

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81731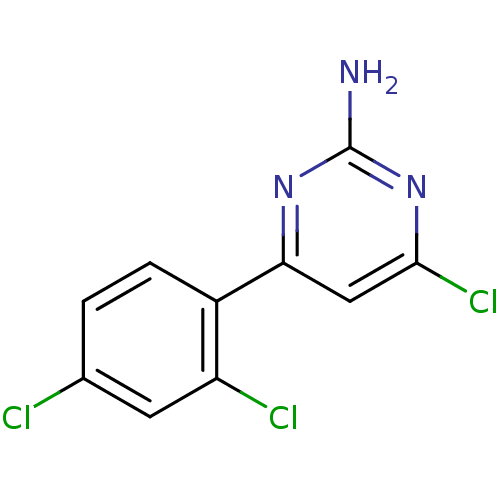 (HSP90 Inhibitor, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 60 | -41.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Abbott Laboratories | Assay Description HSP90 inhibitors identified using Fluorescence resonance energy transfer assay. | Chem Biol Drug Des 70: 1-12 (2007) Article DOI: 10.1111/j.1747-0285.2007.00535.x BindingDB Entry DOI: 10.7270/Q25X27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81730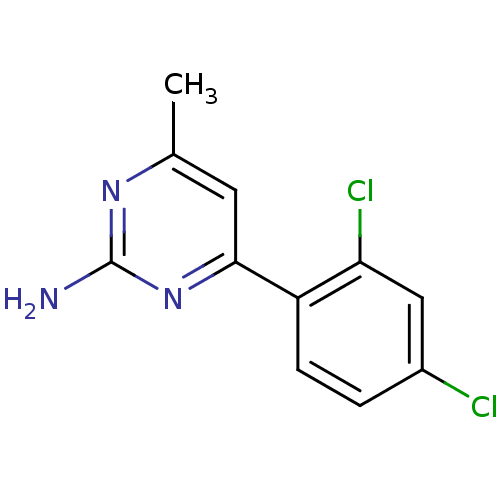 (HSP90 Inhibitor, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 170 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Abbott Laboratories | Assay Description HSP90 inhibitors identified using Fluorescence resonance energy transfer assay. | Chem Biol Drug Des 70: 1-12 (2007) Article DOI: 10.1111/j.1747-0285.2007.00535.x BindingDB Entry DOI: 10.7270/Q25X27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81729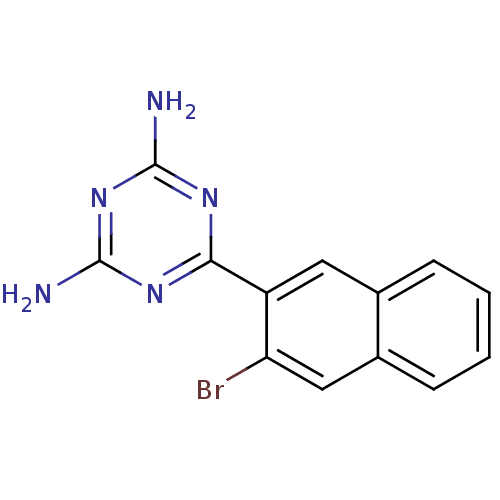 (HSP90 Inhibitor, 1 | hsp90_125) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | 320 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Abbott Laboratories | Assay Description HSP90 inhibitors identified using Fluorescence resonance energy transfer assay. | Chem Biol Drug Des 70: 1-12 (2007) Article DOI: 10.1111/j.1747-0285.2007.00535.x BindingDB Entry DOI: 10.7270/Q25X27DH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81732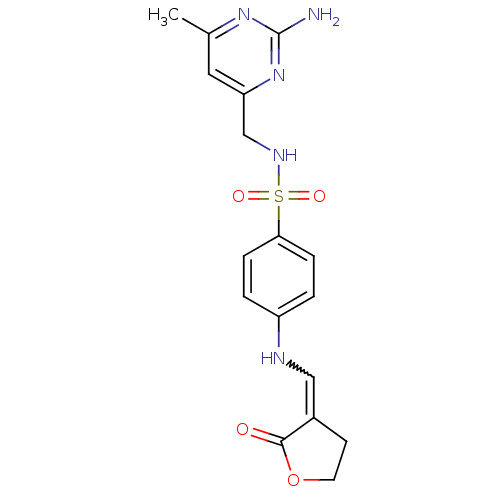 (HSP90 Inhibitor, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Abbott Laboratories | Assay Description HSP90 inhibitors identified using Fluorescence resonance energy