 Found 475 hits with Last Name = 'liao' and Initial = 'm'
Found 475 hits with Last Name = 'liao' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM370121
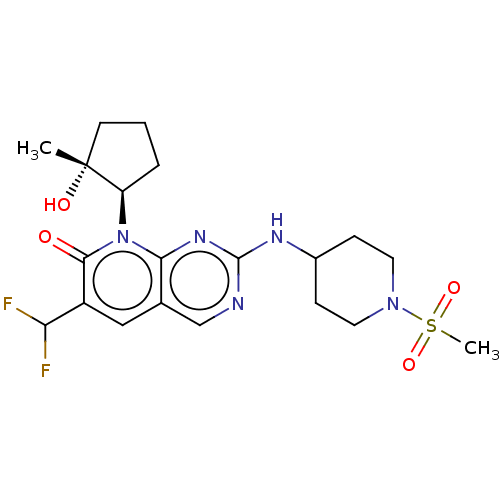
(6-(difluoromethyl)-8-[(1R,2R)-2-hydroxy-2-methylcy...)Show SMILES C[C@@]1(O)CCC[C@H]1n1c2nc(NC3CCN(CC3)S(C)(=O)=O)ncc2cc(C(F)F)c1=O |r| Show InChI InChI=1S/C20H27F2N5O4S/c1-20(29)7-3-4-15(20)27-17-12(10-14(16(21)22)18(27)28)11-23-19(25-17)24-13-5-8-26(9-6-13)32(2,30)31/h10-11,13,15-16,29H,3-9H2,1-2H3,(H,23,24,25)/t15-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM370121

(6-(difluoromethyl)-8-[(1R,2R)-2-hydroxy-2-methylcy...)Show SMILES C[C@@]1(O)CCC[C@H]1n1c2nc(NC3CCN(CC3)S(C)(=O)=O)ncc2cc(C(F)F)c1=O |r| Show InChI InChI=1S/C20H27F2N5O4S/c1-20(29)7-3-4-15(20)27-17-12(10-14(16(21)22)18(27)28)11-23-19(25-17)24-13-5-8-26(9-6-13)32(2,30)31/h10-11,13,15-16,29H,3-9H2,1-2H3,(H,23,24,25)/t15-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6
(Homo sapiens (Human)) | BDBM370121

(6-(difluoromethyl)-8-[(1R,2R)-2-hydroxy-2-methylcy...)Show SMILES C[C@@]1(O)CCC[C@H]1n1c2nc(NC3CCN(CC3)S(C)(=O)=O)ncc2cc(C(F)F)c1=O |r| Show InChI InChI=1S/C20H27F2N5O4S/c1-20(29)7-3-4-15(20)27-17-12(10-14(16(21)22)18(27)28)11-23-19(25-17)24-13-5-8-26(9-6-13)32(2,30)31/h10-11,13,15-16,29H,3-9H2,1-2H3,(H,23,24,25)/t15-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM209932
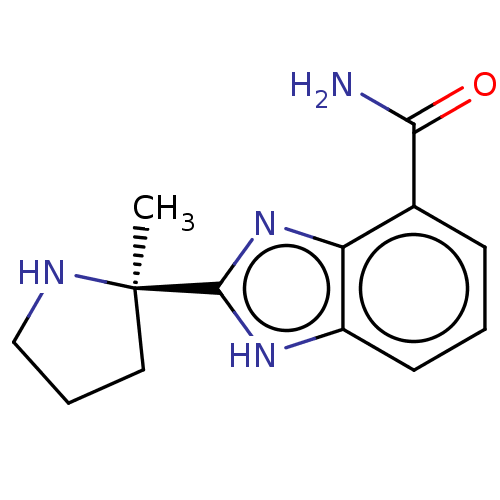
(2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-...)Show InChI InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency against histamine H3 receptor in an electrically stimulated guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency against histamine H3 receptor in an electrically stimulated guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM209932

(2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-...)Show InChI InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency against histamine H3 receptor in an electrically stimulated guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM532292
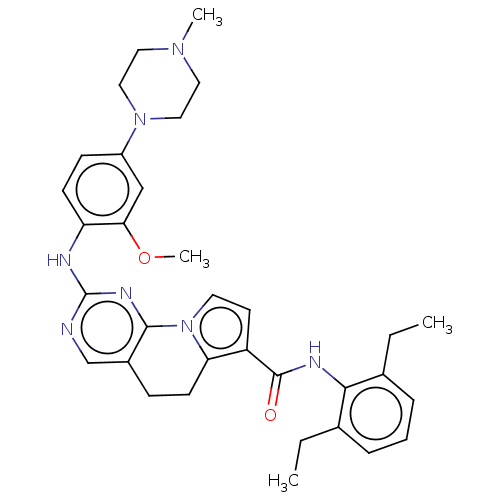
(JGS79C | N-(2,6-diethylphenyl)-2-[2-methoxy-4-(4-m...)Show SMILES CCc1cccc(CC)c1NC(=O)c1ccn-2c1CCc1cnc(Nc3ccc(cc3OC)N3CCN(C)CC3)nc-21 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency against histamine H3 receptor in an electrically stimulated guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency against histamine H3 receptor in an electrically stimulated guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency against histamine H3 receptor in an electrically stimulated guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM457842

