

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM23165 (CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1R expressed in CHO cell membranes | ACS Med Chem Lett 2: 97-101 (2011) Article DOI: 10.1021/ml100227q BindingDB Entry DOI: 10.7270/Q2WM1DP6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM22209 (1,2,4-oxadiazole based compound, 26 | 1-[(4-{5-[4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1R expressed in CHO cell membranes | ACS Med Chem Lett 2: 97-101 (2011) Article DOI: 10.1021/ml100227q BindingDB Entry DOI: 10.7270/Q2WM1DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM23165 (CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P3R expressed in CHO cell membranes | ACS Med Chem Lett 2: 97-101 (2011) Article DOI: 10.1021/ml100227q BindingDB Entry DOI: 10.7270/Q2WM1DP6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50112600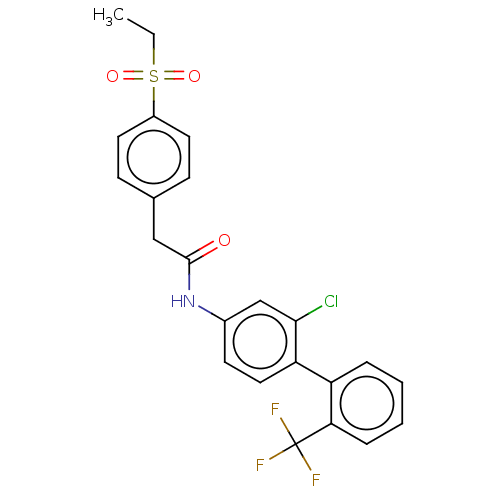 (CHEMBL3609392) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at biotinylated RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bi... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM22203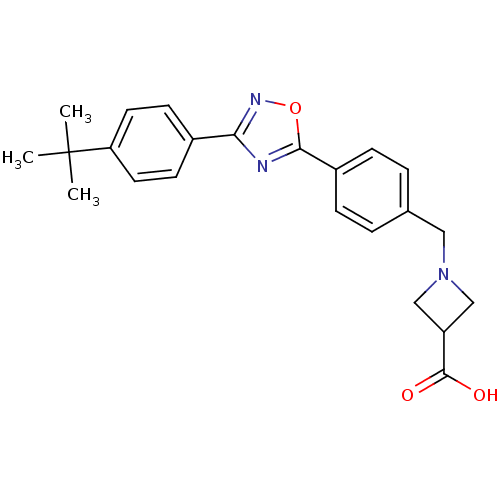 (1,2,4-oxadiazole based compound, 13 | 1-({4-[3-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1R expressed in CHO cell membranes | ACS Med Chem Lett 2: 97-101 (2011) Article DOI: 10.1021/ml100227q BindingDB Entry DOI: 10.7270/Q2WM1DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50445895 (CHEMBL3105674) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at biotinylated RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bi... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50158345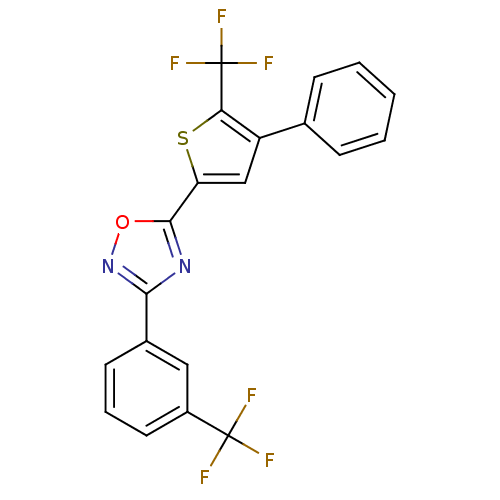 (5-(4-phenyl-5-(trifluoromethyl)thiophen-2-yl)-3-(3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P3R expressed in CHO cell membranes | ACS Med Chem Lett 2: 97-101 (2011) Article DOI: 10.1021/ml100227q BindingDB Entry DOI: 10.7270/Q2WM1DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287460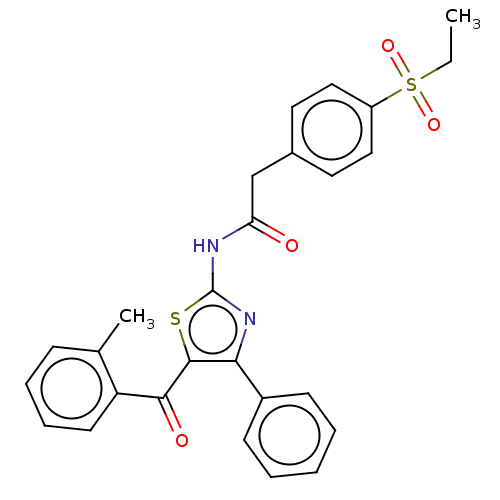 (CHEMBL4171547) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at biotinylated RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bi... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM22203 (1,2,4-oxadiazole based compound, 13 | 1-({4-[3-(4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P3R expressed in CHO cell membranes | ACS Med Chem Lett 2: 97-101 (2011) Article DOI: 10.1021/ml100227q BindingDB Entry DOI: 10.7270/Q2WM1DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM22201 (1,3,4-oxadiazole based compound, 9 | 1-({4-[5-(4-t...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P3R expressed in CHO cell membranes | ACS Med Chem Lett 2: 97-101 (2011) Article DOI: 10.1021/ml100227q BindingDB Entry DOI: 10.7270/Q2WM1DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM22209 (1,2,4-oxadiazole based compound, 26 | 1-[(4-{5-[4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P3R expressed in CHO cell membranes | ACS Med Chem Lett 2: 97-101 (2011) Article DOI: 10.1021/ml100227q BindingDB Entry DOI: 10.7270/Q2WM1DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50112600 (CHEMBL3609392) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at APC-labeled RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bip... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287460 (CHEMBL4171547) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at APC-labeled RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bip... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM50287483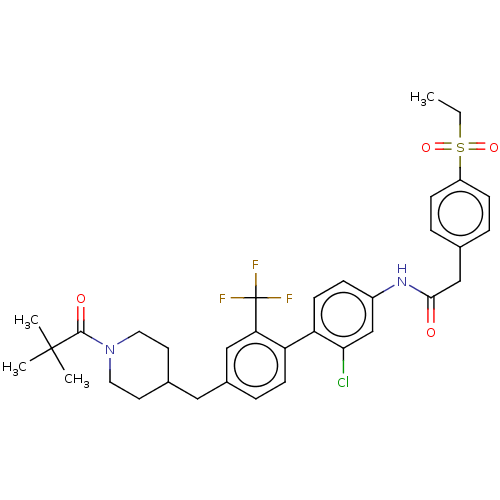 (CHEMBL4173830) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at RORgammat in mouse CD positive T cells assessed as inhibition of Th17 cell differentiation by measuring 1L-17 release aft... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287483 (CHEMBL4173830) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at APC-labeled RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bip... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287463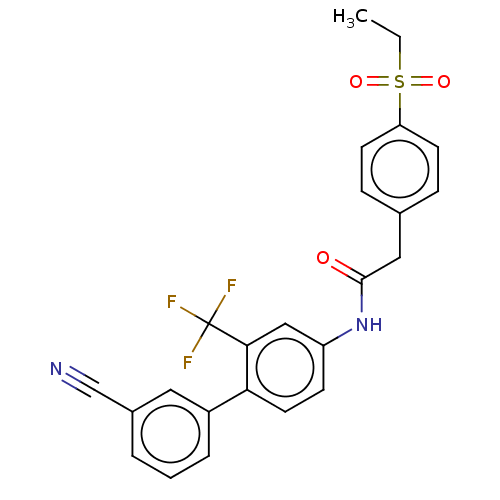 (CHEMBL4163226) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at APC-labeled RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bip... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287470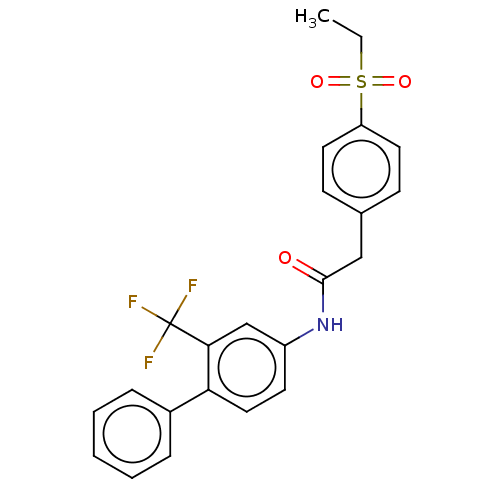 (CHEMBL4172665) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at APC-labeled RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bip... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287483 (CHEMBL4173830) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at biotinylated RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bi... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287470 (CHEMBL4172665) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at biotinylated RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bi... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50158345 (5-(4-phenyl-5-(trifluoromethyl)thiophen-2-yl)-3-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1R expressed in CHO cell membranes | ACS Med Chem Lett 2: 97-101 (2011) Article DOI: 10.1021/ml100227q BindingDB Entry DOI: 10.7270/Q2WM1DP6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50445895 (CHEMBL3105674) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at APC-labeled RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bip... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287466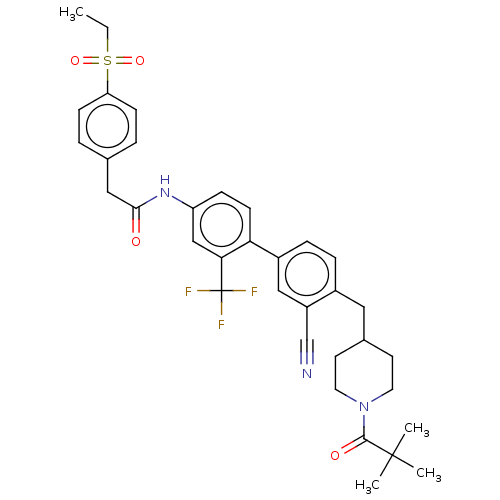 (CHEMBL4163533) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at biotinylated RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bi... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287467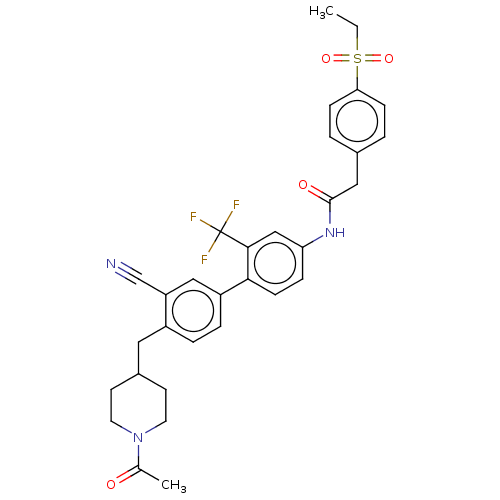 (CHEMBL4159083) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at biotinylated RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bi... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287469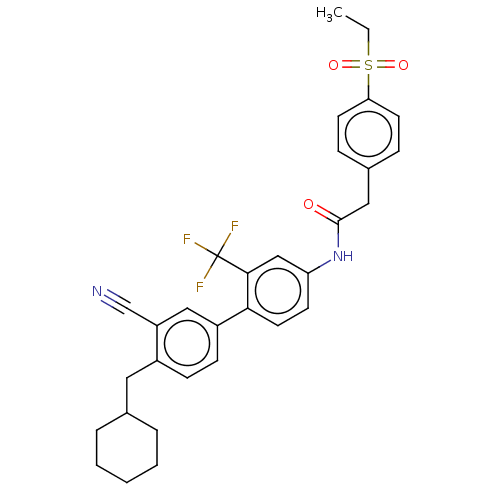 (CHEMBL4166984) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at APC-labeled RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bip... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287464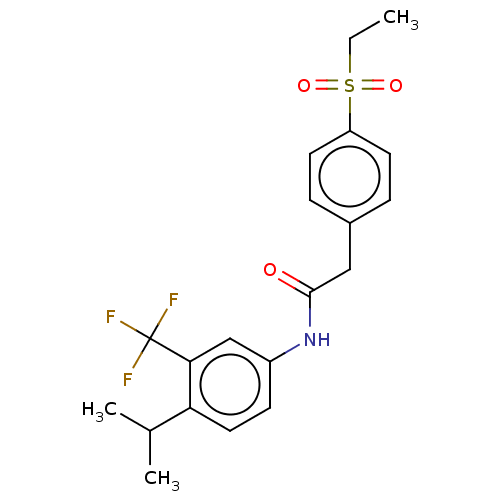 (CHEMBL4164766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at biotinylated RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bi... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287466 (CHEMBL4163533) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at APC-labeled RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bip... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM22201 (1,3,4-oxadiazole based compound, 9 | 1-({4-[5-(4-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1R expressed in CHO cell membranes | ACS Med Chem Lett 2: 97-101 (2011) Article DOI: 10.1021/ml100227q BindingDB Entry DOI: 10.7270/Q2WM1DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287467 (CHEMBL4159083) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at APC-labeled RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bip... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287464 (CHEMBL4164766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at APC-labeled RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bip... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM50112600 (CHEMBL3609392) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at RORgammat in mouse CD positive T cells assessed as inhibition of Th17 cell differentiation by measuring 1L-17 release aft... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287468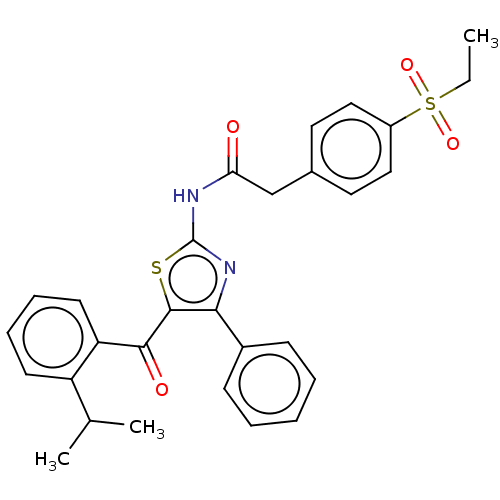 (CHEMBL4160934) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at APC-labeled RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bip... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287463 (CHEMBL4163226) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Antagonist activity at biotinylated RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bipheny... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50336946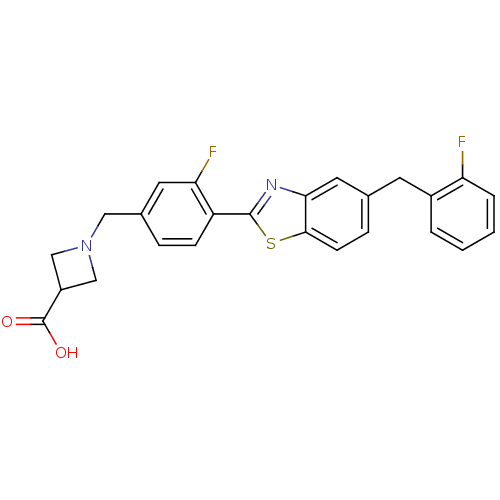 (1-((4-(5-(2-Fluorobenzyl)benzo[d]thiazol-2-yl)-3-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | ACS Med Chem Lett 2: 102-106 (2011) Article DOI: 10.1021/ml100228m BindingDB Entry DOI: 10.7270/Q2T72HR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287469 (CHEMBL4166984) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Antagonist activity at biotinylated RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bipheny... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50335462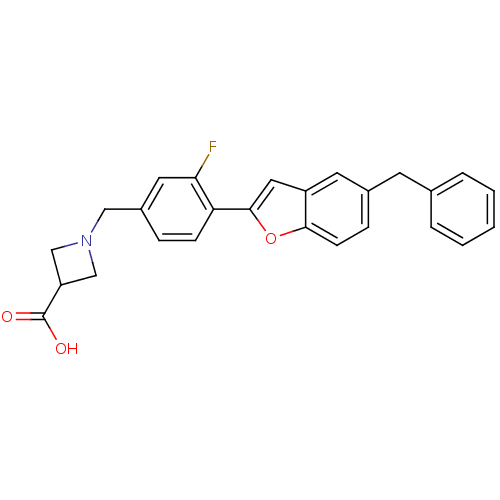 (1-(4-(5-benzylbenzofuran-2-yl)-3-fluorobenzyl)azet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human ERG by patch-clamp method | ACS Med Chem Lett 2: 97-101 (2011) Article DOI: 10.1021/ml100227q BindingDB Entry DOI: 10.7270/Q2WM1DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287462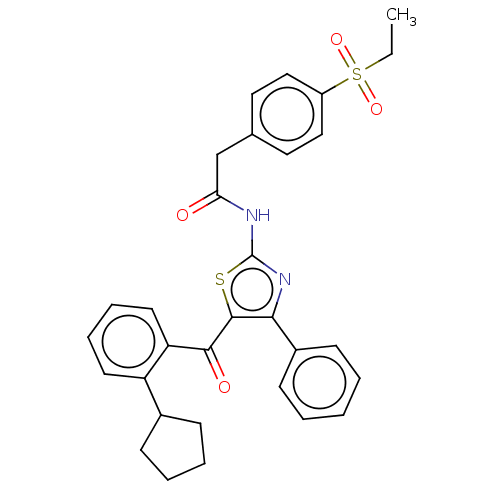 (CHEMBL4169276) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at APC-labeled RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bip... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287461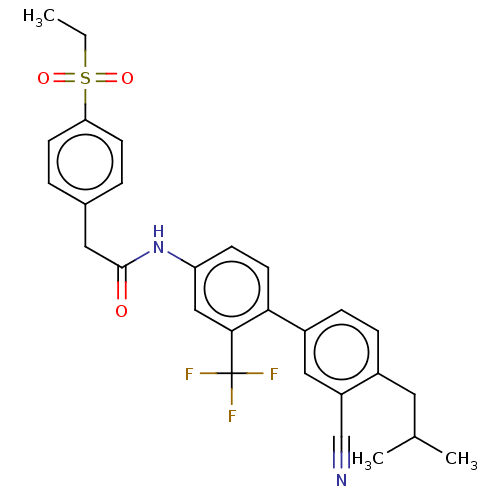 (CHEMBL4171142) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at APC-labeled RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bip... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50287465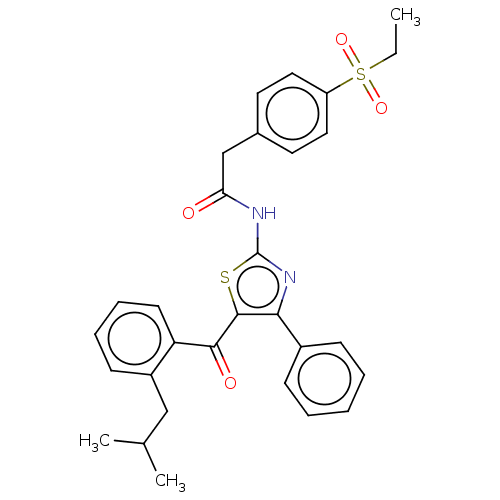 (CHEMBL4161349) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at APC-labeled RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bip... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50336938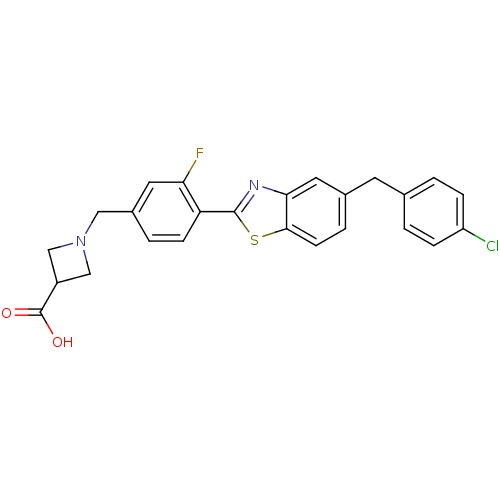 (1-((4-(5-(4-Chlorobenzyl)benzo[d]thiazol-2-yl)-3-f...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells co-expressing Gq/i5 G-protein assessed as calcium mobilization by FLIPR assay | ACS Med Chem Lett 2: 102-106 (2011) Article DOI: 10.1021/ml100228m BindingDB Entry DOI: 10.7270/Q2T72HR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50336939 (1-((4-(5-(2,6-Difluorobenzyl)benzo[d]thiazol-2-yl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells co-expressing Gq/i5 G-protein assessed as calcium mobilization by FLIPR assay | ACS Med Chem Lett 2: 102-106 (2011) Article DOI: 10.1021/ml100228m