transfer assay. | Chem Biol Drug Des 70: 1-12 (2007) Article DOI: 10.1111/j.1747-0285.2007.00535.x BindingDB Entry DOI: 10.7270/Q25X27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81733 (HSP90 Inhibitor, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.00E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Abbott Laboratories | Assay Description HSP90 inhibitors identified using Fluorescence resonance energy transfer assay. | Chem Biol Drug Des 70: 1-12 (2007) Article DOI: 10.1111/j.1747-0285.2007.00535.x BindingDB Entry DOI: 10.7270/Q25X27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50270588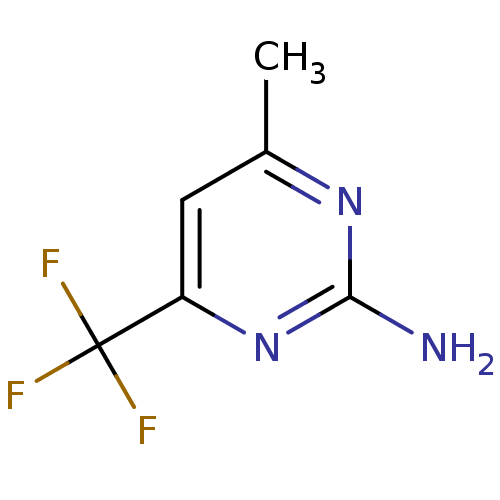 (4-METHYL-6-(TRIFLUOROMETHYL)PYRIMIDIN-2-AMINE | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | 1.80E+4 | -27.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Abbott Laboratories | Assay Description HSP90 inhibitors identified using Fluorescence resonance energy transfer assay. | Chem Biol Drug Des 70: 1-12 (2007) Article DOI: 10.1111/j.1747-0285.2007.00535.x BindingDB Entry DOI: 10.7270/Q25X27DH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121838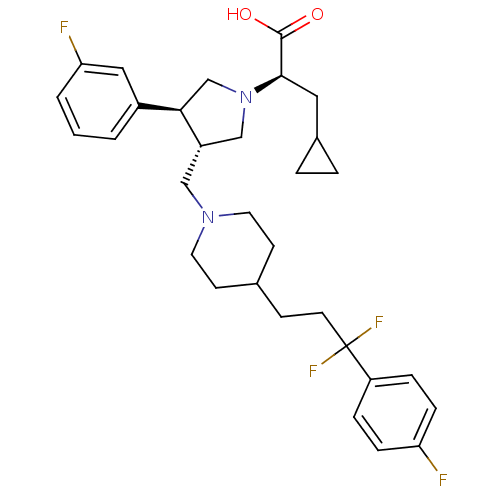 ((R)-3-cyclopropyl-2-((3S,4S)-3-((4-(3,3-difluoro-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119349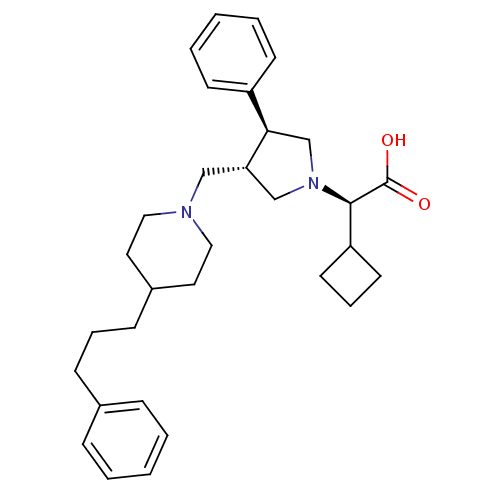 ((R)-2-cyclobutyl-2-((3S,4S)-3-phenyl-4-((4-(3-phen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. | Bioorg Med Chem Lett 12: 3001-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6C71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119338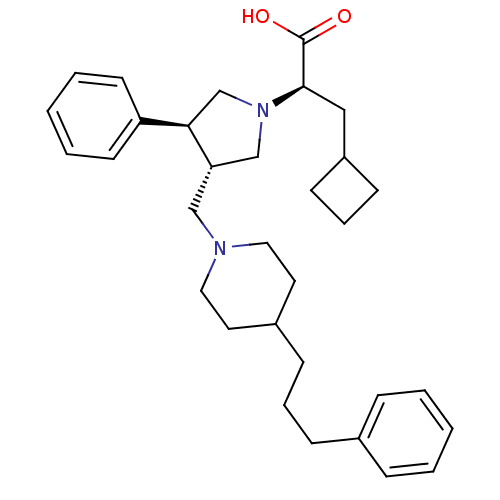 ((R)-3-Cyclobutyl-2-{(3S,4S)-3-phenyl-4-[4-(3-pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-MIP-1 