 Found 497 hits with Last Name = 'lesuisse' and Initial = 'd'
Found 497 hits with Last Name = 'lesuisse' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM50005878

(4-Chloromethylsulfanyl-10,13-dimethyl-1,6,7,8,10,1...)Show SMILES C[C@]12CC=C3C(CCC4=C(SCCl)C(=O)CC[C@]34C)C1CCC2=O |c:3,8| Show InChI InChI=1S/C20H25ClO2S/c1-19-10-8-16(22)18(24-11-21)15(19)4-3-12-13-5-6-17(23)20(13,2)9-7-14(12)19/h7,12-13H,3-6,8-11H2,1-2H3/t12?,13?,19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Roussel-UCLAF
Curated by ChEMBL
| Assay Description
Inhibition constant for human placental cytochrome P450 19A1 |
J Med Chem 35: 1588-97 (1992)
BindingDB Entry DOI: 10.7270/Q2VD7022 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50240798
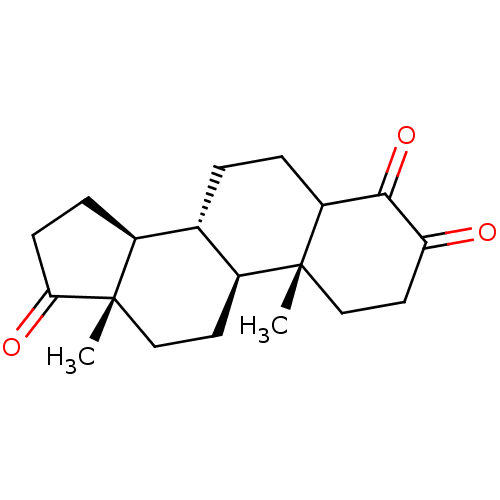
((8R,9S,10R,13S,14S)-4-Hydroxy-10,13-dimethyl-1,6,7...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4C(=O)C(=O)CC[C@]34C)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H26O3/c1-18-10-8-15(20)17(22)14(18)4-3-11-12-5-6-16(21)19(12,2)9-7-13(11)18/h11-14H,3-10H2,1-2H3/t11-,12-,13-,14?,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Roussel-UCLAF
Curated by ChEMBL
| Assay Description
Inhibition constant for human placental cytochrome P450 19A1 |
J Med Chem 35: 1588-97 (1992)
BindingDB Entry DOI: 10.7270/Q2VD7022 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50005876
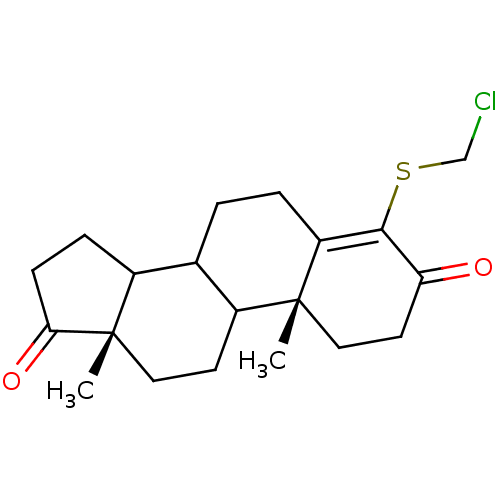
(4-Chloromethylsulfanyl-10,13-dimethyl-1,6,7,8,9,10...)Show SMILES C[C@]12CCC3C(CCC4=C(SCCl)C(=O)CC[C@]34C)C1CCC2=O |c:8| Show InChI InChI=1S/C20H27ClO2S/c1-19-10-8-16(22)18(24-11-21)15(19)4-3-12-13-5-6-17(23)20(13,2)9-7-14(12)19/h12-14H,3-11H2,1-2H3/t12?,13?,14?,19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Roussel-UCLAF
Curated by ChEMBL
| Assay Description
Inhibition constant for human placental cytochrome P450 19A1 |
J Med Chem 35: 1588-97 (1992)
BindingDB Entry DOI: 10.7270/Q2VD7022 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50005874
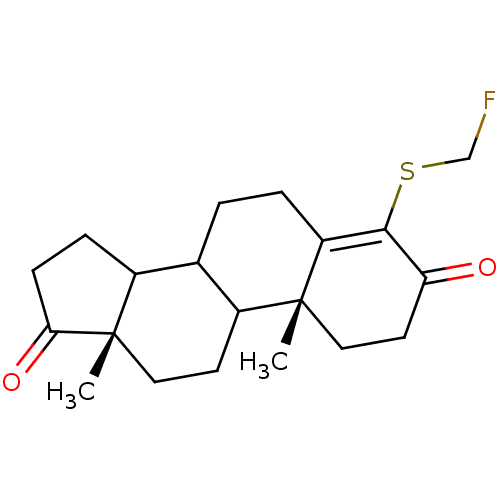
(4-Fluoromethylsulfanyl-10,13-dimethyl-1,6,7,8,9,10...)Show SMILES C[C@]12CCC3C(CCC4=C(SCF)C(=O)CC[C@]34C)C1CCC2=O |c:8| Show InChI InChI=1S/C20H27FO2S/c1-19-10-8-16(22)18(24-11-21)15(19)4-3-12-13-5-6-17(23)20(13,2)9-7-14(12)19/h12-14H,3-11H2,1-2H3/t12?,13?,14?,19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Roussel-UCLAF
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 19A1 |
J Med Chem 35: 1588-97 (1992)
BindingDB Entry DOI: 10.7270/Q2VD7022 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50005875
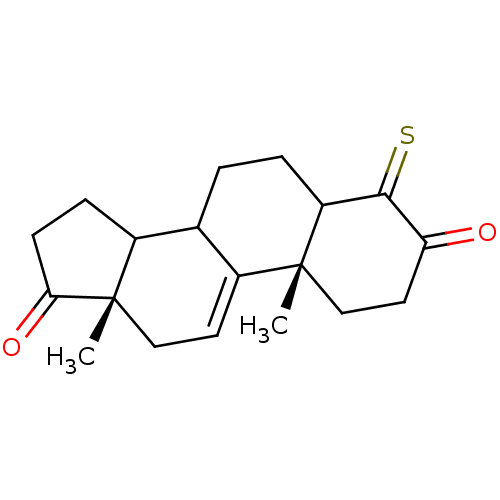
(4-Mercapto-10,13-dimethyl-1,6,7,8,10,12,13,14,15,1...)Show SMILES C[C@]12CC=C3C(CCC4C(=S)C(=O)CC[C@]34C)C1CCC2=O |c:3| Show InChI InChI=1S/C19H24O2S/c1-18-10-8-15(20)17(22)14(18)4-3-11-12-5-6-16(21)19(12,2)9-7-13(11)18/h7,11-12,14H,3-6,8-10H2,1-2H3/t11?,12?,14?,18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Roussel-UCLAF
Curated by ChEMBL
| Assay Description
Inhibition constant for human placental cytochrome P450 19A1 |
J Med Chem 35: 1588-97 (1992)
BindingDB Entry DOI: 10.7270/Q2VD7022 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50406730
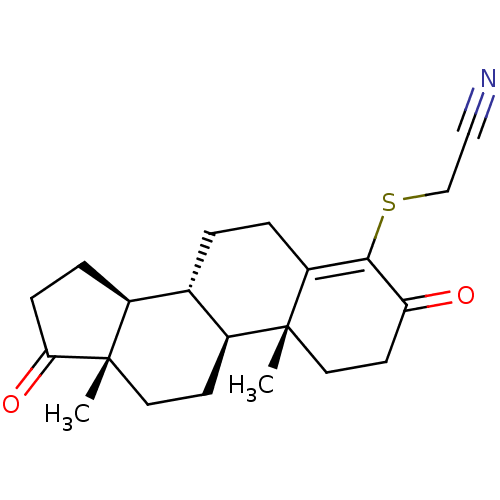
(CHEMBL2113619)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=C(SCC#N)C(=O)CC[C@]34C)[C@@H]1CCC2=O |c:8| Show InChI InChI=1S/C21H27NO2S/c1-20-10-8-17(23)19(25-12-11-22)16(20)4-3-13-14-5-6-18(24)21(14,2)9-7-15(13)20/h13-15H,3-10,12H2,1-2H3/t13-,14-,15-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Roussel-UCLAF
Curated by ChEMBL
| Assay Description
Inhibition constant for human placental Cytochrome P450 19A1 |
J Med Chem 35: 1588-97 (1992)
BindingDB Entry DOI: 10.7270/Q2VD7022 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM14691
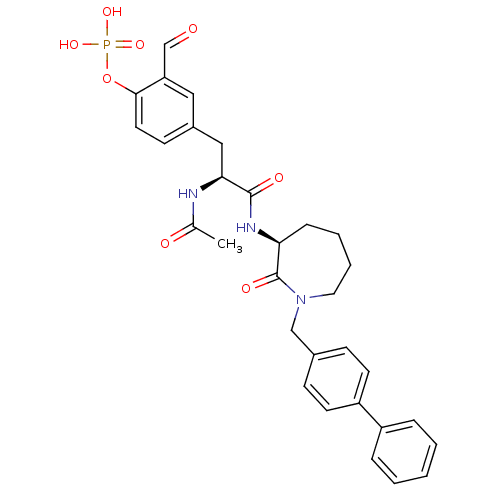
(4-[(2S)-2-acetamido-2-{[(3S)-2-oxo-1-[(4-phenylphe...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)c(C=O)c1)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2ccccc2)C1=O |r| Show InChI InChI=1S/C31H34N3O8P/c1-21(36)32-28(18-23-12-15-29(26(17-23)20-35)42-43(39,40)41)30(37)33-27-9-5-6-16-34(31(27)38)19-22-10-13-25(14-11-22)24-7-3-2-4-8-24/h2-4,7-8,10-15,17,20,27-28H,5-6,9,16,18-19H2,1H3,(H,32,36)(H,33,37)(H2,39,40,41)/t27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory concentration against SH2 domain of human p60 Src tyrosine kinase |
J Med Chem 45: 2915-22 (2002)
BindingDB Entry DOI: 10.7270/Q2ZW1MNM |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM14691

