(CC1)C(CCN1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ncnc1C)c1ccccc1 |r| Show InChI InChI=1S/C30H41N5O2/c1-4-28(36)34-14-10-24(11-15-34)27(23-8-6-5-7-9-23)12-13-33-16-25-18-35(19-26(25)17-33)30(37)29-21(2)31-20-32-22(29)3/h5-9,20,24-27H,4,10-19H2,1-3H3/t25-,26+,27? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50313884
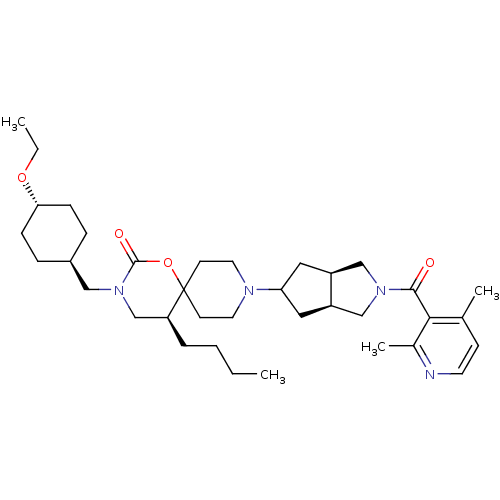
(CHEMBL1077363 | trans-(5S)-5-butyl-9-((3aR,6aS)-2-...)Show SMILES CCCC[C@H]1CN(C[C@H]2CC[C@@H](CC2)OCC)C(=O)OC11CCN(CC1)C1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ccnc1C |r,wU:8.7,wD:28.31,32.34,4.3,11.14,(6,-35.54,;4.46,-35.54,;3.69,-36.88,;2.15,-36.88,;1.38,-38.22,;-.15,-38.22,;-.93,-39.56,;-2.47,-39.57,;-3.24,-38.23,;-4.78,-38.24,;-5.55,-36.9,;-4.78,-35.57,;-3.24,-35.57,;-2.47,-36.9,;-5.55,-34.23,;-4.78,-32.9,;-5.54,-31.57,;-.15,-40.9,;-.92,-42.24,;1.4,-40.9,;2.16,-39.56,;2.95,-38.24,;4.48,-38.25,;5.25,-39.59,;4.47,-40.92,;2.93,-40.91,;6.79,-39.59,;7.69,-40.84,;9.16,-40.38,;10.62,-40.86,;11.53,-39.62,;10.63,-38.37,;9.17,-38.84,;7.71,-38.35,;13.07,-39.63,;13.85,-38.3,;13.84,-40.96,;13.06,-42.29,;11.52,-42.28,;13.82,-43.62,;15.36,-43.63,;16.14,-42.3,;15.37,-40.96,;16.14,-39.63,)| Show InChI InChI=1S/C36H56N4O4/c1-5-7-8-30-24-40(21-27-9-11-32(12-10-27)43-6-2)35(42)44-36(30)14-17-38(18-15-36)31-19-28-22-39(23-29(28)20-31)34(41)33-25(3)13-16-37-26(33)4/h13,16,27-32H,5-12,14-15,17-24H2,1-4H3/t27-,28-,29+,30-,31?,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Displacement of [128I]-RANTES from CCR5 receptor receptors expressed in CHO cells |
Bioorg Med Chem Lett 20: 1830-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.004
BindingDB Entry DOI: 10.7270/Q25Q4W7T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data