((S)-2-(1-((6-amino-5-(3-methyl-1,2,4-oxadiazol-5-y...)Show SMILES CC#Cc1cccc2nc([C@H](C)Nc3ncnc(N)c3-c3nc(C)no3)n(-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C26H22N8O2/c1-4-9-17-10-8-13-19-20(17)26(35)34(18-11-6-5-7-12-18)24(32-19)15(2)30-23-21(22(27)28-14-29-23)25-31-16(3)33-36-25/h5-8,10-15H,1-3H3,(H3,27,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SUNSHINE LAKE PHARMA CO., LTD.; CALITOR SCIENCES, LLC
US Patent
| Assay Description
PI3K (p110δ/p85α) (h) is incubated in assay buffer containing 10 μM phosphatidylinositol-4, 5-bisphosphate and MgATP (concentration as... |
US Patent US10751339 (2020)
BindingDB Entry DOI: 10.7270/Q2GX4FM7 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50407812
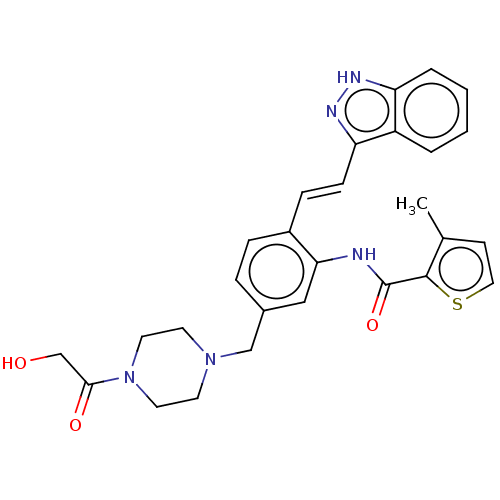
(KW-2450 | Kw-2450)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](CO)NC(=O)[C@H](Cc2cccs2)NC(=O)CCCNC1=O |r| Show InChI InChI=1S/C39H53N9O7S/c40-39(41)43-16-5-12-27-34(51)42-15-6-14-33(50)44-28(20-26-11-7-17-56-26)35(52)46-29(22-49)37(54)47-21-25-10-2-1-8-23(25)18-32(47)38(55)48-30-13-4-3-9-24(30)19-31(48)36(53)45-27/h1-2,7-8,10-11,17,24,27-32,49H,3-6,9,12-16,18-22H2,(H,42,51)(H,44,50)(H,45,53)(H,46,52)(H4,40,41,43)/t24?,27-,28-,29-,30?,31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency at cloned recombinant human adenosine A2A receptor transfected in CHO cells by cAMP assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50110183
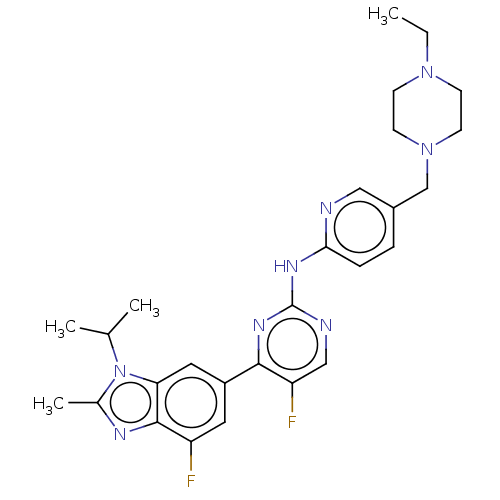
(Abemaciclib | LY-2835219 | US10626107, Example LY2...)Show SMILES CCN1CCN(Cc2ccc(Nc3ncc(F)c(n3)-c3cc(F)c4nc(C)n(C(C)C)c4c3)nc2)CC1 Show InChI InChI=1S/C27H32F2N8/c1-5-35-8-10-36(11-9-35)16-19-6-7-24(30-14-19)33-27-31-15-22(29)25(34-27)20-12-21(28)26-23(13-20)37(17(2)3)18(4)32-26/h6-7,12-15,17H,5,8-11,16H2,1-4H3,(H,30,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency at cloned recombinant human adenosine A2A receptor transfected in CHO cells by cAMP assay |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50407812

(KW-2450 | Kw-2450)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](CO)NC(=O)[C@H](Cc2cccs2)NC(=O)CCCNC1=O |r| Show InChI InChI=1S/C39H53N9O7S/c40-39(41)43-16-5-12-27-34(51)42-15-6-14-33(50)44-28(20-26-11-7-17-56-26)35(52)46-29(22-49)37(54)47-21-25-10-2-1-8-23(25)18-32(47)38(55)48-30-13-4-3-9-24(30)19-31(48)36(53)45-27/h1-2,7-8,10-11,17,24,27-32,49H,3-6,9,12-16,18-22H2,(H,42,51)(H,44,50)(H,45,53)(H,46,52)(H4,40,41,43)/t24?,27-,28-,29-,30?,31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency at cloned recombinant human adenosine A2B receptor transfected in CHO cells by cAMP assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM378887
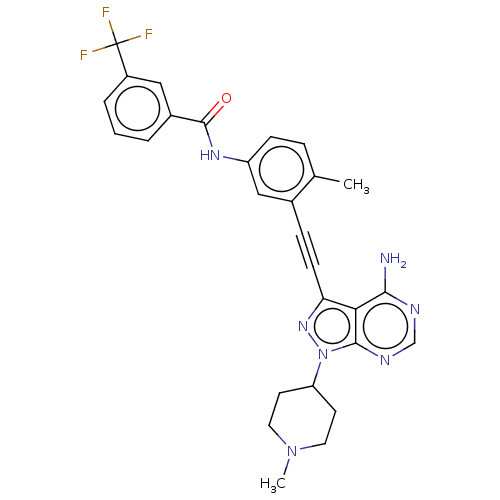
(US10266537, Compound 31)Show SMILES CN1CCC(CC1)n1nc(C#Cc2cc(NC(=O)c3cccc(c3)C(F)(F)F)ccc2C)c2c(N)ncnc12 Show InChI InChI=1S/C28H26F3N7O/c1-17-6-8-21(35-27(39)19-4-3-5-20(14-19)28(29,30)31)15-18(17)7-9-23-24-25(32)33-16-34-26(24)38(36-23)22-10-12-37(2)13-11-22/h3-6,8,14-16,22H,10-13H2,1-2H3,(H,35,39)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency at cloned recombinant human adenosine A2A receptor transfected in CHO cells by cAMP assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Complement factor D
(Homo sapiens (Human)) | BDBM50524347

