

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141931 ((R)-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-ethyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141911 ((R)-2-[(2S,3S)-3-{4-[5-(4-Cyano-benzyl)-2-ethyl-2H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141908 ((R)-2-[(2S,3S)-3-{4-[5-(4-tert-Butyl-benzyl)-2-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141905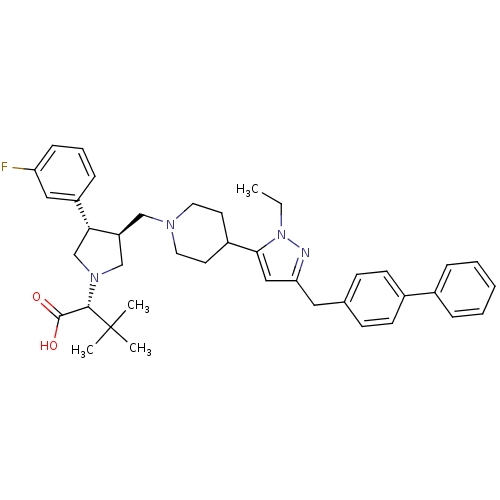 ((R)-2-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141978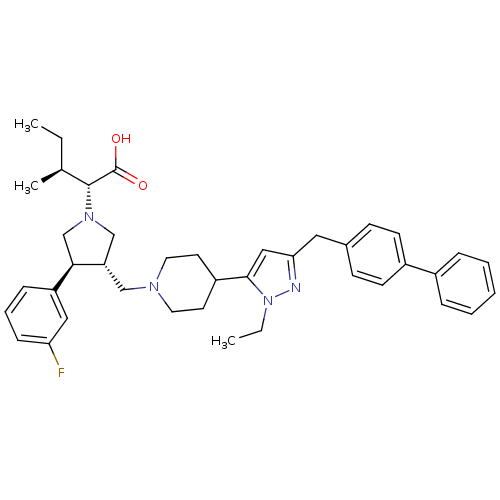 ((2R,4S)-2-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141935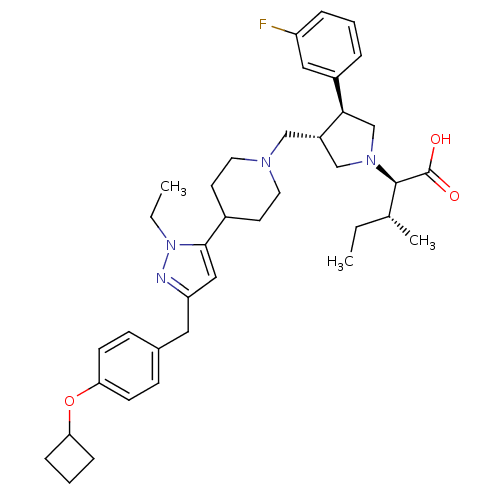 ((2R,4R)-2-[(2S,3S)-3-{4-[5-(4-Cyclobutoxy-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141951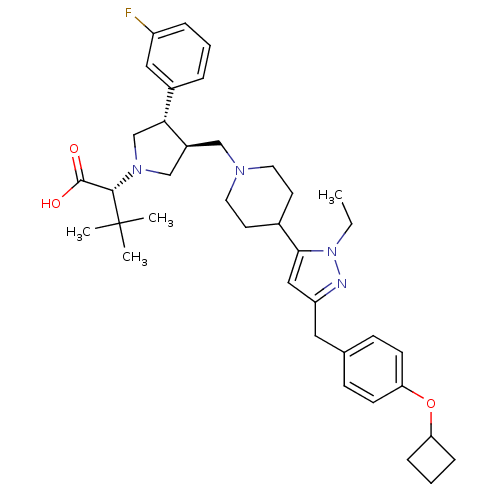 ((R)-2-[(2S,3S)-3-{4-[5-(4-Cyclobutoxy-benzyl)-2-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141913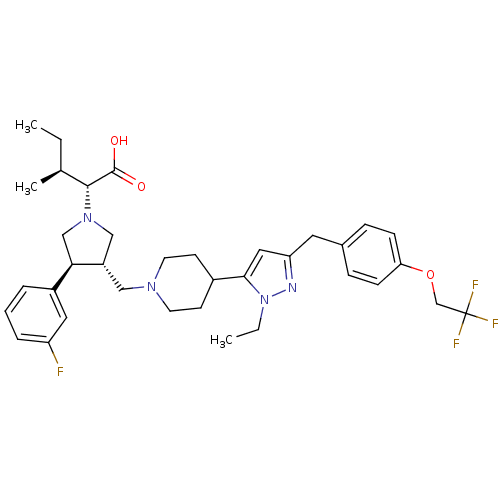 ((2R,4S)-2-[(2S,3S)-3-(4-{2-Ethyl-5-[4-(2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141941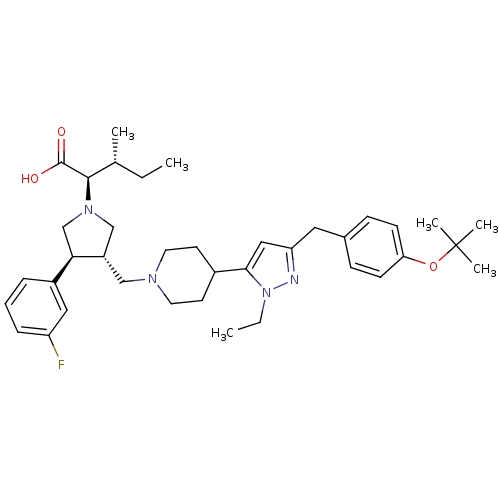 ((2R,4R)-2-[(2S,3S)-3-{4-[5-(4-tert-Butoxy-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141906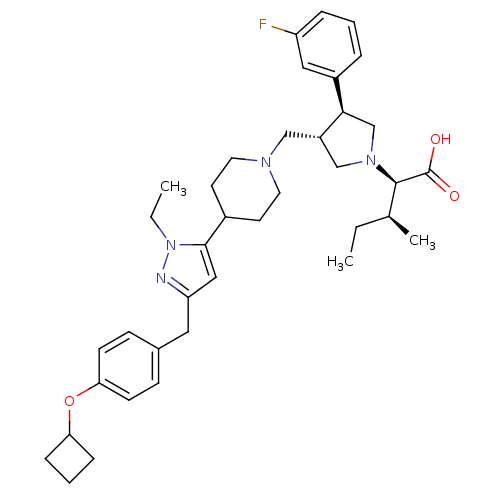 ((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Cyclobutoxy-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141973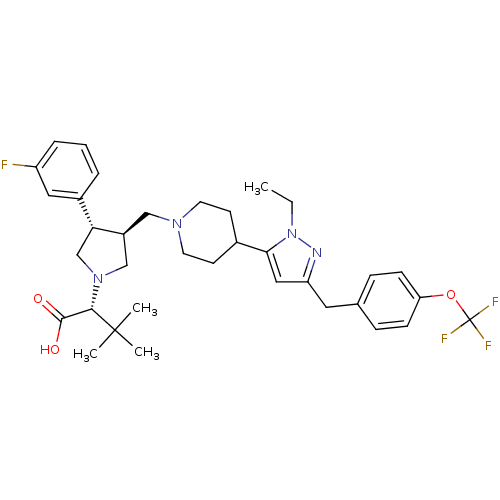 (2-[3-{4-[2-Ethyl-5-(4-trifluoromethoxy-benzyl)-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141910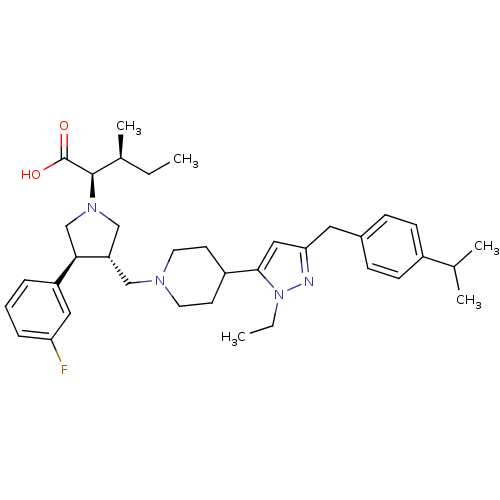 ((2R,4S)-2-[(2S,3S)-3-{4-[2-Ethyl-5-(4-isopropyl-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141979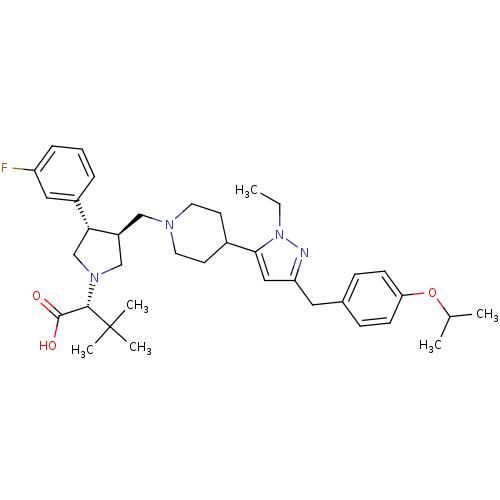 ((R)-2-[(2S,3S)-3-{4-[2-Ethyl-5-(4-isopropoxy-benzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141883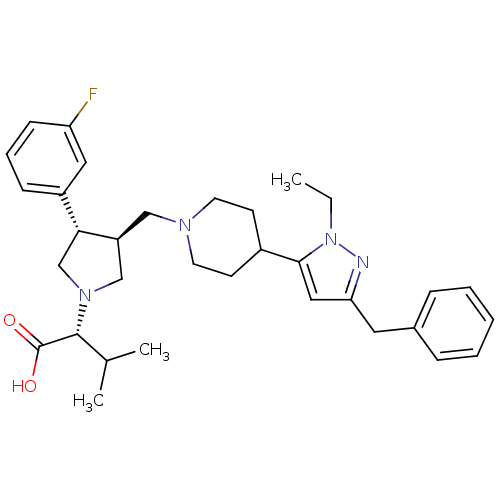 ((R)-2-((3S,4S)-3-((4-(3-benzyl-1-ethyl-1H-pyrazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141984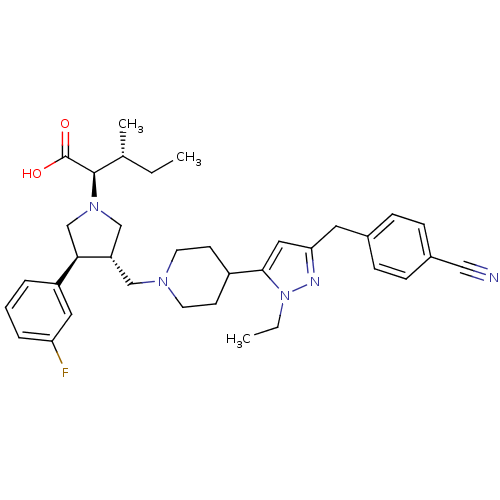 ((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Cyano-benzyl)-2-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141972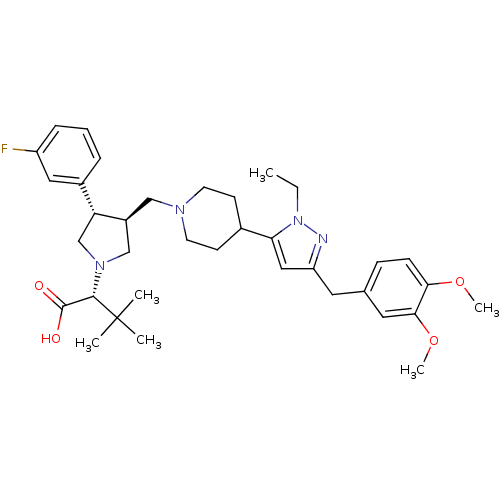 ((R)-2-[(2S,3S)-3-{4-[5-(3,4-Dimethoxy-benzyl)-2-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141874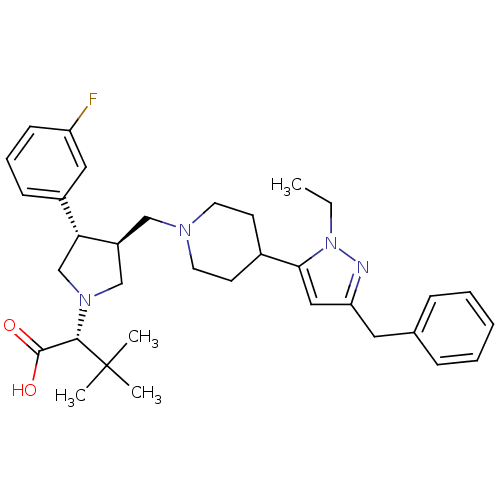 ((R)-2-((3S,4S)-3-((4-(3-benzyl-1-ethyl-1H-pyrazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141945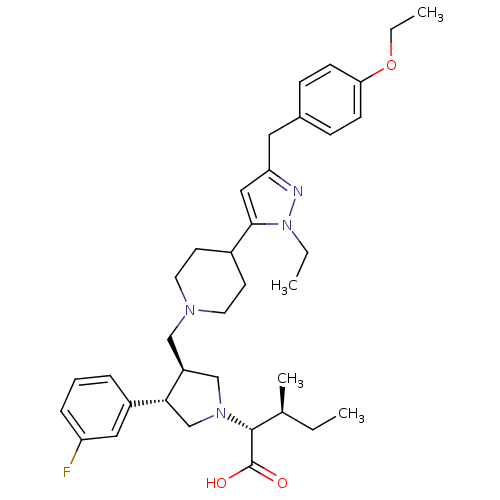 ((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Ethoxy-benzyl)-2-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141914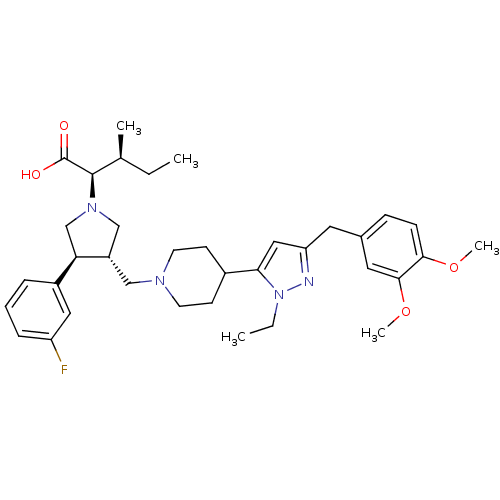 ((2R,4S)-2-[(2S,3S)-3-{4-[5-(3,4-Dimethoxy-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141875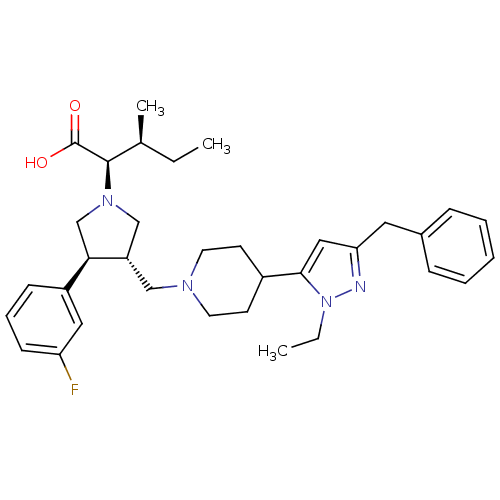 ((2R,3S)-2-[(2S,3S)-3-[4-(5-Benzyl-2-ethyl-2H-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141887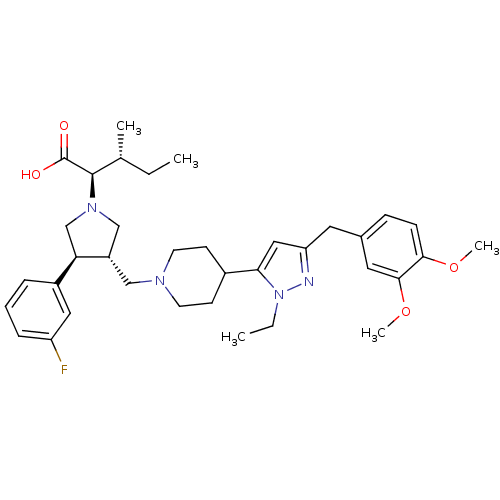 ((2R,4R)-2-[(2S,3S)-3-{4-[5-(3,4-Dimethoxy-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50101187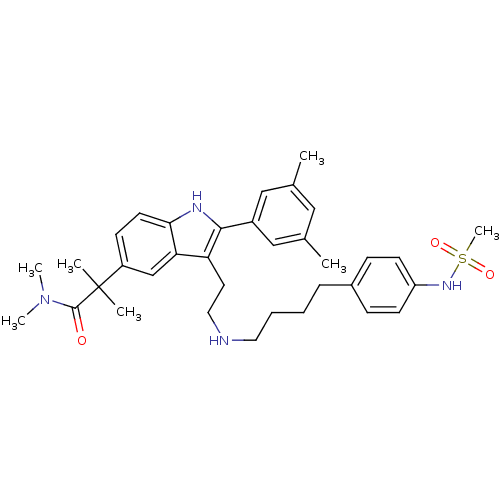 (2-(2-(3,5-Dimethyl-phenyl)-3-{2-[4-(4-methanesulfo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin. | Bioorg Med Chem Lett 11: 1723-6 (2001) BindingDB Entry DOI: 10.7270/Q2KH0MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50101189 (CHEMBL49891 | N-{4-[4-(2-{2-(3,5-Dimethyl-phenyl)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin. | Bioorg Med Chem Lett 11: 1723-6 (2001) BindingDB Entry DOI: 10.7270/Q2KH0MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50155363 (7-Chloro-2-oxo-4-[2-(4-oxo-azetidin-2-yl)-ethoxy]-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human gonadotropin releasing hormone receptor expressed in CHO cells was determined by using [125I]-buserelin as radioligand | Bioorg Med Chem Lett 14: 5599-603 (2004) Article DOI: 10.1016/j.bmcl.2004.08.056 