(4-[(2S)-2-acetamido-2-{[(3S)-2-oxo-1-[(4-phenylphe...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)c(C=O)c1)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2ccccc2)C1=O |r| Show InChI InChI=1S/C31H34N3O8P/c1-21(36)32-28(18-23-12-15-29(26(17-23)20-35)42-43(39,40)41)30(37)33-27-9-5-6-16-34(31(27)38)19-22-10-13-25(14-11-22)24-7-3-2-4-8-24/h2-4,7-8,10-15,17,20,27-28H,5-6,9,16,18-19H2,1H3,(H,32,36)(H,33,37)(H2,39,40,41)/t27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Binding affinity for Src SH2 domain in scintillation proximity binding assay (SPA) |
J Med Chem 45: 2379-87 (2002)
BindingDB Entry DOI: 10.7270/Q21J992G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src [145-252]
(Homo sapiens (Human)) | BDBM14691

(4-[(2S)-2-acetamido-2-{[(3S)-2-oxo-1-[(4-phenylphe...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)c(C=O)c1)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2ccccc2)C1=O |r| Show InChI InChI=1S/C31H34N3O8P/c1-21(36)32-28(18-23-12-15-29(26(17-23)20-35)42-43(39,40)41)30(37)33-27-9-5-6-16-34(31(27)38)19-22-10-13-25(14-11-22)24-7-3-2-4-8-24/h2-4,7-8,10-15,17,20,27-28H,5-6,9,16,18-19H2,1H3,(H,32,36)(H,33,37)(H2,39,40,41)/t27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
SPA uses 125I as energy donor and scintillant-coated beads as energy acceptor. The labeled ligand is captured by the biotinylated Src-SH2 protein imm... |
J Med Chem 46: 5184-95 (2003)
Article DOI: 10.1021/jm020970s
BindingDB Entry DOI: 10.7270/Q2XW4H14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50080177
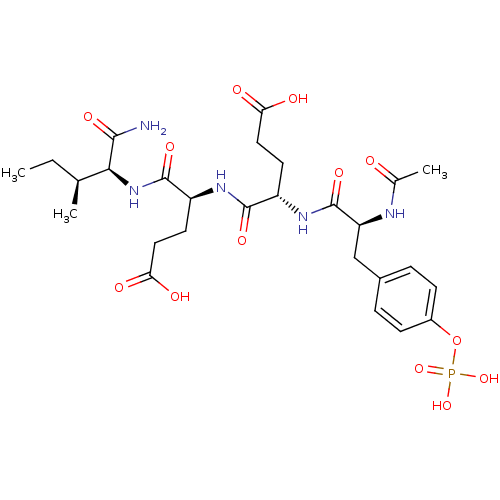
((S)-4-{(S)-2-[(S)-2-Acetylamino-3-(4-phosphonooxy-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O)C(N)=O Show InChI InChI=1S/C27H40N5O13P/c1-4-14(2)23(24(28)38)32-26(40)19(10-12-22(36)37)30-25(39)18(9-11-21(34)35)31-27(41)20(29-15(3)33)13-16-5-7-17(8-6-16)45-46(42,43)44/h5-8,14,18-20,23H,4,9-13H2,1-3H3,(H2,28,38)(H,29,33)(H,30,39)(H,31,41)(H,32,40)(H,34,35)(H,36,37)(H2,42,43,44)/t14-,18-,19-,20-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Binding affinity for Src protein tryrosine kinase SH2 domain using surface plasmon resonance (SPR) assay |
J Med Chem 45: 2379-87 (2002)
BindingDB Entry DOI: 10.7270/Q21J992G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50080177