alpha from human C-C chemokine receptor type 5 expressed on CHO cell membranes | Bioorg Med Chem Lett 15: 2129-34 (2005) Article DOI: 10.1016/j.bmcl.2005.02.030 BindingDB Entry DOI: 10.7270/Q21C1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119338 ((R)-3-Cyclobutyl-2-{(3S,4S)-3-phenyl-4-[4-(3-pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. | Bioorg Med Chem Lett 12: 3001-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6C71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121836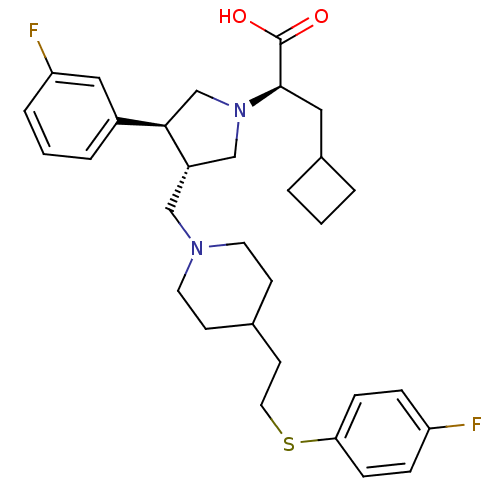 ((R)-3-Cyclobutyl-2-((S)-3-(3-fluoro-phenyl)-4-{4-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119341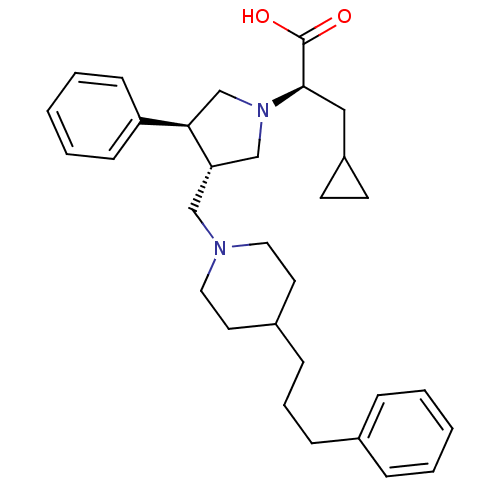 ((R)-3-cyclopropyl-2-((3S,4S)-3-phenyl-4-((4-(3-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. | Bioorg Med Chem Lett 12: 3001-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6C71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119336 (2-cyclohexyl-2-{3-phenyl-4-[4-(3-phenylpropyl)hexa...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. | Bioorg Med Chem Lett 12: 3001-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6C71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119338 ((R)-3-Cyclobutyl-2-{(3S,4S)-3-phenyl-4-[4-(3-pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50110088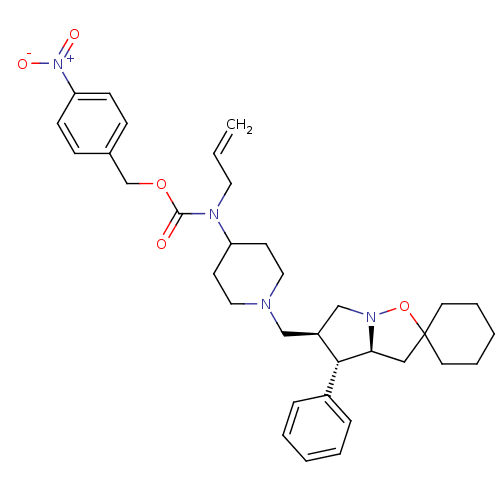 (4N-allyl-4N-[4-nitrobenzyloxycarboyl]-1-[4'-phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]-labeled MIP-1alpha from the C-C chemokine receptor type 5 expressed on CHO cell membranes | Bioorg Med Chem Lett 12: 677-9 (2002) BindingDB Entry DOI: 10.7270/Q2DF6QHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50152322 (CHEMBL184879 | {2-[5-(4-Nonyl-phenyl)-pyrrolidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes | Bioorg Med Chem Lett 14: 4861-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.049 BindingDB Entry DOI: 10.7270/Q2BK1D38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50152328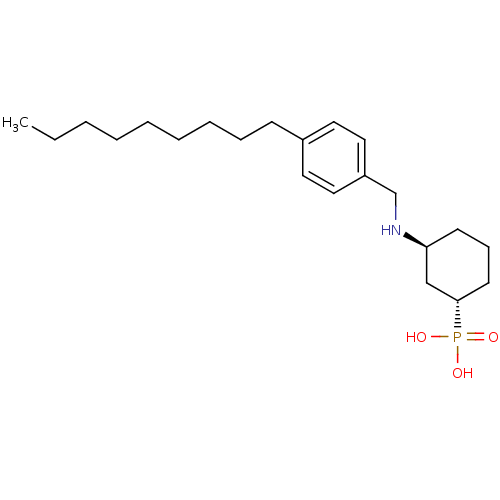 (CHEMBL181597 | [(1S,3S)-3-(4-Nonyl-benzylamino)-cy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes | Bioorg Med Chem Lett 14: 4861-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.049 BindingDB Entry DOI: 10.7270/Q2BK1D38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50152322 (CHEMBL184879 | {2-[5-(4-Nonyl-phenyl)-pyrrolidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes | Bioorg Med Chem Lett 14: 4861-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.049 BindingDB Entry DOI: 10.7270/Q2BK1D38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119321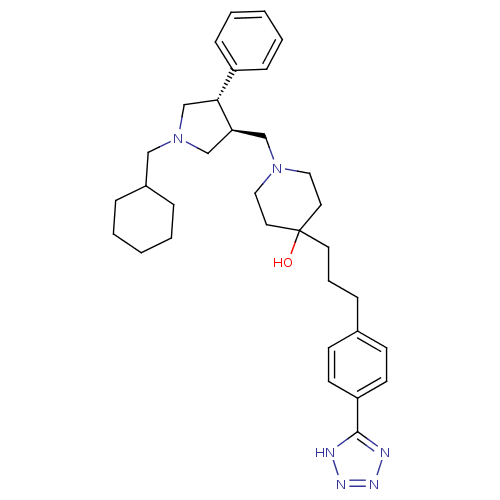 (1-(1-Cyclohexylmethyl-4-phenyl-pyrrolidin-3-ylmeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. | Bioorg Med Chem Lett 12: 3001-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6C71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119342 ((R)-2-cyclopentyl-2-((3S,4S)-3-phenyl-4-((4-(3-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. | Bioorg Med Chem Lett 12: 3001-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6C71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121833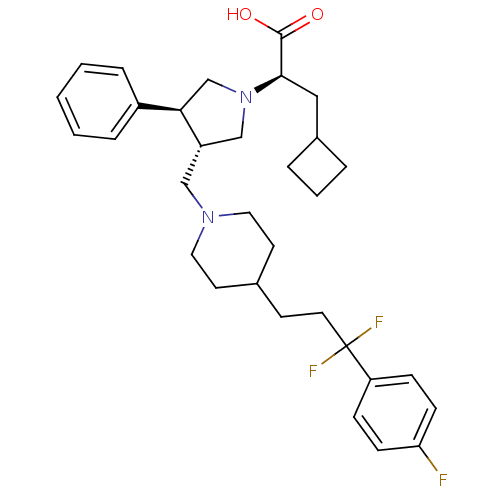 ((R)-3-cyclobutyl-2-((3S,4S)-3-((4-(3,3-difluoro-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121828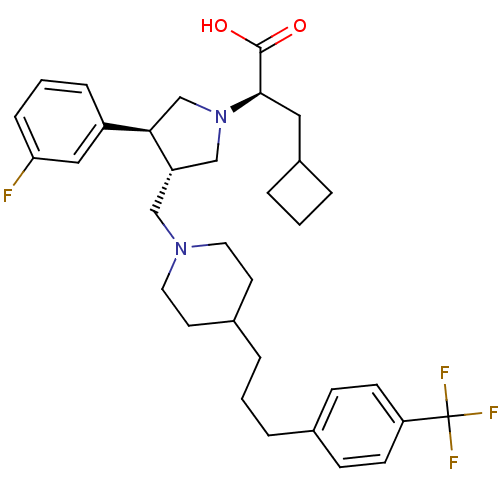 ((R)-3-Cyclobutyl-2-((S)-3-(3-fluoro-phenyl)-4-{4-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50158348 ((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine 1 phosphate from human S1P3 receptor expressed in CHO cells | J Med Chem 47: 6662-5 (2004) Article DOI: 10.1021/jm0492507 BindingDB Entry DOI: 10.7270/Q2TQ611F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM23163 (CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine 1 phosphate from human S1P1 receptor expressed in CHO cells | J Med Chem 47: 6662-5 (2004) Article DOI: 10.1021/jm0492507 BindingDB Entry DOI: 10.7270/Q2TQ611F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50152337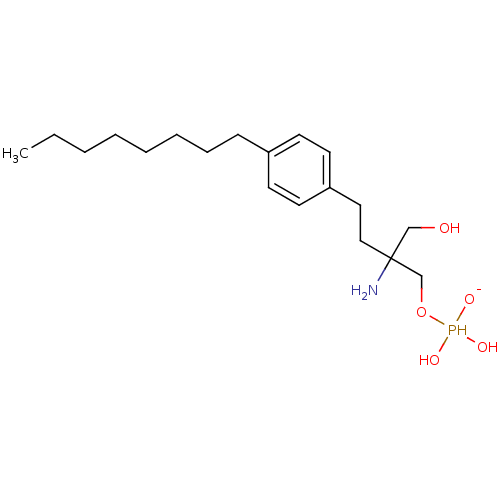 (2-amino-2-({[dihydroxy(oxido)--phosphanyl]oxy}meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes | Bioorg Med Chem Lett 14: 4861-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.049 BindingDB Entry DOI: 10.7270/Q2BK1D38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121819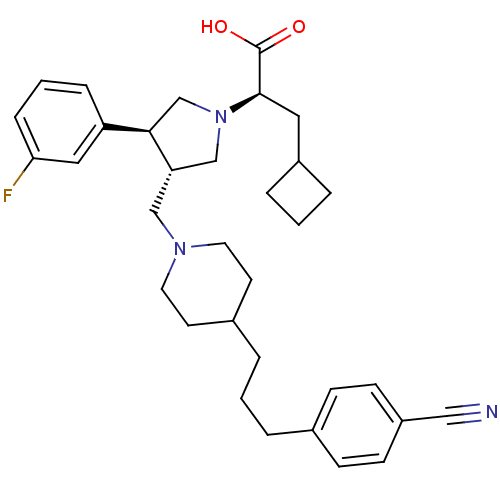 ((R)-2-[(S)-3-{4-[3-(4-Cyano-phenyl)-propyl]-piperi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121829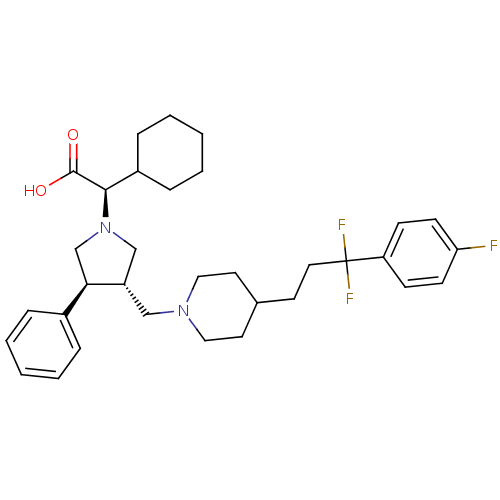 ((R)-Cyclohexyl-((3S,4S)-3-{4-[3,3-difluoro-3-(4-fl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121818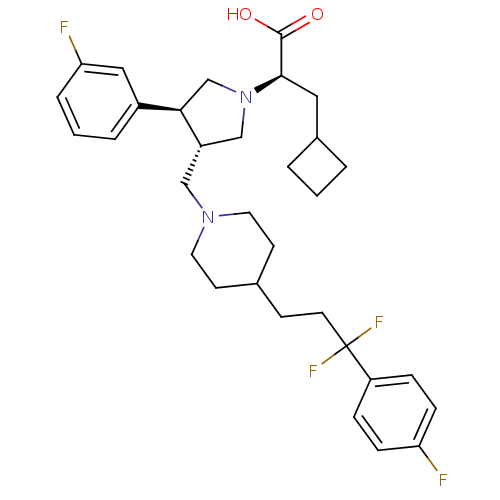 ((R)-3-cyclobutyl-2-((3S,4S)-3-((4-(3,3-difluoro-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50165072 ((R)-3-Cyclobutyl-2-[(3S,4S)-3-[4-(6-fluoro-imidazo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-MIP-1 alpha from human C-C chemokine receptor type 5 expressed on CHO cell membranes | Bioorg Med Chem Lett 15: 2129-34 (2005) Article DOI: 10.1016/j.bmcl.2005.02.030 