(CHEMBL4535197)Show SMILES N[C@H](CO)c1cccc(c1)-c1cc(Br)cc(COc2ccccc2CC(O)=O)c1 |r| Show InChI InChI=1S/C23H22BrNO4/c24-20-9-15(14-29-22-7-2-1-4-18(22)12-23(27)28)8-19(11-20)16-5-3-6-17(10-16)21(25)13-26/h1-11,21,26H,12-14,25H2,(H,27,28)/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of 2-((1E,3E,5E)-5-(1-(6-((((3S,5S)-1-((1-carbamoyl-1H-indol-3-yl)carbamoyl)-5-((3-chloro-2-fluorobenzyl)carbamoyl)-3-fluoropyrrolidin-3-y... |
J Med Chem 62: 4656-4668 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00271
BindingDB Entry DOI: 10.7270/Q2CC144N |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50591583

(CHEMBL5169760)Show SMILES CN1CCN(Cc2ccc(Nc3ncc(Cl)c(Nc4ccccc4-c4n[nH]c(C)n4)n3)cc2)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00153e
BindingDB Entry DOI: 10.7270/Q2ZS31HH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM457868

((S)-2-(1-((6-amino-5-(3-methyl-1,2,4-oxadiazol-5-y...)Show SMILES C[C@H](Nc1ncnc(N)c1-c1nc(C)no1)c1nc2cccc(C#CCO)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C26H22N8O3/c1-15(30-23-21(22(27)28-14-29-23)25-31-16(2)33-37-25)24-32-19-12-6-8-17(9-7-13-35)20(19)26(36)34(24)18-10-4-3-5-11-18/h3-6,8,10-12,14-15,35H,13H2,1-2H3,(H3,27,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
SUNSHINE LAKE PHARMA CO., LTD.; CALITOR SCIENCES, LLC
US Patent
| Assay Description
PI3K (p110δ/p85α) (h) is incubated in assay buffer containing 10 μM phosphatidylinositol-4, 5-bisphosphate and MgATP (concentration as... |
US Patent US10751339 (2020)
BindingDB Entry DOI: 10.7270/Q2GX4FM7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM457871

((S)-3-(1-((6-amino-5-(3-methyl-1,2,4-oxadiazol-5-y...)Show SMILES C[C@H](Nc1ncnc(N)c1-c1nc(C)no1)c1cc2cccc(C#CCO)c2c(=O)n1C |r| Show InChI InChI=1S/C22H21N7O3/c1-12(26-20-18(19(23)24-11-25-20)21-27-13(2)28-32-21)16-10-15-7-4-6-14(8-5-9-30)17(15)22(31)29(16)3/h4,6-7,10-12,30H,9H2,1-3H3,(H3,23,24,25,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SUNSHINE LAKE PHARMA CO., LTD.; CALITOR SCIENCES, LLC
US Patent
| Assay Description
PI3K (p110δ/p85α) (h) is incubated in assay buffer containing 10 μM phosphatidylinositol-4, 5-bisphosphate and MgATP (concentration as... |
US Patent US10751339 (2020)
BindingDB Entry DOI: 10.7270/Q2GX4FM7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50591595
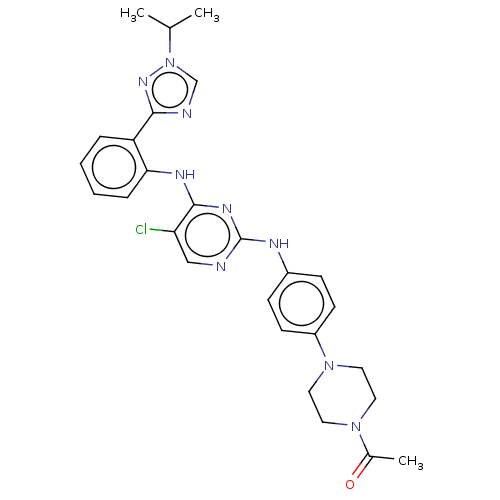
(CHEMBL5184642)Show SMILES CC(C)n1cnc(n1)-c1ccccc1Nc1nc(Nc2ccc(cc2)N2CCN(CC2)C(C)=O)ncc1Cl | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00153e
BindingDB Entry DOI: 10.7270/Q2ZS31HH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50591605
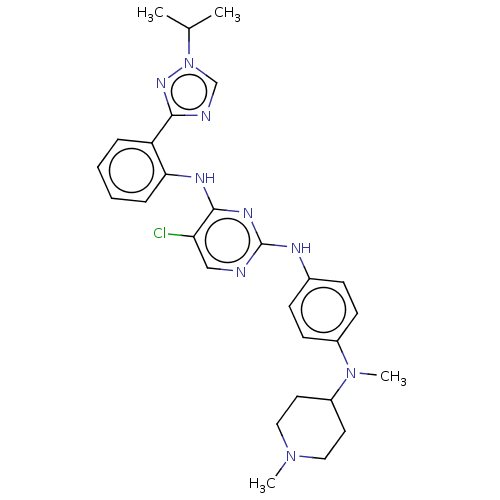
(CHEMBL5185599)Show SMILES CC(C)n1cnc(n1)-c1ccccc1Nc1nc(Nc2ccc(cc2)N(C)C2CCN(C)CC2)ncc1Cl | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00153e
BindingDB Entry DOI: 10.7270/Q2ZS31HH |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency against histamine H3 receptor in an electrically stimulated guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50088378
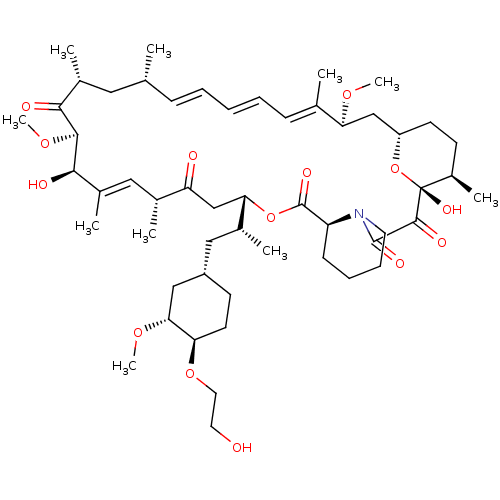
(Afinitor | Afinitor Disperz | CHEBI:68478 | Everol...)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@@]1([H])C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](OCCO)[C@@H](C1)OC |r,c:32,51,t:47,49| Show InChI InChI=1S/C53H83NO14/c1-32-16-12-11-13-17-33(2)44(63-8)30-40-21-19-38(7)53(62,68-40)50(59)51(60)54-23-15-14-18-41(54)52(61)67-45(35(4)28-39-20-22-43(66-25-24-55)46(29-39)64-9)31-42(56)34(3)27-37(6)48(58)49(65-10)47(57)36(5)26-32/h11-13,16-17,27,32,34-36,38-41,43-46,48-49,55,58,62H,14-15,18-26,28-31H2,1-10H3/b13-11+,16-12+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43-,44+,45+,46-,48-,49+,53-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency against histamine H3 receptor in an electrically stimulated guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50313650
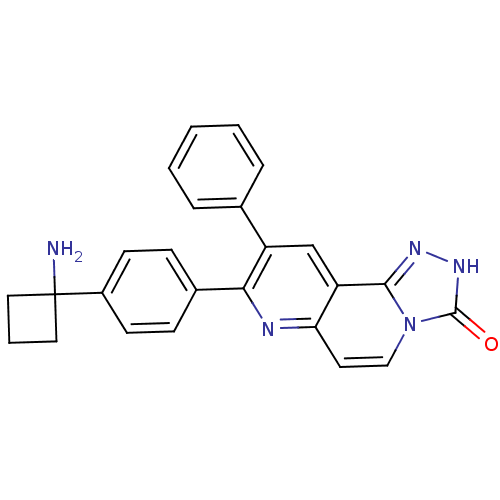
(8-(4-(1-aminocyclobutyl)phenyl)-9-phenyl-[1,2,4]tr...)Show SMILES NC1(CCC1)c1ccc(cc1)-c1nc2ccn3c(n[nH]c3=O)c2cc1-c1ccccc1 Show InChI InChI=1S/C25H21N5O/c26-25(12-4-13-25)18-9-7-17(8-10-18)22-19(16-5-2-1-3-6-16)15-20-21(27-22)11-14-30-23(20)28-29-24(30)31/h1-3,5-11,14-15H,4,12-13,26H2,(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Complement factor D
(Homo sapiens (Human)) | BDBM50462108