((S)-4-{(S)-2-[(S)-2-Acetylamino-3-(4-phosphonooxy-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O)C(N)=O Show InChI InChI=1S/C27H40N5O13P/c1-4-14(2)23(24(28)38)32-26(40)19(10-12-22(36)37)30-25(39)18(9-11-21(34)35)31-27(41)20(29-15(3)33)13-16-5-7-17(8-6-16)45-46(42,43)44/h5-8,14,18-20,23H,4,9-13H2,1-3H3,(H2,28,38)(H,29,33)(H,30,39)(H,31,41)(H,32,40)(H,34,35)(H,36,37)(H2,42,43,44)/t14-,18-,19-,20-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Binding affinity for Src protein tryrosine kinase SH2 domain using surface plasmon resonance (SPR) assay |
J Med Chem 45: 2379-87 (2002)
BindingDB Entry DOI: 10.7270/Q21J992G |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50366682
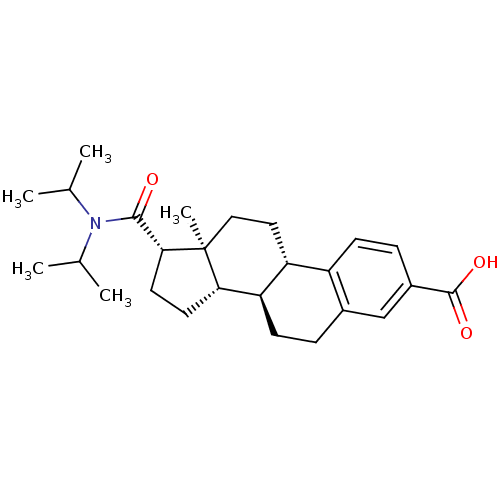
(CHEMBL1627395)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CC[C@H]2[C@@H]3CCc4cc(ccc4[C@H]3CC[C@]12C)C(O)=O Show InChI InChI=1S/C26H37NO3/c1-15(2)27(16(3)4)24(28)23-11-10-22-21-9-6-17-14-18(25(29)30)7-8-19(17)20(21)12-13-26(22,23)5/h7-8,14-16,20-23H,6,9-13H2,1-5H3,(H,29,30)/t20-,21-,22+,23-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of type 2 5-alpha-reductase of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50112427
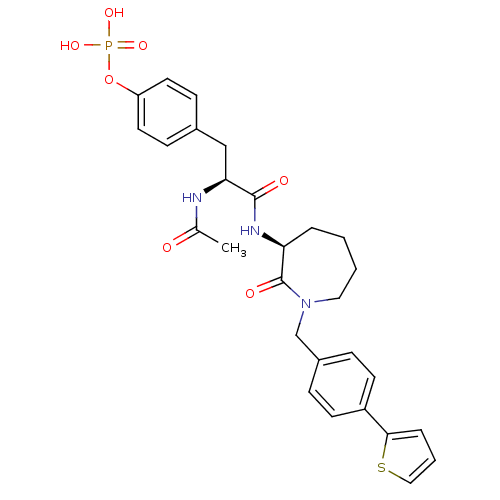
(CHEMBL281775 | Phosphoric acid mono-(4-{2-acetylam...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)cc1)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2cccs2)C1=O Show InChI InChI=1S/C28H32N3O7PS/c1-19(32)29-25(17-20-9-13-23(14-10-20)38-39(35,36)37)27(33)30-24-5-2-3-15-31(28(24)34)18-21-7-11-22(12-8-21)26-6-4-16-40-26/h4,6-14,16,24-25H,2-3,5,15,17-18H2,1H3,(H,29,32)(H,30,33)(H2,35,36,37)/t24-,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Binding affinity towards p60 Src tyrosine kinase using scintillation proximity assay |
Bioorg Med Chem Lett 12: 1291-4 (2002)
BindingDB Entry DOI: 10.7270/Q2DZ07MZ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src [145-252]
(Homo sapiens (Human)) | BDBM14690
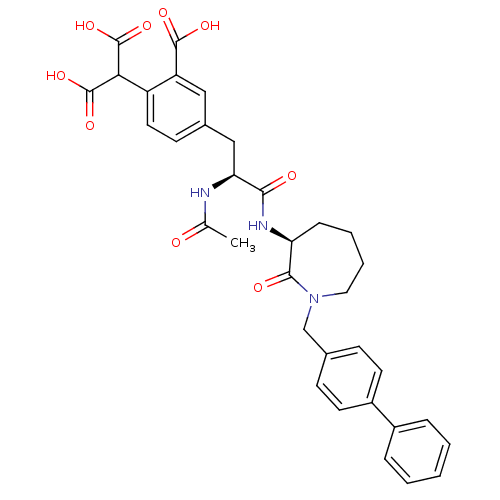
(2-{2-carboxy-4-[(2S)-2-acetamido-2-{[(3S)-2-oxo-1-...)Show SMILES CC(=O)N[C@@H](Cc1ccc(C(C(O)=O)C(O)=O)c(c1)C(O)=O)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2ccccc2)C1=O |r| Show InChI InChI=1S/C34H35N3O9/c1-20(38)35-28(18-22-12-15-25(26(17-22)32(41)42)29(33(43)44)34(45)46)30(39)36-27-9-5-6-16-37(31(27)40)19-21-10-13-24(14-11-21)23-7-3-2-4-8-23/h2-4,7-8,10-15,17,27-29H,5-6,9,16,18-19H2,1H3,(H,35,38)(H,36,39)(H,41,42)(H,43,44)(H,45,46)/t27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
SPA uses 125I as energy donor and scintillant-coated beads as energy acceptor. The labeled ligand is captured by the biotinylated Src-SH2 protein imm... |
J Med Chem 46: 5184-95 (2003)
Article DOI: 10.1021/jm020970s
BindingDB Entry DOI: 10.7270/Q2XW4H14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM14690

(2-{2-carboxy-4-[(2S)-2-acetamido-2-{[(3S)-2-oxo-1-...)Show SMILES CC(=O)N[C@@H](Cc1ccc(C(C(O)=O)C(O)=O)c(c1)C(O)=O)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2ccccc2)C1=O |r| Show InChI InChI=1S/C34H35N3O9/c1-20(38)35-28(18-22-12-15-25(26(17-22)32(41)42)29(33(43)44)34(45)46)30(39)36-27-9-5-6-16-37(31(27)40)19-21-10-13-24(14-11-21)23-7-3-2-4-8-23/h2-4,7-8,10-15,17,27-29H,5-6,9,16,18-19H2,1H3,(H,35,38)(H,36,39)(H,41,42)(H,43,44)(H,45,46)/t27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory concentration against SH2 domain of human p60 Src tyrosine kinase |
J Med Chem 45: 2915-22 (2002)
BindingDB Entry DOI: 10.7270/Q2ZW1MNM |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src [145-252]
(Homo sapiens (Human)) | BDBM14693

(({4-[(2S)-2-acetamido-2-{[(3S)-2-oxo-1-[(4-phenylp...)Show SMILES CC(=O)N[C@@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2ccccc2)C1=O |r| Show InChI InChI=1S/C31H34F2N3O6P/c1-21(37)34-28(19-22-12-16-26(17-13-22)31(32,33)43(40,41)42)29(38)35-27-9-5-6-18-36(30(27)39)20-23-10-14-25(15-11-23)24-7-3-2-4-8-24/h2-4,7-8,10-17,27-28H,5-6,9,18-20H2,1H3,(H,34,37)(H,35,38)(H2,40,41,42)/t27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
SPA uses 125I as energy donor and scintillant-coated beads as energy acceptor. The labeled ligand is captured by the biotinylated Src-SH2 protein imm... |
J Med Chem 46: 5184-95 (2003)
Article DOI: 10.1021/jm020970s
BindingDB Entry DOI: 10.7270/Q2XW4H14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM14690

(2-{2-carboxy-4-[(2S)-2-acetamido-2-{[(3S)-2-oxo-1-...)Show SMILES CC(=O)N[C@@H](Cc1ccc(C(C(O)=O)C(O)=O)c(c1)C(O)=O)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2ccccc2)C1=O |r| Show InChI InChI=1S/C34H35N3O9/c1-20(38)35-28(18-22-12-15-25(26(17-22)32(41)42)29(33(43)44)34(45)46)30(39)36-27-9-5-6-16-37(31(27)40)19-21-10-13-24(14-11-21)23-7-3-2-4-8-23/h2-4,7-8,10-15,17,27-29H,5-6,9,16,18-19H2,1H3,(H,35,38)(H,36,39)(H,41,42)(H,43,44)(H,45,46)/t27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Binding affinity towards Src protein tryrosine kinase SH2 domain using scintillation proximity binding assay (SPA) |
J Med Chem 45: 2379-87 (2002)
BindingDB Entry DOI: 10.7270/Q21J992G |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313678