BindingDB Entry DOI: 10.7270/Q20V8C8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50101170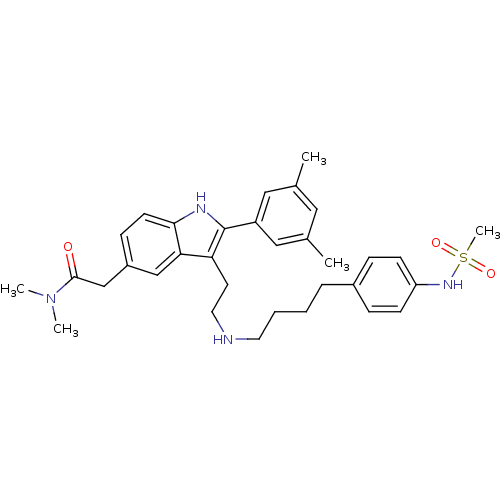 (2-(2-(3,5-Dimethyl-phenyl)-3-{2-[4-(4-methanesulfo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin. | Bioorg Med Chem Lett 11: 1723-6 (2001) BindingDB Entry DOI: 10.7270/Q2KH0MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50101192 (CHEMBL45597 | N-[4-(4-{2-[5-(1,1-Dimethyl-2-morpho...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition GnRH-stimulated luteinizing hormone (LH) release from rat pituitary cells | Bioorg Med Chem Lett 11: 1723-6 (2001) BindingDB Entry DOI: 10.7270/Q2KH0MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50141904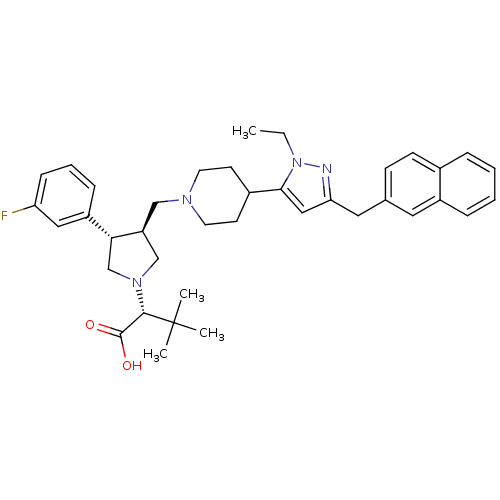 ((R)-2-[(2S,3S)-3-[4-(2-Ethyl-5-naphthalen-2-ylmeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50101184 (CHEMBL47761 | N-[4-(4-{2-[5-(1,1-Dimethyl-2-oxo-2-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin. | Bioorg Med Chem Lett 11: 1723-6 (2001) BindingDB Entry DOI: 10.7270/Q2KH0MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50101195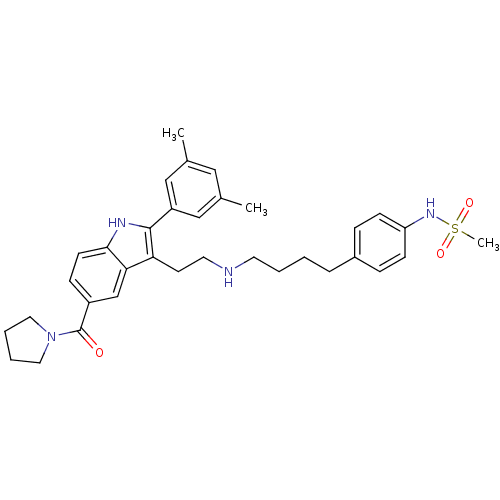 (CHEMBL47862 | N-[4-(4-{2-[2-(3,5-Dimethyl-phenyl)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin. | Bioorg Med Chem Lett 11: 1723-6 (2001) BindingDB