(CHEMBL4246585)Show SMILES N[C@H](CO)c1cccc(c1)-c1cc(CO)cc(COc2ccccc2CC(O)=O)c1 |r| Show InChI InChI=1S/C24H25NO5/c25-22(14-27)19-6-3-5-18(11-19)21-9-16(13-26)8-17(10-21)15-30-23-7-2-1-4-20(23)12-24(28)29/h1-11,22,26-27H,12-15,25H2,(H,28,29)/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of 2-((1E,3E,5E)-5-(1-(6-((((3S,5S)-1-((1-carbamoyl-1H-indol-3-yl)carbamoyl)-5-((3-chloro-2-fluorobenzyl)carbamoyl)-3-fluoropyrrolidin-3-y... |
J Med Chem 62: 4656-4668 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00271
BindingDB Entry DOI: 10.7270/Q2CC144N |
More data for this
Ligand-Target Pair | |
Complement factor D
(Homo sapiens (Human)) | BDBM171332
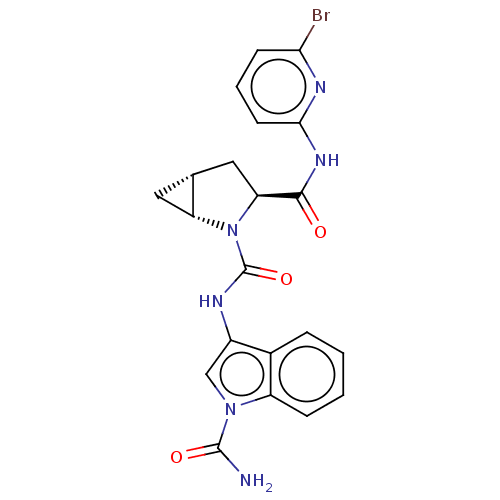
(US9085555, 762)Show SMILES NC(=O)n1cc(NC(=O)N2[C@@H]3C[C@@H]3C[C@H]2C(=O)Nc2cccc(Br)n2)c2ccccc12 |r| Show InChI InChI=1S/C21H19BrN6O3/c22-17-6-3-7-18(25-17)26-19(29)16-9-11-8-15(11)28(16)21(31)24-13-10-27(20(23)30)14-5-2-1-4-12(13)14/h1-7,10-11,15-16H,8-9H2,(H2,23,30)(H,24,31)(H,25,26,29)/t11-,15-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... |
J Med Chem 60: 5717-5735 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00425
BindingDB Entry DOI: 10.7270/Q2TB195V |
More data for this
Ligand-Target Pair | |
Complement factor B
(Homo sapiens (Human)) | BDBM50540314
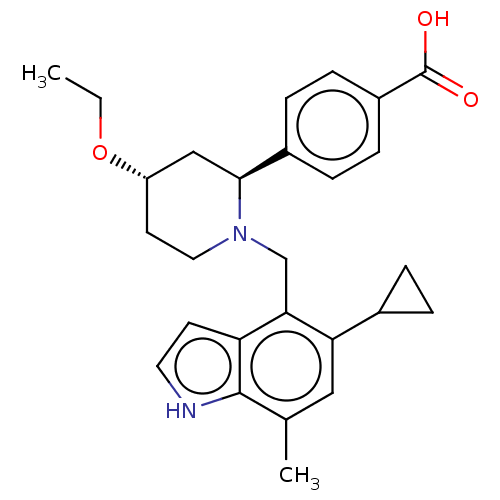
(CHEMBL4639592)Show SMILES CCO[C@H]1CCN(Cc2c(cc(C)c3[nH]ccc23)C2CC2)[C@@H](C1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C27H32N2O3/c1-3-32-21-11-13-29(25(15-21)19-6-8-20(9-7-19)27(30)31)16-24-22-10-12-28-26(22)17(2)14-23(24)18-4-5-18/h6-10,12,14,18,21,25,28H,3-5,11,13,15-16H2,1-2H3,(H,30,31)/t21-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human serine protease factor B by TR-FRET based competition binding assay |
J Med Chem 63: 5697-5722 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01870
BindingDB Entry DOI: 10.7270/Q21N84P4 |
More data for this
Ligand-Target Pair | |
Complement factor D [24-253]
(Homo sapiens (Human)) | BDBM171350