(CHEMBL1093747 | N-(5-bromo-6-(4-hydroxyphenyl)-1H-...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(c(Br)cc12)-c1ccc(O)cc1 Show InChI InChI=1S/C17H16BrN3O2/c1-2-3-16(23)19-17-13-8-14(18)12(9-15(13)20-21-17)10-4-6-11(22)7-5-10/h4-9,22H,2-3H2,1H3,(H2,19,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta using gamma[33P]-ATP after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1985-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.114
BindingDB Entry DOI: 10.7270/Q2QN66XJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM14693

(({4-[(2S)-2-acetamido-2-{[(3S)-2-oxo-1-[(4-phenylp...)Show SMILES CC(=O)N[C@@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2ccccc2)C1=O |r| Show InChI InChI=1S/C31H34F2N3O6P/c1-21(37)34-28(19-22-12-16-26(17-13-22)31(32,33)43(40,41)42)29(38)35-27-9-5-6-18-36(30(27)39)20-23-10-14-25(15-11-23)24-7-3-2-4-8-24/h2-4,7-8,10-17,27-28H,5-6,9,18-20H2,1H3,(H,34,37)(H,35,38)(H2,40,41,42)/t27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory concentration against SH2 domain of human p60 Src tyrosine kinase |
J Med Chem 45: 2915-22 (2002)
BindingDB Entry DOI: 10.7270/Q2ZW1MNM |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313696
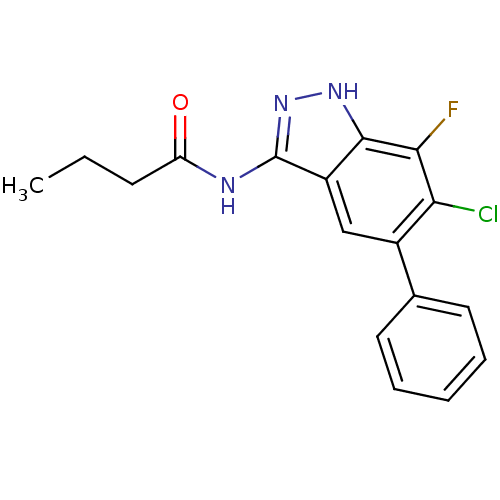
(CHEMBL1086427 | N-(6-chloro-7-fluoro-5-phenyl-1H-i...)Show SMILES CCCC(=O)Nc1n[nH]c2c(F)c(Cl)c(cc12)-c1ccccc1 Show InChI InChI=1S/C17H15ClFN3O/c1-2-6-13(23)20-17-12-9-11(10-7-4-3-5-8-10)14(18)15(19)16(12)21-22-17/h3-5,7-9H,2,6H2,1H3,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta using gamma[33P]-ATP after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1985-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.114
BindingDB Entry DOI: 10.7270/Q2QN66XJ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50340502

(CHEMBL1762178 | N-(4-((5,5-dimethyl-2,4-dioxo-3-(4...)Show SMILES CC(=O)Nc1cc(CN2C(=O)N(C(=O)C2(C)C)c2ccc(SC(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C20H19F3N4O3S/c1-12(28)25-16-10-13(8-9-24-16)11-26-18(30)27(17(29)19(26,2)3)14-4-6-15(7-5-14)31-20(21,22)23/h4-10H,11H2,1-3H3,(H,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of IGF-induced autophosphosphorylation of GST-tagged human IGF1R expressed in baculovirus-SF21 cell system preincubated for 60 mins by tim... |
Bioorg Med Chem Lett 21: 2224-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.003
BindingDB Entry DOI: 10.7270/Q2CC11PK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50113760

(2-{4-[2-Acetylamino-2-(1-biphenyl-4-ylmethyl-2-oxo...)Show SMILES COC(=O)c1cc(C[C@@H](NC(C)=O)C(=O)N[C@H]2CCCCN(Cc3ccc(cc3)-c3ccccc3)C2=O)ccc1C(C(O)=O)C(O)=O Show InChI InChI=1S/C35H37N3O9/c1-21(39)36-29(19-23-13-16-26(27(18-23)35(46)47-2)30(33(42)43)34(44)45)31(40)37-28-10-6-7-17-38(32(28)41)20-22-11-14-25(15-12-22)24-8-4-3-5-9-24/h3-5,8-9,11-16,18,28-30H,6-7,10,17,19-20H2,1-2H3,(H,36,39)(H,37,40)(H,42,43)(H,44,45)/t28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Binding affinity towards Src protein tryrosine kinase SH2 domain using surface plasmon resonance (SPR) assay |
J Med Chem 45: 2379-87 (2002)
BindingDB Entry DOI: 10.7270/Q21J992G |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313661
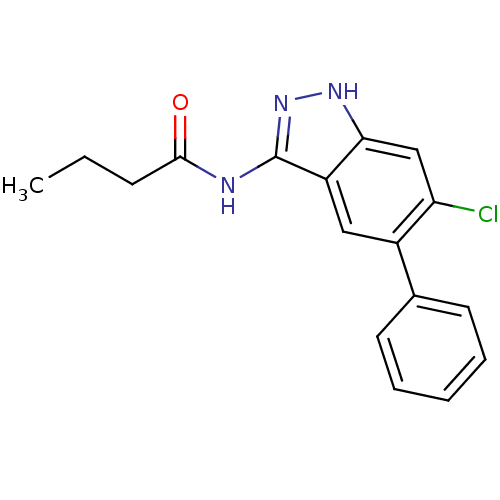
(CHEMBL1095040 | N-(6-chloro-5-phenyl-1H-indazol-3-...)Show InChI InChI=1S/C17H16ClN3O/c1-2-6-16(22)19-17-13-9-12(11-7-4-3-5-8-11)14(18)10-15(13)20-21-17/h3-5,7-10H,2,6H2,1H3,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta using gamma[33P]-ATP after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1985-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.114
BindingDB Entry DOI: 10.7270/Q2QN66XJ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50340502

(CHEMBL1762178 | N-(4-((5,5-dimethyl-2,4-dioxo-3-(4...)Show SMILES CC(=O)Nc1cc(CN2C(=O)N(C(=O)C2(C)C)c2ccc(SC(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C20H19F3N4O3S/c1-12(28)25-16-10-13(8-9-24-16)11-26-18(30)27(17(29)19(26,2)3)14-4-6-15(7-5-14)31-20(21,22)23/h4-10H,11H2,1-3H3,(H,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of autophosphosphorylation of human IGF1R expressed in IGF1-stimulated MCF7 cells preincubated for 60 mins by ELISA |
Bioorg Med Chem Lett 21: 2224-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.003
BindingDB Entry DOI: 10.7270/Q2CC11PK |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50340502