BindingDB Entry DOI: 10.7270/Q2T72HR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50336940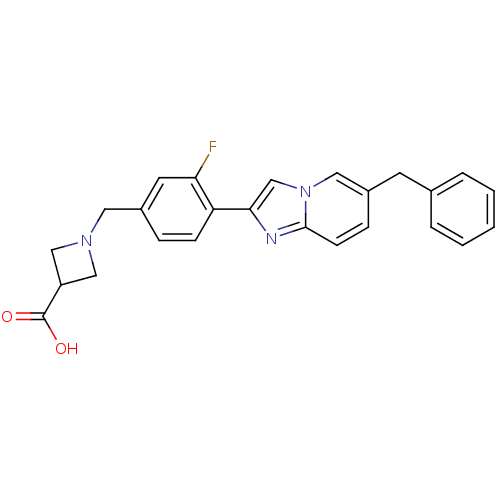 (1-(4-(6-Benzylimidazo[1,2-a]pyridin-2-yl)-3-fluoro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells co-expressing Gq/i5 G-protein assessed as calcium mobilization by FLIPR assay | ACS Med Chem Lett 2: 102-106 (2011) Article DOI: 10.1021/ml100228m BindingDB Entry DOI: 10.7270/Q2T72HR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50336941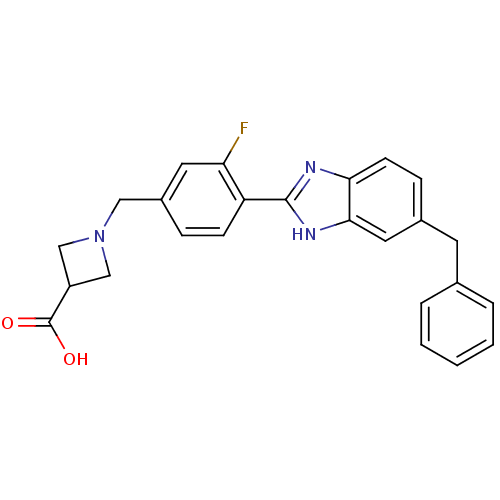 (1-(4-(6-Benzyl-1H-benzo[d]imidazol-2-yl)-3-fluorob...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells co-expressing Gq/i5 G-protein assessed as calcium mobilization by FLIPR assay | ACS Med Chem Lett 2: 102-106 (2011) Article DOI: 10.1021/ml100228m BindingDB Entry DOI: 10.7270/Q2T72HR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50336942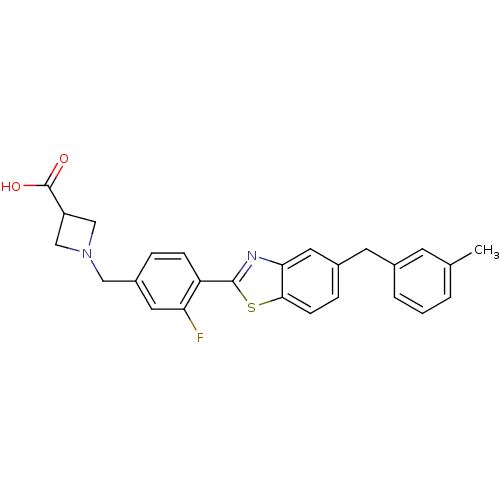 (1-(3-Fluoro-4-(5-(3-methylbenzyl)benzo[d]thiazol-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells co-expressing Gq/i5 G-protein assessed as calcium mobilization by FLIPR assay | ACS Med Chem Lett 2: 102-106 (2011) Article DOI: 10.1021/ml100228m BindingDB Entry DOI: 10.7270/Q2T72HR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50336943 (1-(4-(7-Benzylimidazo[1,2-a]pyridin-2-yl)-3-fluoro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.09E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells co-expressing Gq/i5 G-protein assessed as calcium mobilization by FLIPR assay | ACS Med Chem Lett 2: 102-106 (2011) Article DOI: 10.1021/ml100228m BindingDB Entry DOI: 10.7270/Q2T72HR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50336944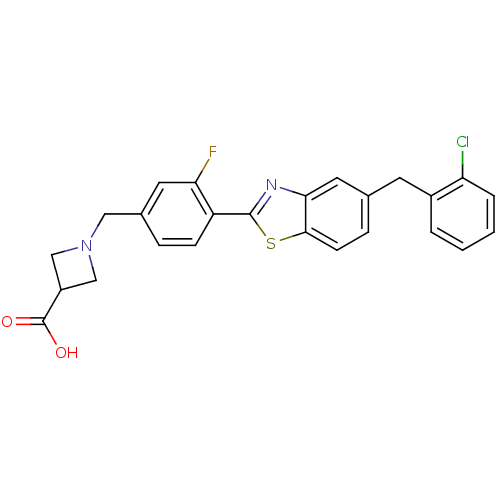 (1-((4-(5-(2-Chlorobenzyl)benzo[d]thiazol-2-yl)-3-f...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.88E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells co-expressing Gq/i5 G-protein assessed as calcium mobilization by FLIPR assay | ACS Med Chem Lett 2: 102-106 (2011) Article DOI: 10.1021/ml100228m BindingDB Entry DOI: 10.7270/Q2T72HR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50336945 (1-((4-(5-(3-Chlorobenzyl)benzo[d]thiazol-2-yl)-3-f...