Entry DOI: 10.7270/Q2KH0MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50141937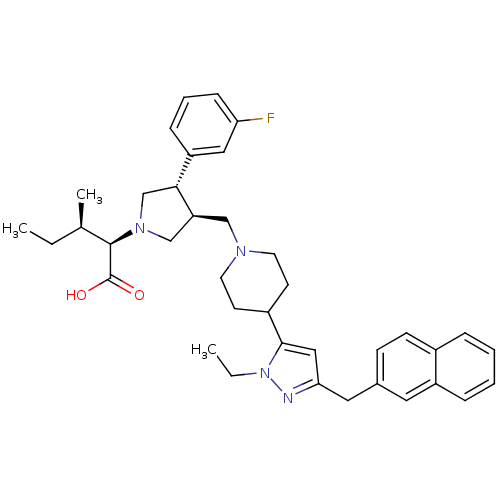 ((2R,4R)-2-[(2S,3S)-3-[4-(2-Ethyl-5-naphthalen-2-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50141946 ((2R,4R)-2-[(2S,3S)-3-[4-(2-Ethyl-5-naphthalen-1-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50141948 ((R)-2-[(2S,3S)-3-[4-(2-Ethyl-5-naphthalen-1-ylmeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50141990 ((R)-Cyclohexyl-[(2S,3S)-3-{4-[5-(4-cyclopropoxy-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50101200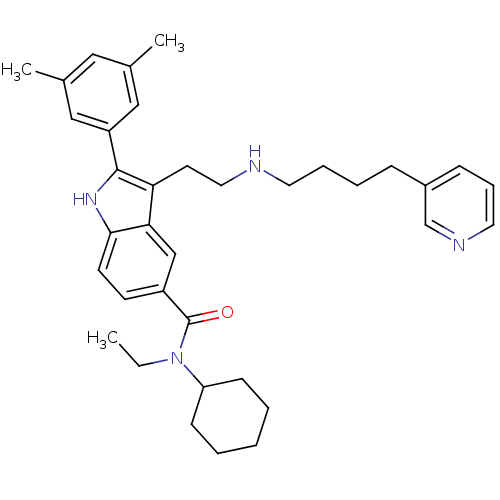 (2-(3,5-Dimethyl-phenyl)-3-[2-(4-pyridin-3-yl-butyl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to rat Gonadotropin-releasing hormone receptor. | Bioorg Med Chem Lett 11: 1727-31 (2001) BindingDB Entry DOI: 10.7270/Q2FQ9VW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50101165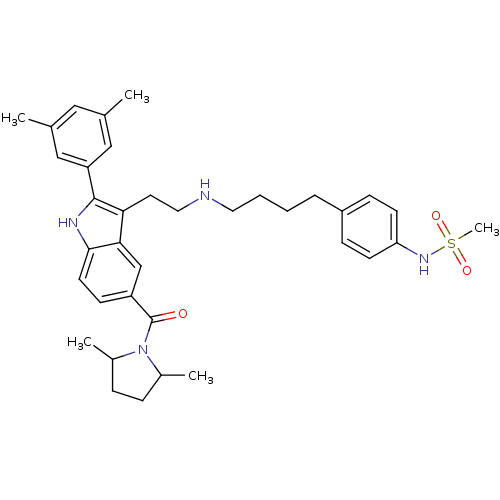 (CHEMBL239108 | N-[4-(4-{2-[2-(3,5-Dimethyl-phenyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin. | Bioorg Med Chem Lett 11: 1723-6 (2001) BindingDB Entry DOI: 10.7270/Q2KH0MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50101191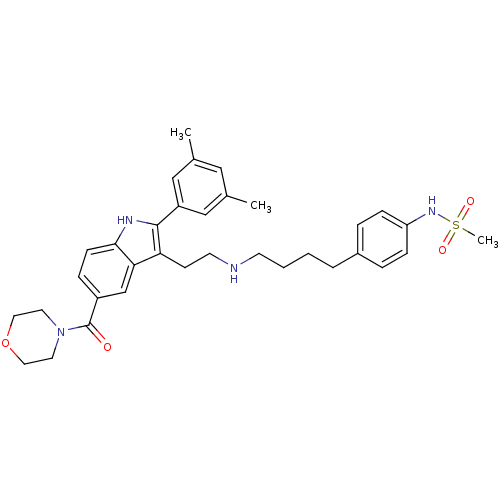 (CHEMBL300045 | N-[4-(4-{2-[2-(3,5-Dimethyl-phenyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition GnRH-stimulated luteinizing hormone (LH) release from rat pituitary cells | Bioorg Med Chem Lett 11: 1723-6 (2001) BindingDB Entry DOI: 10.7270/Q2KH0MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50101171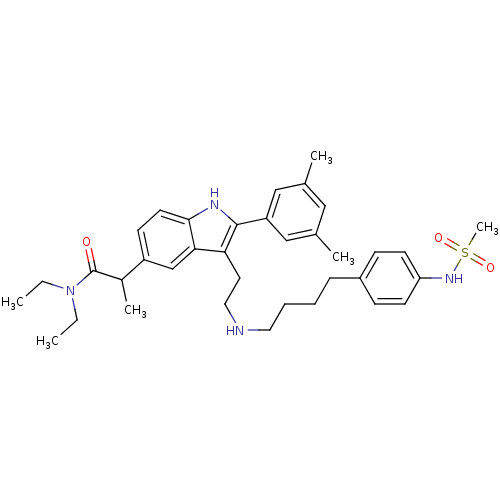 (2-(2-(3,5-Dimethyl-phenyl)-3-{2-[4-(4-methanesulfo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the absence of bovine serum albumin (BSA) | Bioorg Med Chem Lett 11: 1723-6 (2001) BindingDB Entry DOI: 10.7270/Q2KH0MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50101188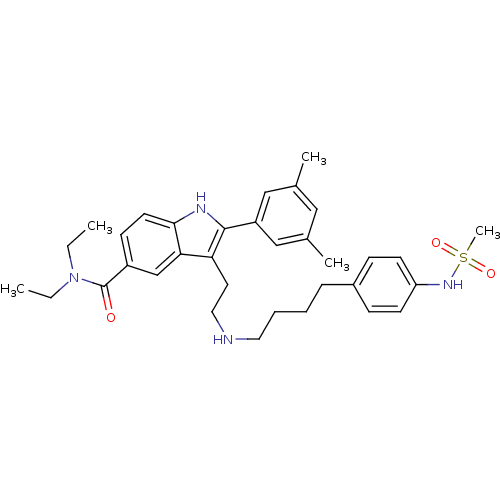 (2-(3,5-Dimethyl-phenyl)-3-{2-[4-(4-methanesulfonyl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition GnRH-stimulated luteinizing hormone (LH) release from rat pituitary cells | Bioorg Med Chem Lett 11: 1723-6 (2001) BindingDB Entry DOI: 10.7270/Q2KH0MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50101194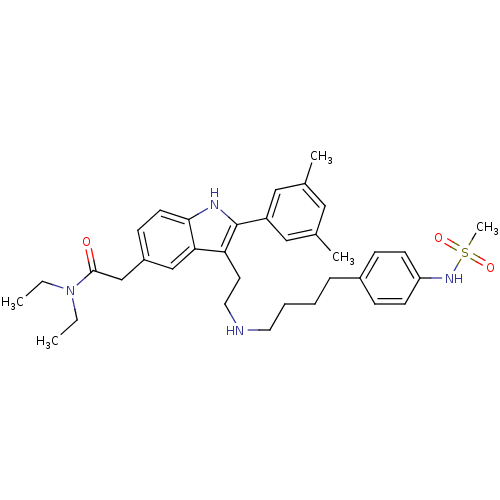 (2-(2-(3,5-Dimethyl-phenyl)-3-{2-[4-(4-methanesulfo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin. | Bioorg Med Chem Lett 11: 1723-6 (2001) BindingDB Entry DOI: 10.7270/Q2KH0MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50101164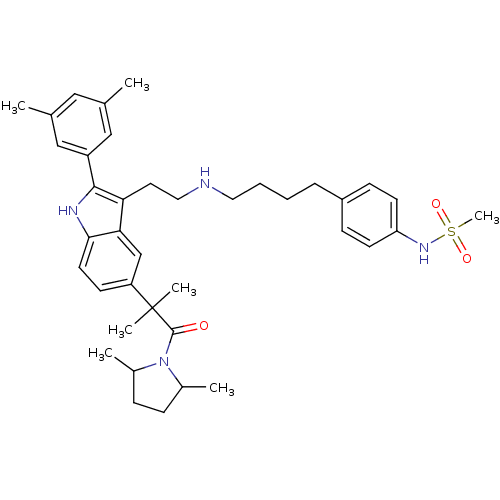 (CHEMBL295152 | N-{4-[4-(2-{2-(3,5-Dimethyl-phenyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the absence of bovine serum albumin (BSA) | Bioorg Med Chem Lett 11: 1723-6 (2001) BindingDB Entry DOI: 10.7270/Q2KH0MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50101169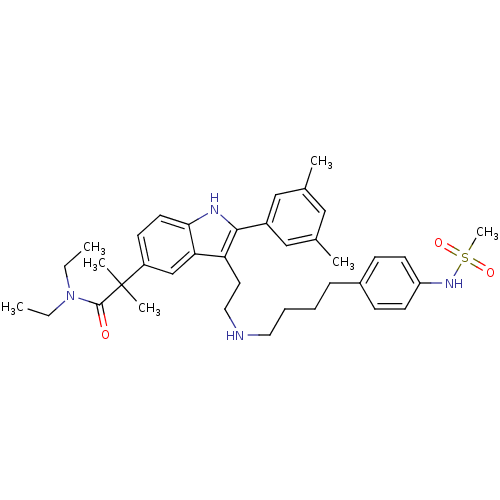 (2-(2-(3,5-Dimethyl-phenyl)-3-{2-[4-(4-methanesulfo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin. | Bioorg Med Chem Lett 11: 1723-6 (2001) BindingDB Entry DOI: 10.7270/Q2KH0MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046798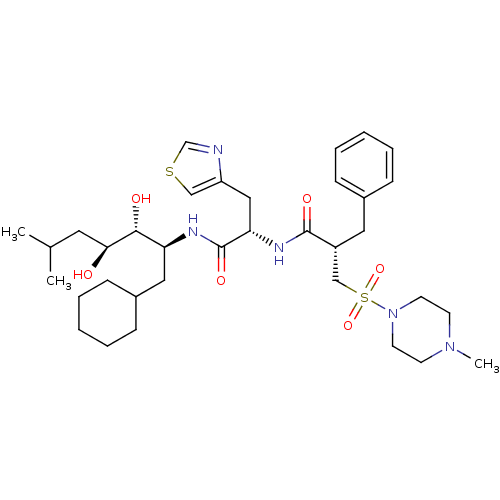 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory concentration against monkey plasma renin at pH 7.4 | J Med Chem 36: 460-7 (1993) BindingDB Entry DOI: 10.7270/Q2VT1R5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50141888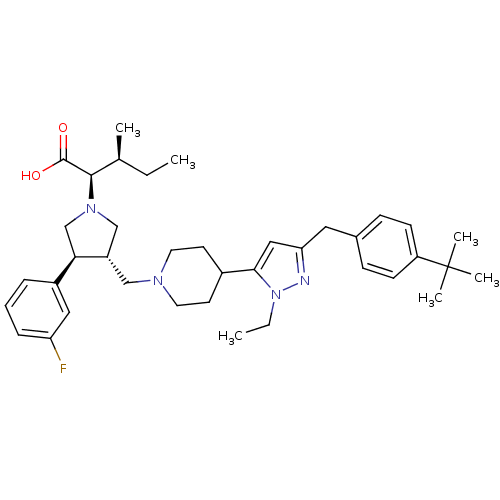 ((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-tert-Butyl-benzyl)-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50155355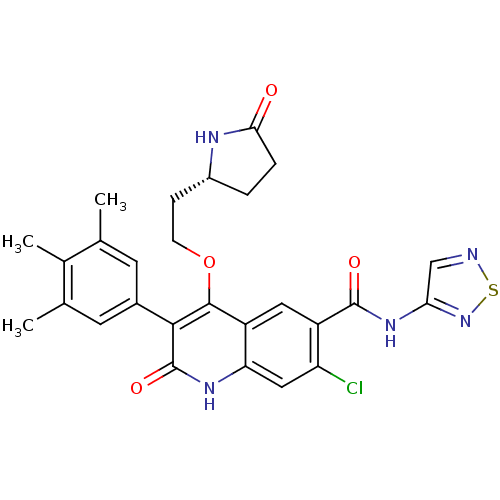 (7-Chloro-2-oxo-4-[2-(5-oxo-pyrrolidin-2-yl)-ethoxy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human gonadotropin releasing hormone receptor expressed in CHO cells was determined by using [125I]-buserelin as radioligand | Bioorg Med Chem Lett 14: 