(CHEMBL1762178 | N-(4-((5,5-dimethyl-2,4-dioxo-3-(4...)Show SMILES CC(=O)Nc1cc(CN2C(=O)N(C(=O)C2(C)C)c2ccc(SC(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C20H19F3N4O3S/c1-12(28)25-16-10-13(8-9-24-16)11-26-18(30)27(17(29)19(26,2)3)14-4-6-15(7-5-14)31-20(21,22)23/h4-10H,11H2,1-3H3,(H,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of autophosphosphorylation of human IGF1R expressed in IGF1-stimulated MCF7 cells by ELISA |
Bioorg Med Chem Lett 21: 2224-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.003
BindingDB Entry DOI: 10.7270/Q2CC11PK |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313662
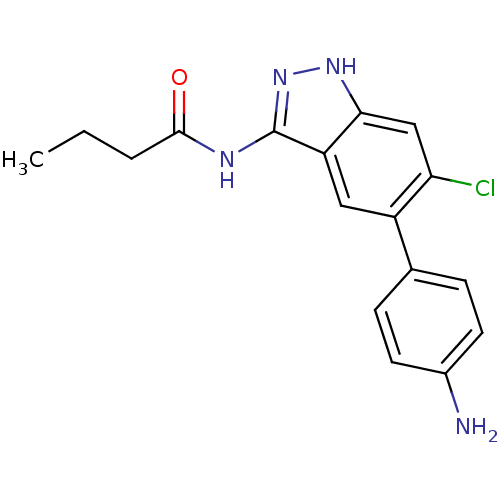
(CHEMBL1095041 | N-(5-(4-aminophenyl)-6-chloro-1H-i...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(Cl)c(cc12)-c1ccc(N)cc1 Show InChI InChI=1S/C17H17ClN4O/c1-2-3-16(23)20-17-13-8-12(10-4-6-11(19)7-5-10)14(18)9-15(13)21-22-17/h4-9H,2-3,19H2,1H3,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta using gamma[33P]-ATP after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1985-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.114
BindingDB Entry DOI: 10.7270/Q2QN66XJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50074242
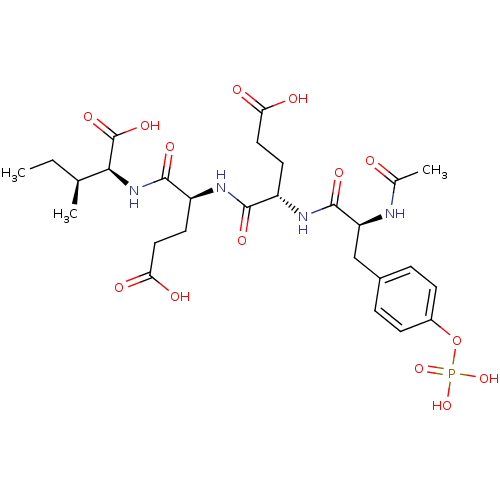
((2S,3S)-2-((S)-2-{(S)-2-[(S)-2-Acetylamino-3-(4-ph...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O)C(O)=O Show InChI InChI=1S/C27H39N4O14P/c1-4-14(2)23(27(40)41)31-25(38)19(10-12-22(35)36)29-24(37)18(9-11-21(33)34)30-26(39)20(28-15(3)32)13-16-5-7-17(8-6-16)45-46(42,43)44/h5-8,14,18-20,23H,4,9-13H2,1-3H3,(H,28,32)(H,29,37)(H,30,39)(H,31,38)(H,33,34)(H,35,36)(H,40,41)(H2,42,43,44)/t14-,18-,19-,20-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Binding affinity for Src SH2 domain in scintillation proximity binding assay (SPA) |
J Med Chem 45: 2379-87 (2002)
BindingDB Entry DOI: 10.7270/Q21J992G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM14689
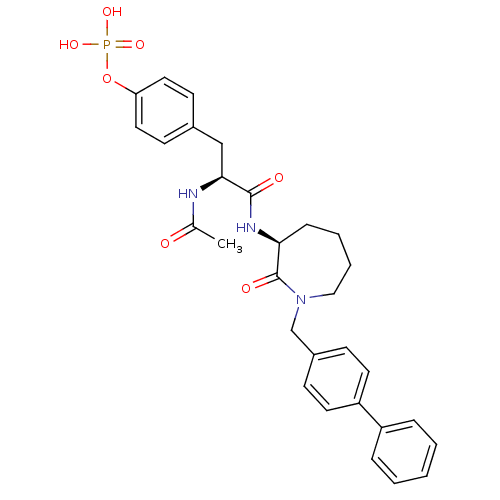
(4-[(2S)-2-acetamido-2-{[(3S)-2-oxo-1-[(4-phenylphe...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)cc1)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2ccccc2)C1=O |r| Show InChI InChI=1S/C30H34N3O7P/c1-21(34)31-28(19-22-12-16-26(17-13-22)40-41(37,38)39)29(35)32-27-9-5-6-18-33(30(27)36)20-23-10-14-25(15-11-23)24-7-3-2-4-8-24/h2-4,7-8,10-17,27-28H,5-6,9,18-20H2,1H3,(H,31,34)(H,32,35)(H2,37,38,39)/t27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory concentration against SH2 domain of human p60 Src tyrosine kinase |
J Med Chem 45: 2915-22 (2002)
BindingDB Entry DOI: 10.7270/Q2ZW1MNM |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM14689

(4-[(2S)-2-acetamido-2-{[(3S)-2-oxo-1-[(4-phenylphe...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)cc1)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2ccccc2)C1=O |r| Show InChI InChI=1S/C30H34N3O7P/c1-21(34)31-28(19-22-12-16-26(17-13-22)40-41(37,38)39)29(35)32-27-9-5-6-18-33(30(27)36)20-23-10-14-25(15-11-23)24-7-3-2-4-8-24/h2-4,7-8,10-17,27-28H,5-6,9,18-20H2,1H3,(H,31,34)(H,32,35)(H2,37,38,39)/t27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Binding affinity towards p60 Src tyrosine kinase using scintillation proximity assay |
Bioorg Med Chem Lett 12: 1291-4 (2002)
BindingDB Entry DOI: 10.7270/Q2DZ07MZ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src [145-252]
(Homo sapiens (Human)) | BDBM14689

(4-[(2S)-2-acetamido-2-{[(3S)-2-oxo-1-[(4-phenylphe...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)cc1)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2ccccc2)C1=O |r| Show InChI InChI=1S/C30H34N3O7P/c1-21(34)31-28(19-22-12-16-26(17-13-22)40-41(37,38)39)29(35)32-27-9-5-6-18-33(30(27)36)20-23-10-14-25(15-11-23)24-7-3-2-4-8-24/h2-4,7-8,10-17,27-28H,5-6,9,18-20H2,1H3,(H,31,34)(H,32,35)(H2,37,38,39)/t27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
SPA uses 125I as energy donor and scintillant-coated beads as energy acceptor. The labeled ligand is captured by the biotinylated Src-SH2 protein imm... |
J Med Chem 46: 5184-95 (2003)
Article DOI: 10.1021/jm020970s
BindingDB Entry DOI: 10.7270/Q2XW4H14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50101143
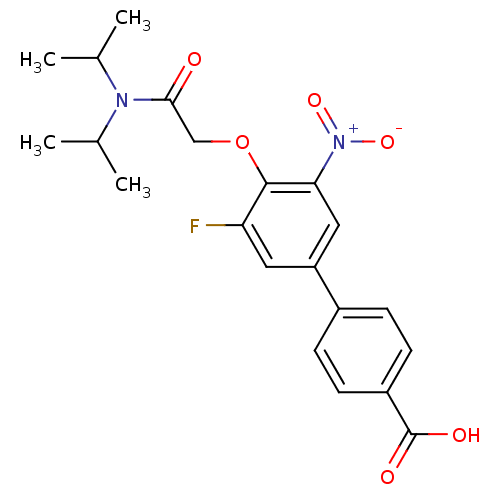
(4'-[(Diisopropylcarbamoyl)-methoxy]-5'-fluoro-3'-n...)Show SMILES CC(C)N(C(C)C)C(=O)COc1c(F)cc(cc1[N+]([O-])=O)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C21H23FN2O6/c1-12(2)23(13(3)4)19(25)11-30-20-17(22)9-16(10-18(20)24(28)29)14-5-7-15(8-6-14)21(26)27/h5-10,12-13H,11H2,1-4H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of type 2 5-alpha-reductase of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src [145-252]
(Homo sapiens (Human)) | BDBM14692