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.88E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells co-expressing Gq/i5 G-protein assessed as calcium mobilization by FLIPR assay | ACS Med Chem Lett 2: 102-106 (2011) Article DOI: 10.1021/ml100228m BindingDB Entry DOI: 10.7270/Q2T72HR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50336946 (1-((4-(5-(2-Fluorobenzyl)benzo[d]thiazol-2-yl)-3-f...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells co-expressing Gq/i5 G-protein assessed as calcium mobilization by FLIPR assay | ACS Med Chem Lett 2: 102-106 (2011) Article DOI: 10.1021/ml100228m BindingDB Entry DOI: 10.7270/Q2T72HR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50335508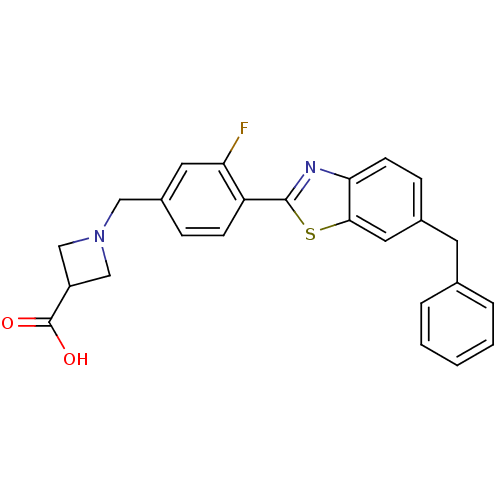 (1-(4-(6-benzylbenzo[d]thiazol-2-yl)-3-fluorobenzyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells co-expressing Gq/i5 G-protein assessed as calcium mobilization by FLIPR assay | ACS Med Chem Lett 2: 102-106 (2011) Article DOI: 10.1021/ml100228m BindingDB Entry DOI: 10.7270/Q2T72HR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50336947 (1-(4-(6-Benzyl-2H-indazol-2-yl)-3-fluorobenzyl)aze...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells co-expressing Gq/i5 G-protein assessed as calcium mobilization by FLIPR assay | ACS Med Chem Lett 2: 102-106 (2011) Article DOI: 10.1021/ml100228m BindingDB Entry DOI: 10.7270/Q2T72HR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50336948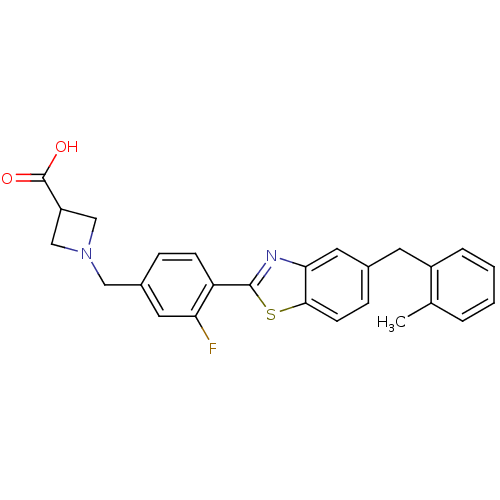 (1-(3-Fluoro-4-(5-(2-methylbenzyl)benzo[d]thiazol-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.44E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells co-expressing Gq/i5 G-protein assessed as calcium mobilization by FLIPR assay | ACS Med Chem Lett 2: 102-106 (2011) Article DOI: 10.1021/ml100228m BindingDB Entry DOI: 10.7270/Q2T72HR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 142 total ) | Next | Last >> |