(1-(2-((1R,3S,5R)-3-((6-Bromopyridin-2-yl)carbamoyl...)Show SMILES NC(=O)c1nn(CC(=O)N2[C@@H]3C[C@@H]3C[C@H]2C(=O)Nc2cccc(Br)n2)c2ccccc12 Show InChI InChI=1S/C21H19BrN6O3/c22-16-6-3-7-17(24-16)25-21(31)15-9-11-8-14(11)28(15)18(29)10-27-13-5-2-1-4-12(13)19(26-27)20(23)30/h1-7,11,14-15H,8-10H2,(H2,23,30)(H,24,25,31)/t11-,14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis Pharma AG
| Assay Description
Briefly, recombinant human or murine FD catalytic domain (10 nM concentration) were incubated with compound at various concentrations for 1 h at room... |
Nat Chem Biol 12: 1105-1110 (2016)
Article DOI: 10.1038/nchembio.2208
BindingDB Entry DOI: 10.7270/Q2KD1WR1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM457852

((S)-2-(1-((6-amino-5-(5-methyl-1,3,4-oxadiazol-2-y...)Show SMILES CC#Cc1cccc2nc([C@H](C)Nc3ncnc(N)c3-c3nnc(C)o3)n(-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C26H22N8O2/c1-4-9-17-10-8-13-19-20(17)26(35)34(18-11-6-5-7-12-18)24(31-19)15(2)30-23-21(22(27)28-14-29-23)25-33-32-16(3)36-25/h5-8,10-15H,1-3H3,(H3,27,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
SUNSHINE LAKE PHARMA CO., LTD.; CALITOR SCIENCES, LLC
US Patent
| Assay Description
PI3K (p110δ/p85α) (h) is incubated in assay buffer containing 10 μM phosphatidylinositol-4, 5-bisphosphate and MgATP (concentration as... |
US Patent US10751339 (2020)
BindingDB Entry DOI: 10.7270/Q2GX4FM7 |
More data for this
Ligand-Target Pair | |
Complement factor D
(Homo sapiens (Human)) | BDBM171332

(US9085555, 762)Show SMILES NC(=O)n1cc(NC(=O)N2[C@@H]3C[C@@H]3C[C@H]2C(=O)Nc2cccc(Br)n2)c2ccccc12 |r| Show InChI InChI=1S/C21H19BrN6O3/c22-17-6-3-7-18(25-17)26-19(29)16-9-11-8-15(11)28(16)21(31)24-13-10-27(20(23)30)14-5-2-1-4-12(13)14/h1-7,10-11,15-16H,8-9H2,(H2,23,30)(H,24,31)(H,25,26,29)/t11-,15-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... |
J Med Chem 60: 5717-5735 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00425
BindingDB Entry DOI: 10.7270/Q2TB195V |
More data for this
Ligand-Target Pair | |
Gamma-secretase-activating protein
(Homo sapiens (Human)) | BDBM50458159