(2-{4-[(2S)-2-acetamido-2-{[(3S)-2-oxo-1-[(4-phenyl...)Show SMILES COC(=O)c1cc(C[C@H](NC(C)=O)C(=O)N[C@H]2CCCCN(Cc3ccc(cc3)-c3ccccc3)C2=O)ccc1C(F)(C(O)=O)C(O)=O |r| Show InChI InChI=1S/C35H36FN3O9/c1-21(40)37-29(19-23-13-16-27(26(18-23)32(43)48-2)35(36,33(44)45)34(46)47)30(41)38-28-10-6-7-17-39(31(28)42)20-22-11-14-25(15-12-22)24-8-4-3-5-9-24/h3-5,8-9,11-16,18,28-29H,6-7,10,17,19-20H2,1-2H3,(H,37,40)(H,38,41)(H,44,45)(H,46,47)/t28-,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
SPA uses 125I as energy donor and scintillant-coated beads as energy acceptor. The labeled ligand is captured by the biotinylated Src-SH2 protein imm... |
J Med Chem 46: 5184-95 (2003)
Article DOI: 10.1021/jm020970s
BindingDB Entry DOI: 10.7270/Q2XW4H14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313663
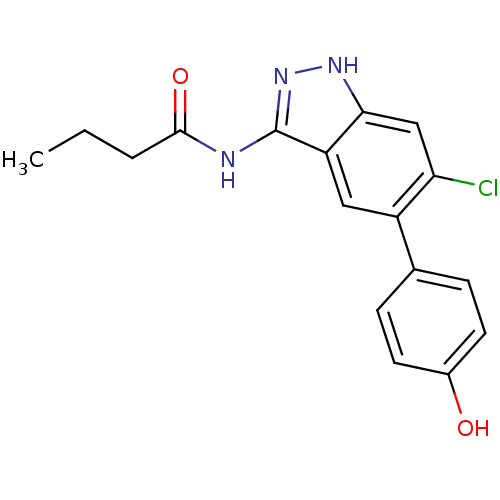
(CHEMBL1095366 | N-(6-chloro-5-(4-hydroxyphenyl)-1H...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(Cl)c(cc12)-c1ccc(O)cc1 Show InChI InChI=1S/C17H16ClN3O2/c1-2-3-16(23)19-17-13-8-12(10-4-6-11(22)7-5-10)14(18)9-15(13)20-21-17/h4-9,22H,2-3H2,1H3,(H2,19,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta using gamma[33P]-ATP after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1985-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.114
BindingDB Entry DOI: 10.7270/Q2QN66XJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313664
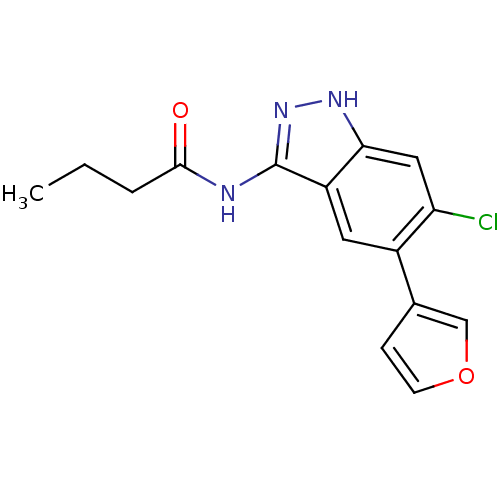
(CHEMBL1077218 | N-(6-chloro-5-(furan-3-yl)-1H-inda...)Show InChI InChI=1S/C15H14ClN3O2/c1-2-3-14(20)17-15-11-6-10(9-4-5-21-8-9)12(16)7-13(11)18-19-15/h4-8H,2-3H2,1H3,(H2,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta using gamma[33P]-ATP after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1985-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.114
BindingDB Entry DOI: 10.7270/Q2QN66XJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313665
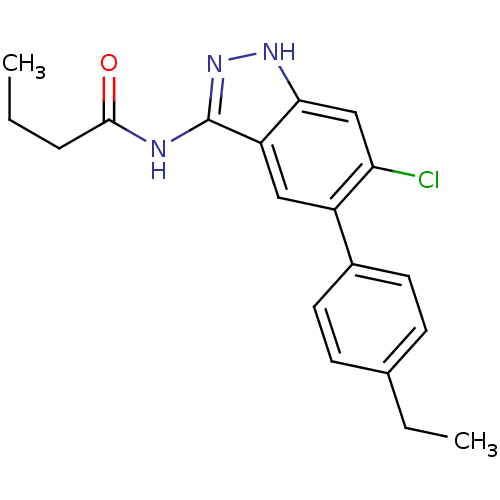
(CHEMBL1077213 | N-(6-chloro-5-(4-ethylphenyl)-1H-i...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(Cl)c(cc12)-c1ccc(CC)cc1 Show InChI InChI=1S/C19H20ClN3O/c1-3-5-18(24)21-19-15-10-14(16(20)11-17(15)22-23-19)13-8-6-12(4-2)7-9-13/h6-11H,3-5H2,1-2H3,(H2,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta using gamma[33P]-ATP after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1985-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.114
BindingDB Entry DOI: 10.7270/Q2QN66XJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313697
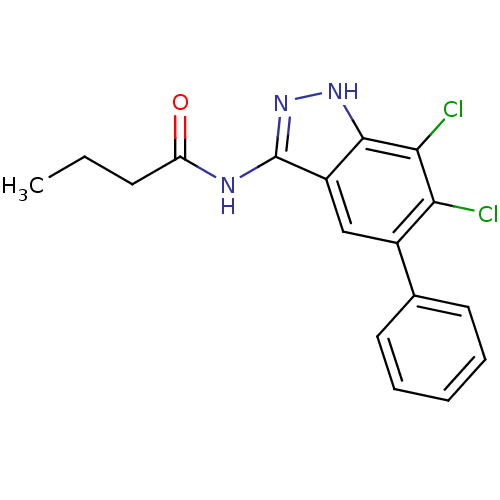
(CHEMBL1097003 | N-(6,7-dichloro-5-phenyl-1H-indazo...)Show SMILES CCCC(=O)Nc1n[nH]c2c(Cl)c(Cl)c(cc12)-c1ccccc1 Show InChI InChI=1S/C17H15Cl2N3O/c1-2-6-13(23)20-17-12-9-11(10-7-4-3-5-8-10)14(18)15(19)16(12)21-22-17/h3-5,7-9H,2,6H2,1H3,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta using gamma[33P]-ATP after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1985-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.114
BindingDB Entry DOI: 10.7270/Q2QN66XJ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10045
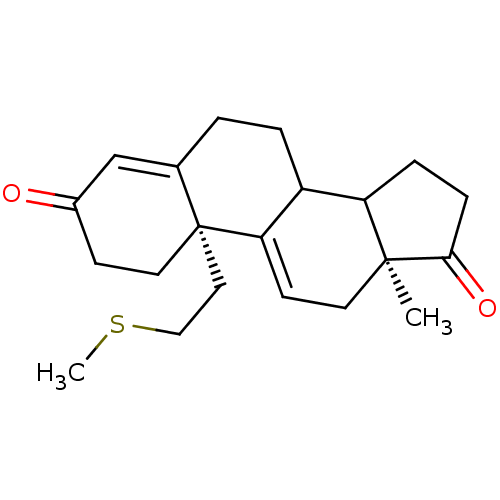
((2R,15S)-15-methyl-2-[2-(methylsulfanyl)ethyl]tetr...)Show SMILES CSCC[C@]12CCC(=O)C=C1CCC1C3CCC(=O)[C@@]3(C)CC=C21 |r,c:9,t:24| Show InChI InChI=1S/C21H28O2S/c1-20-9-8-18-16(17(20)5-6-19(20)23)4-3-14-13-15(22)7-10-21(14,18)11-12-24-2/h8,13,16-17H,3-7,9-12H2,1-2H3/t16?,17?,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf
| Assay Description
The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... |
J Med Chem 39: 757-72 (1996)
Article DOI: 10.1021/jm950539l
BindingDB Entry DOI: 10.7270/Q28C9TGH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313670