5599-603 (2004) Article DOI: 10.1016/j.bmcl.2004.08.056 BindingDB Entry DOI: 10.7270/Q20V8C8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50141910 ((2R,4S)-2-[(2S,3S)-3-{4-[2-Ethyl-5-(4-isopropyl-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50141912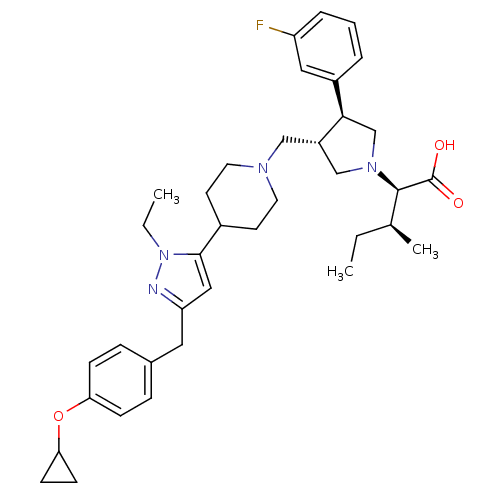 ((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Cyclopropoxy-benzyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50141915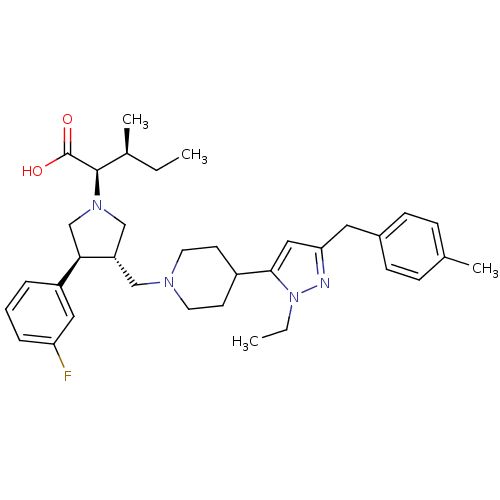 ((2R,4S)-2-[(2S,3S)-3-{4-[2-Ethyl-5-(4-methyl-benzy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50141925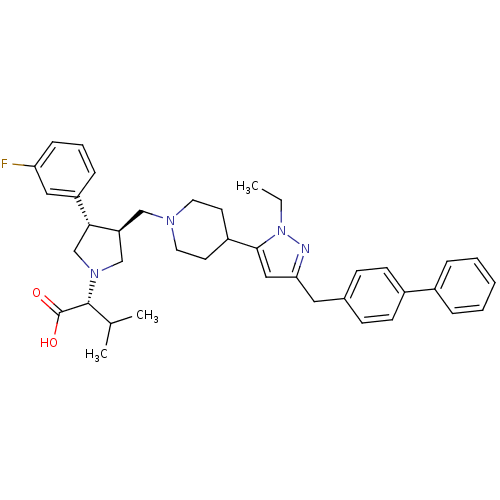 ((R)-2-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-ethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50141931 ((R)-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-ethyl-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50101167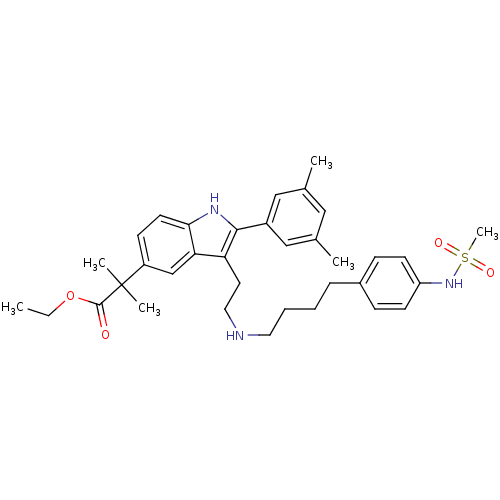 (2-(2-(3,5-Dimethyl-phenyl)-3-{2-[4-(4-methanesulfo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to rat pituitary Gonadotropin-releasing hormone receptor, in the presence of 0.1% bovine serum albumin. | Bioorg Med Chem Lett 11: 1723-6 (2001) BindingDB Entry DOI: 10.7270/Q2KH0MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1740 total ) | Next | Last >> |