(Nirogacestat | PF 03084014 | PF 3084014 | PF-03084...)Show SMILES CCC[C@H](N[C@H]1CCc2cc(F)cc(F)c2C1)C(=O)Nc1cn(cn1)C(C)(C)CNCC(C)(C)C |r| Show InChI InChI=1S/C27H41F2N5O/c1-7-8-23(32-20-10-9-18-11-19(28)12-22(29)21(18)13-20)25(35)33-24-14-34(17-31-24)27(5,6)16-30-15-26(2,3)4/h11-12,14,17,20,23,30,32H,7-10,13,15-16H2,1-6H3,(H,33,35)/t20-,23-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM457853
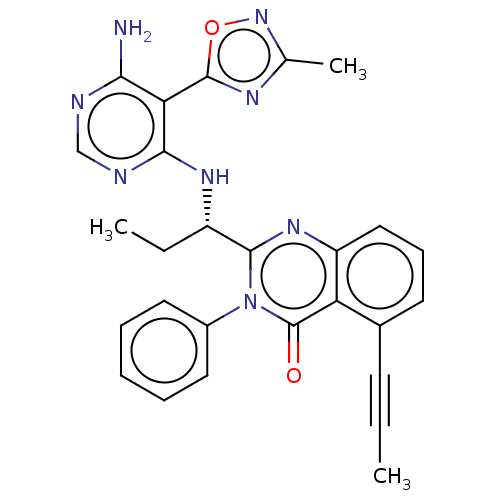
((S)-2-(1-((6-amino-5-(3-methyl-1,2,4-oxadiazol-5-y...)Show SMILES CC[C@H](Nc1ncnc(N)c1-c1nc(C)no1)c1nc2cccc(C#CC)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C27H24N8O2/c1-4-10-17-11-9-14-20-21(17)27(36)35(18-12-7-6-8-13-18)25(33-20)19(5-2)32-24-22(23(28)29-15-30-24)26-31-16(3)34-37-26/h6-9,11-15,19H,5H2,1-3H3,(H3,28,29,30,32)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
SUNSHINE LAKE PHARMA CO., LTD.; CALITOR SCIENCES, LLC
US Patent
| Assay Description
PI3K (p110δ/p85α) (h) is incubated in assay buffer containing 10 μM phosphatidylinositol-4, 5-bisphosphate and MgATP (concentration as... |
US Patent US10751339 (2020)
BindingDB Entry DOI: 10.7270/Q2GX4FM7 |
More data for this
Ligand-Target Pair | |
Complement factor D
(Homo sapiens (Human)) | BDBM50524338
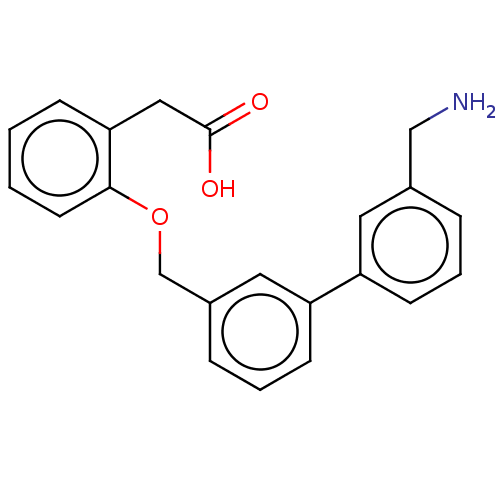
(CHEMBL4468000)Show InChI InChI=1S/C22H21NO3/c23-14-16-5-3-8-18(11-16)19-9-4-6-17(12-19)15-26-21-10-2-1-7-20(21)13-22(24)25/h1-12H,13-15,23H2,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of 2-((1E,3E,5E)-5-(1-(6-((((3S,5S)-1-((1-carbamoyl-1H-indol-3-yl)carbamoyl)-5-((3-chloro-2-fluorobenzyl)carbamoyl)-3-fluoropyrrolidin-3-y... |
J Med Chem 62: 4656-4668 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00271
BindingDB Entry DOI: 10.7270/Q2CC144N |
More data for this
Ligand-Target Pair | |
Complement factor D
(Homo sapiens (Human)) | BDBM50524344
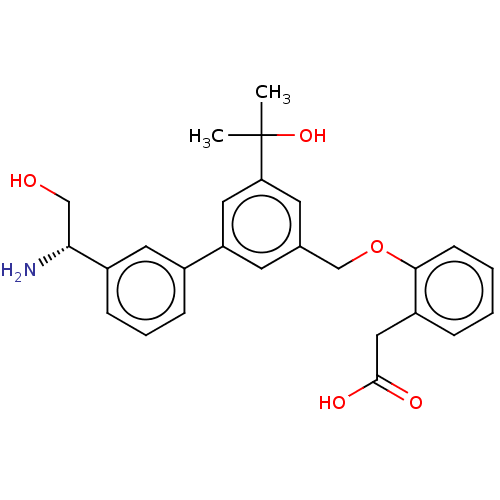
(CHEMBL4584532)Show SMILES CC(C)(O)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1cccc(c1)[C@H](N)CO |r| Show InChI InChI=1S/C26H29NO5/c1-26(2,31)22-11-17(16-32-24-9-4-3-6-20(24)14-25(29)30)10-21(13-22)18-7-5-8-19(12-18)23(27)15-28/h3-13,23,28,31H,14-16,27H2,1-2H3,(H,29,30)/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of 2-((1E,3E,5E)-5-(1-(6-((((3S,5S)-1-((1-carbamoyl-1H-indol-3-yl)carbamoyl)-5-((3-chloro-2-fluorobenzyl)carbamoyl)-3-fluoropyrrolidin-3-y... |
J Med Chem 62: 4656-4668 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00271
BindingDB Entry DOI: 10.7270/Q2CC144N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50591590

(CHEMBL5187900)Show SMILES CN1CCN(CC1)C(=O)c1ccc(Nc2ncc(Cl)c(Nc3ccccc3-c3nc(C)n(C)n3)n2)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00153e
BindingDB Entry DOI: 10.7270/Q2ZS31HH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50591572

(CHEMBL5201640)Show SMILES CN1CCN(CC1)C(=O)c1ccc(Nc2ncc(Cl)c(Nc3ccccc3-c3n[nH]c(C)n3)n2)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00153e
BindingDB Entry DOI: 10.7270/Q2ZS31HH |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency against adenosine A2A receptor of Sprague-Dawley rat aortic smooth muscle |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50591598
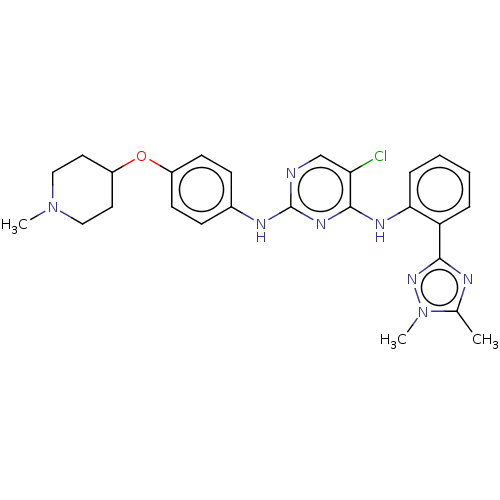
(CHEMBL5192037)Show SMILES CN1CCC(CC1)Oc1ccc(Nc2ncc(Cl)c(Nc3ccccc3-c3nc(C)n(C)n3)n2)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00153e
BindingDB Entry DOI: 10.7270/Q2ZS31HH |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50139171
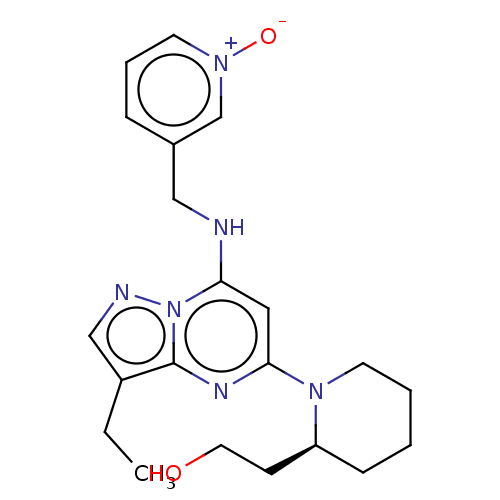
(Dinaciclib | MK-7965 | SCH-727965 | US11643396, Ex...)Show SMILES CCc1cnn2c(NCc3ccc[n+]([O-])c3)cc(nc12)N1CCCC[C@H]1CCO |r| Show InChI InChI=1S/C21H28N6O2/c1-2-17-14-23-27-19(22-13-16-6-5-9-25(29)15-16)12-20(24-21(17)27)26-10-4-3-7-18(26)8-11-28/h5-6,9,12,14-15,18,22,28H,2-4,7-8,10-11,13H2,1H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM27344
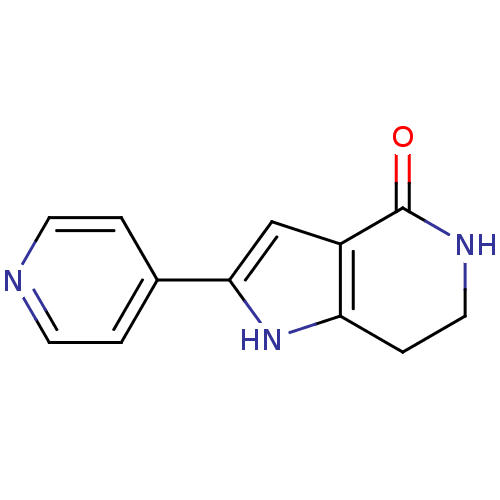
(2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,2-c]pyri...)Show InChI InChI=1S/C12H11N3O/c16-12-9-7-11(8-1-4-13-5-2-8)15-10(9)3-6-14-12/h1-2,4-5,7,15H,3,6H2,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency at cloned recombinant human adenosine A2A receptor transfected in CHO cells by cAMP assay |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50591588