(CHEMBL1089723 | N-(5-(furan-2-yl)-6-(4-hydroxyphen...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(-c3ccc(O)cc3)c(cc12)-c1ccco1 Show InChI InChI=1S/C21H19N3O3/c1-2-4-20(26)22-21-17-11-16(19-5-3-10-27-19)15(12-18(17)23-24-21)13-6-8-14(25)9-7-13/h3,5-12,25H,2,4H2,1H3,(H2,22,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta using gamma[33P]-ATP after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1985-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.114
BindingDB Entry DOI: 10.7270/Q2QN66XJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313666
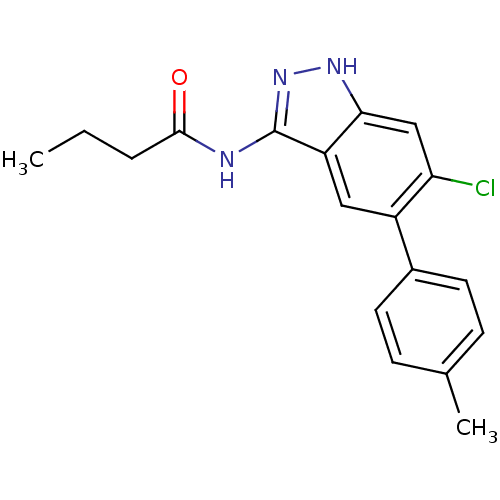
(CHEMBL1097694 | N-(6-chloro-5-p-tolyl-1H-indazol-3...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(Cl)c(cc12)-c1ccc(C)cc1 Show InChI InChI=1S/C18H18ClN3O/c1-3-4-17(23)20-18-14-9-13(12-7-5-11(2)6-8-12)15(19)10-16(14)21-22-18/h5-10H,3-4H2,1-2H3,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta using gamma[33P]-ATP after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1985-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.114
BindingDB Entry DOI: 10.7270/Q2QN66XJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313667

(CHEMBL1091081 | N-(6-chloro-5-(4-nitrophenyl)-1H-i...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(Cl)c(cc12)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C17H15ClN4O3/c1-2-3-16(23)19-17-13-8-12(14(18)9-15(13)20-21-17)10-4-6-11(7-5-10)22(24)25/h4-9H,2-3H2,1H3,(H2,19,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta using gamma[33P]-ATP after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1985-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.114
BindingDB Entry DOI: 10.7270/Q2QN66XJ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10046
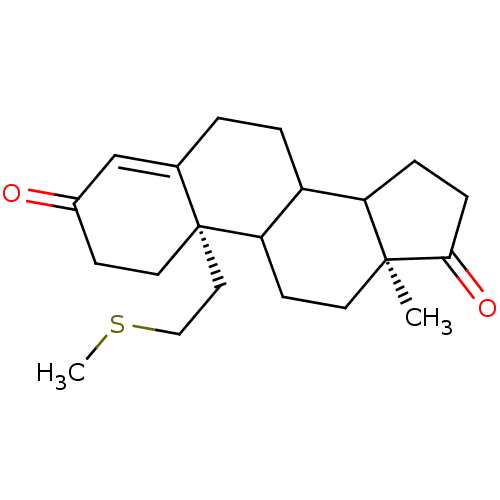
((2S,15S)-15-methyl-2-[2-(methylsulfanyl)ethyl]tetr...)Show SMILES CSCC[C@]12CCC(=O)C=C1CCC1C3CCC(=O)[C@@]3(C)CCC21 |r,c:9| Show InChI InChI=1S/C21H30O2S/c1-20-9-8-18-16(17(20)5-6-19(20)23)4-3-14-13-15(22)7-10-21(14,18)11-12-24-2/h13,16-18H,3-12H2,1-2H3/t16?,17?,18?,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf
| Assay Description
The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... |
J Med Chem 39: 757-72 (1996)
Article DOI: 10.1021/jm950539l
BindingDB Entry DOI: 10.7270/Q28C9TGH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313668
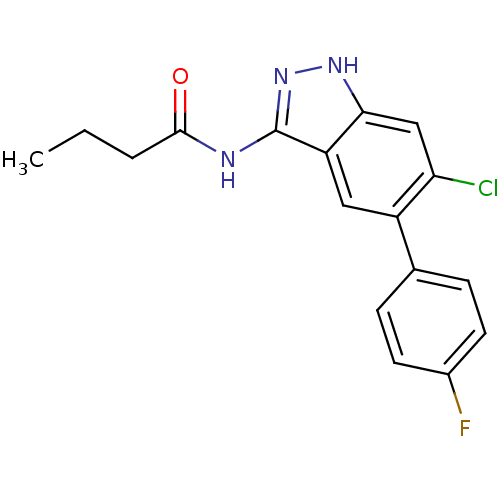
(CHEMBL1091082 | N-(6-chloro-5-(4-fluorophenyl)-1H-...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(Cl)c(cc12)-c1ccc(F)cc1 Show InChI InChI=1S/C17H15ClFN3O/c1-2-3-16(23)20-17-13-8-12(10-4-6-11(19)7-5-10)14(18)9-15(13)21-22-17/h4-9H,2-3H2,1H3,(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta using gamma[33P]-ATP after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1985-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.114
BindingDB Entry DOI: 10.7270/Q2QN66XJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313669

(CHEMBL1091083 | N-(5-(4-(benzyloxy)phenyl)-6-chlor...)Show SMILES CCCC(=O)Nc1n[nH]c2cc(Cl)c(cc12)-c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C24H22ClN3O2/c1-2-6-23(29)26-24-20-13-19(21(25)14-22(20)27-28-24)17-9-11-18(12-10-17)30-15-16-7-4-3-5-8-16/h3-5,7-14H,2,6,15H2,1H3,(H2,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta using gamma[33P]-ATP after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1985-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.114
BindingDB Entry DOI: 10.7270/Q2QN66XJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50112418
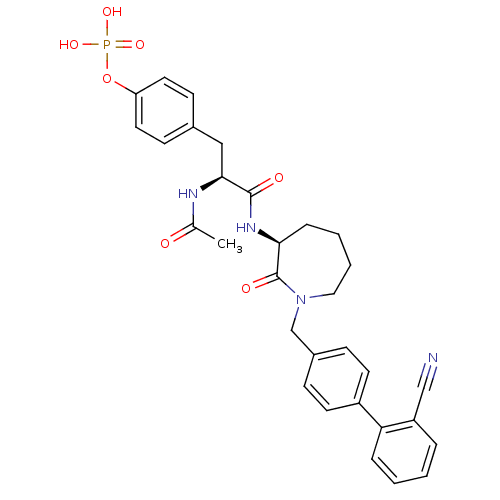
(CHEMBL433046 | Phosphoric acid mono-(4-{2-acetylam...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)cc1)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2ccccc2C#N)C1=O Show InChI InChI=1S/C31H33N4O7P/c1-21(36)33-29(18-22-11-15-26(16-12-22)42-43(39,40)41)30(37)34-28-8-4-5-17-35(31(28)38)20-23-9-13-24(14-10-23)27-7-3-2-6-25(27)19-32/h2-3,6-7,9-16,28-29H,4-5,8,17-18,20H2,1H3,(H,33,36)(H,34,37)(H2,39,40,41)/t28-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Binding affinity towards p60 Src tyrosine kinase using scintillation proximity assay |
Bioorg Med Chem Lett 12: 1291-4 (2002)
BindingDB Entry DOI: 10.7270/Q2DZ07MZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313703
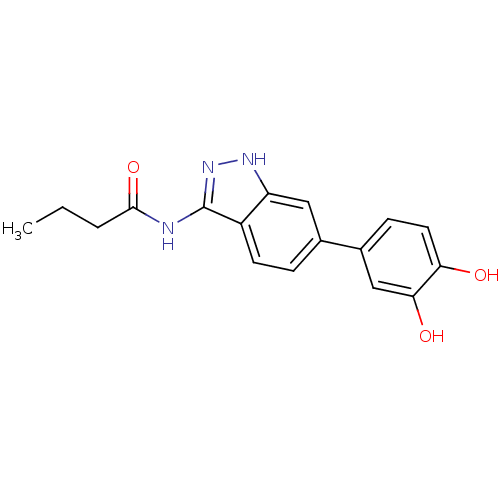
(CHEMBL1082236 | N-(6-(3,4-dihydroxyphenyl)-1H-inda...)Show InChI InChI=1S/C17H17N3O3/c1-2-3-16(23)18-17-12-6-4-10(8-13(12)19-20-17)11-5-7-14(21)15(22)9-11/h4-9,21-22H,2-3H2,1H3,(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta using gamma[33P]-ATP after 30 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1985-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.114
BindingDB Entry DOI: 10.7270/Q2QN66XJ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50340502

(CHEMBL1762178 | N-(4-((5,5-dimethyl-2,4-dioxo-3-(4...)Show SMILES CC(=O)Nc1cc(CN2C(=O)N(C(=O)C2(C)C)c2ccc(SC(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C20H19F3N4O3S/c1-12(28)25-16-10-13(8-9-24-16)11-26-18(30)27(17(29)19(26,2)3)14-4-6-15(7-5-14)31-20(21,22)23/h4-10H,11H2,1-3H3,(H,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of IGF-induced autophosphosphorylation of GST-tagged human IGF1R expressed in baculovirus-SF21 cell system preincubated for 30 mins by tim... |
Bioorg Med Chem Lett 21: 2224-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.003
BindingDB Entry DOI: 10.7270/Q2CC11PK |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313664

(CHEMBL1077218 | N-(6-chloro-5-(furan-3-yl)-1H-inda...)Show InChI InChI=1S/C15H14ClN3O2/c1-2-3-14(20)17-15-11-6-10(9-4-5-21-8-9)12(16)7-13(11)18-19-15/h4-8H,2-3H2,1H3,(H2,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta by kinetic assay |
Bioorg Med Chem Lett 20: 2344-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.132
BindingDB Entry DOI: 10.7270/Q2SB45WF |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50340513
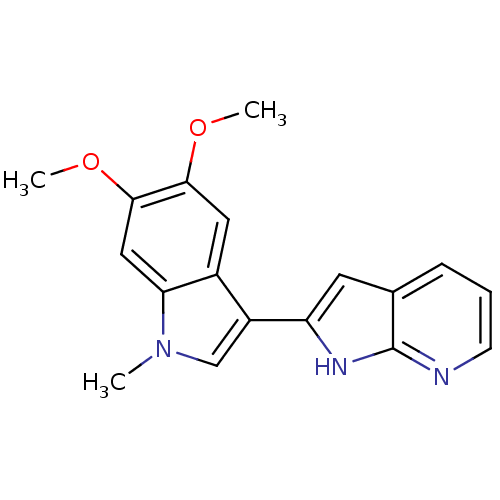
(2-(5,6-dimethoxy-1-methyl-1H-indol-3-yl)-1H-pyrrol...)Show InChI InChI=1S/C18H17N3O2/c1-21-10-13(14-7-11-5-4-6-19-18(11)20-14)12-8-16(22-2)17(23-3)9-15(12)21/h4-10H,1-3H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of IGF-induced autophosphosphorylation of GST-tagged human IGF1R expressed in baculovirus-SF21 cell system preincubated for 30 mins by tim... |
Bioorg Med Chem Lett 21: 2224-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.003
BindingDB Entry DOI: 10.7270/Q2CC11PK |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50340513

(2-(5,6-dimethoxy-1-methyl-1H-indol-3-yl)-1H-pyrrol...)Show InChI InChI=1S/C18H17N3O2/c1-21-10-13(14-7-11-5-4-6-19-18(11)20-14)12-8-16(22-2)17(23-3)9-15(12)21/h4-10H,1-3H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of IGF-induced autophosphosphorylation of GST-tagged human IGF1R expressed in baculovirus-SF21 cell system preincubated for 10 mins by tim... |
Bioorg Med Chem Lett 21: 2224-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.003
BindingDB Entry DOI: 10.7270/Q2CC11PK |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50340503
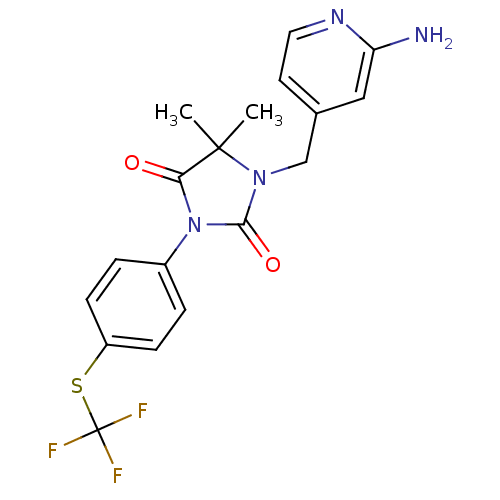
(1-((2-aminopyridin-4-yl)methyl)-5,5-dimethyl-3-(4-...)Show SMILES CC1(C)N(Cc2ccnc(N)c2)C(=O)N(C1=O)c1ccc(SC(F)(F)F)cc1 Show InChI InChI=1S/C18H17F3N4O2S/c1-17(2)15(26)25(12-3-5-13(6-4-12)28-18(19,20)21)16(27)24(17)10-11-7-8-23-14(22)9-11/h3-9H,10H2,1-2H3,(H2,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis
Curated by ChEMBL
| Assay Description
Inhibition of autophosphosphorylation of human IGF1R expressed in IGF1-stimulated MCF7 cells by ELISA |
Bioorg Med Chem Lett 21: 2224-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.003
BindingDB Entry DOI: 10.7270/Q2CC11PK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data