(CHEMBL5171253)Show SMILES CN1CCC(CC1)Oc1ccc(Nc2ncc(Cl)c(Nc3ccccc3-c3n[nH]c(C)n3)n2)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00153e
BindingDB Entry DOI: 10.7270/Q2ZS31HH |
More data for this
Ligand-Target Pair | |
Complement factor D
(Homo sapiens (Human)) | BDBM50524340

(CHEMBL4483713)Show SMILES COCc1cc(COc2ccccc2CC(O)=O)cc(c1)-c1cccc(c1)[C@H](N)CO |r| Show InChI InChI=1S/C25H27NO5/c1-30-15-17-9-18(16-31-24-8-3-2-5-21(24)13-25(28)29)11-22(10-17)19-6-4-7-20(12-19)23(26)14-27/h2-12,23,27H,13-16,26H2,1H3,(H,28,29)/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of 2-((1E,3E,5E)-5-(1-(6-((((3S,5S)-1-((1-carbamoyl-1H-indol-3-yl)carbamoyl)-5-((3-chloro-2-fluorobenzyl)carbamoyl)-3-fluoropyrrolidin-3-y... |
J Med Chem 62: 4656-4668 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00271
BindingDB Entry DOI: 10.7270/Q2CC144N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM457855

((S)-3-(1-((6-amino-5-(3-methyl-1,2,4-oxadiazol-5-y...)Show SMILES CC#Cc1cccc2cc([C@H](C)Nc3ncnc(N)c3-c3nc(C)no3)n(-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C27H23N7O2/c1-4-9-18-10-8-11-19-14-21(34(27(35)22(18)19)20-12-6-5-7-13-20)16(2)31-25-23(24(28)29-15-30-25)26-32-17(3)33-36-26/h5-8,10-16H,1-3H3,(H3,28,29,30,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
SUNSHINE LAKE PHARMA CO., LTD.; CALITOR SCIENCES, LLC
US Patent
| Assay Description
PI3K (p110δ/p85α) (h) is incubated in assay buffer containing 10 μM phosphatidylinositol-4, 5-bisphosphate and MgATP (concentration as... |
US Patent US10751339 (2020)
BindingDB Entry DOI: 10.7270/Q2GX4FM7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM457869

((S)-2-(1-((6-amino-5-(3-methyl-1,2,4-oxadiazol-5-y...)Show SMILES CC[C@H](Nc1ncnc(N)c1-c1nc(C)no1)c1nc2cccc(C#CCO)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C27H24N8O3/c1-3-19(32-24-22(23(28)29-15-30-24)26-31-16(2)34-38-26)25-33-20-13-7-9-17(10-8-14-36)21(20)27(37)35(25)18-11-5-4-6-12-18/h4-7,9,11-13,15,19,36H,3,14H2,1-2H3,(H3,28,29,30,32)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
SUNSHINE LAKE PHARMA CO., LTD.; CALITOR SCIENCES, LLC
US Patent
| Assay Description
PI3K (p110δ/p85α) (h) is incubated in assay buffer containing 10 μM phosphatidylinositol-4, 5-bisphosphate and MgATP (concentration as... |
US Patent US10751339 (2020)
BindingDB Entry DOI: 10.7270/Q2GX4FM7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM148264

(7-cyclopentyl-N,N-dimethyl-2-((5-(piperazin-1-yl)p...)Show SMILES CN(C)C(=O)c1cc2cnc(Nc3ccc(cn3)N3CCNCC3)nc2n1C1CCCC1 Show InChI InChI=1S/C23H30N8O/c1-29(2)22(32)19-13-16-14-26-23(28-21(16)31(19)17-5-3-4-6-17)27-20-8-7-18(15-25-20)30-11-9-24-10-12-30/h7-8,13-15,17,24H,3-6,9-12H2,1-2H3,(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency at cloned recombinant human adenosine A2A receptor transfected in CHO cells by cAMP assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Complement factor D
(Homo sapiens (Human)) | BDBM171239
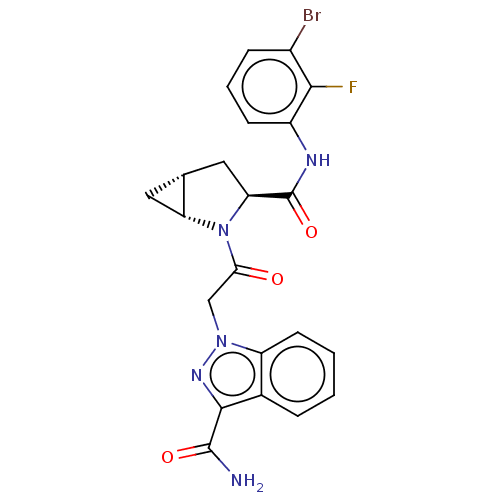
(US9085555, 669)Show SMILES NC(=O)c1nn(CC(=O)N2[C@@H]3C[C@@H]3C[C@H]2C(=O)Nc2cccc(Br)c2F)c2ccccc12 |r| Show InChI InChI=1S/C22H19BrFN5O3/c23-13-5-3-6-14(19(13)24)26-22(32)17-9-11-8-16(11)29(17)18(30)10-28-15-7-2-1-4-12(15)20(27-28)21(25)31/h1-7,11,16-17H,8-10H2,(H2,25,31)(H,26,32)/t11-,16-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human complement factor D catalytic domain using Z-Lys-thiobenzylester as substrate preincubated for 1 hr followed by subst... |
J Med Chem 60: 5717-5735 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00425
BindingDB Entry DOI: 10.7270/Q2TB195V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency against adenosine A2B receptor of guinea pig thoracic aortic smooth muscle |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 6
(Homo sapiens (Human)) | BDBM50110183

(Abemaciclib | LY-2835219 | US10626107, Example LY2...)Show SMILES CCN1CCN(Cc2ccc(Nc3ncc(F)c(n3)-c3cc(F)c4nc(C)n(C(C)C)c4c3)nc2)CC1 Show InChI InChI=1S/C27H32F2N8/c1-5-35-8-10-36(11-9-35)16-19-6-7-24(30-14-19)33-27-31-15-22(29)25(34-27)20-12-21(28)26-23(13-20)37(17(2)3)18(4)32-26/h6-7,12-15,17H,5,8-11,16H2,1-4H3,(H,30,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency at cloned recombinant human adenosine A2A receptor transfected in CHO cells by cAMP assay |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM457864
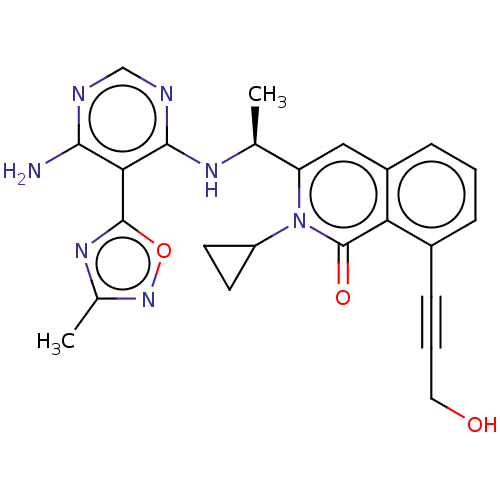
((S)-3-(1-((6-amino-5-(3-methyl-1,2,4-oxadiazol-5-y...)Show SMILES C[C@H](Nc1ncnc(N)c1-c1nc(C)no1)c1cc2cccc(C#CCO)c2c(=O)n1C1CC1 |r| Show InChI InChI=1S/C24H23N7O3/c1-13(28-22-20(21(25)26-12-27-22)23-29-14(2)30-34-23)18-11-16-6-3-5-15(7-4-10-32)19(16)24(33)31(18)17-8-9-17/h3,5-6,11-13,17,32H,8-10H2,1-2H3,(H3,25,26,27,28)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
SUNSHINE LAKE PHARMA CO., LTD.; CALITOR SCIENCES, LLC
US Patent
| Assay Description
PI3K (p110δ/p85α) (h) is incubated in assay buffer containing 10 μM phosphatidylinositol-4, 5-bisphosphate and MgATP (concentration as... |
US Patent US10751339 (2020)
BindingDB Entry DOI: 10.7270/Q2GX4FM7 |
More data for this
Ligand-Target Pair | |
Complement factor D
(Homo sapiens (Human)) | BDBM171350

(1-(2-((1R,3S,5R)-3-((6-Bromopyridin-2-yl)carbamoyl...)Show SMILES NC(=O)c1nn(CC(=O)N2[C@@H]3C[C@@H]3C[C@H]2C(=O)Nc2cccc(Br)n2)c2ccccc12 Show InChI InChI=1S/C21H19BrN6O3/c22-16-6-3-7-17(24-16)25-21(31)15-9-11-8-14(11)28(15)18(29)10-27-13-5-2-1-4-12(13)19(26-27)20(23)30/h1-7,11,14-15H,8-10H2,(H2,23,30)(H,24,25,31)/t11-,14-,15+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of complement factor D in human whole blood assessed as decrease in zymosan-induced AP activation mediated soluble MAC complex formation p... |
J Med Chem 60: 5717-5735 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00425
BindingDB Entry DOI: 10.7270/Q2TB195V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Complement factor D
(Homo sapiens (Human)) | BDBM171350

(1-(2-((1R,3S,5R)-3-((6-Bromopyridin-2-yl)carbamoyl...)Show SMILES NC(=O)c1nn(CC(=O)N2[C@@H]3C[C@@H]3C[C@H]2C(=O)Nc2cccc(Br)n2)c2ccccc12 Show InChI InChI=1S/C21H19BrN6O3/c22-16-6-3-7-17(24-16)25-21(31)15-9-11-8-14(11)28(15)18(29)10-27-13-5-2-1-4-12(13)19(26-27)20(23)30/h1-7,11,14-15H,8-10H2,(H2,23,30)(H,24,25,31)/t11-,14-,15+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for the Non-competitive inhibition constant Ki against TdR varied rat cytoplasmic soluble thymidine kinase |
J Med Chem 60: 5717-5735 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00425
BindingDB Entry DOI: 10.7270/Q2TB195V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data