BindingDB Entry DOI: 10.7270/Q21C1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM22218 (1,2,4-oxadiazole based compound, 35 | 1-[(4-{5-[4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Research Laboratories | Assay Description The assay measures the displacement of [33P]-labeled sphingosine-1-phosphate (S1P) by test compounds from human S1P receptors expressed on CHO cell m... | J Med Chem 48: 6169-73 (2005) Article DOI: 10.1021/jm0503244 BindingDB Entry DOI: 10.7270/Q26971W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50152329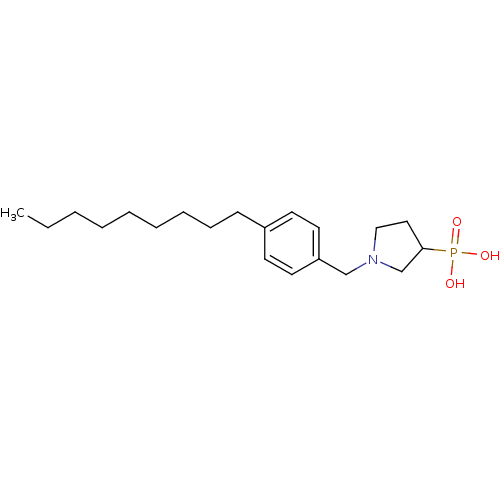 (1-(4-nonylbenzyl)pyrrolidin-3-ylphosphonic acid | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes | Bioorg Med Chem Lett 14: 4861-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.049 BindingDB Entry DOI: 10.7270/Q2BK1D38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50158348 ((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine 1 phosphate from human S1P2 receptor expressed in CHO cells | J Med Chem 47: 6662-5 (2004) Article DOI: 10.1021/jm0492507 BindingDB Entry DOI: 10.7270/Q2TQ611F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50165099 ((R)-3-Cyclobutyl-2-[(3S,4S)-3-[4-(7-ethyl-imidazo[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-MIP-1 alpha from human C-C chemokine receptor type 5 expressed on CHO cell membranes | Bioorg Med Chem Lett 15: 2129-34 (2005) Article DOI: 10.1016/j.bmcl.2005.02.030 BindingDB Entry DOI: 10.7270/Q21C1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121837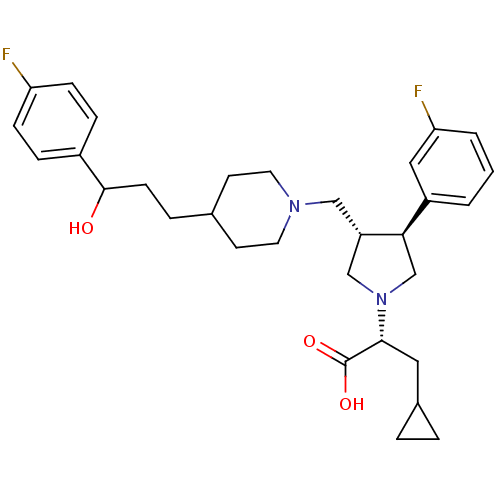 ((2R)-3-cyclopropyl-2-((3S,4S)-3-(3-fluorophenyl)-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM22215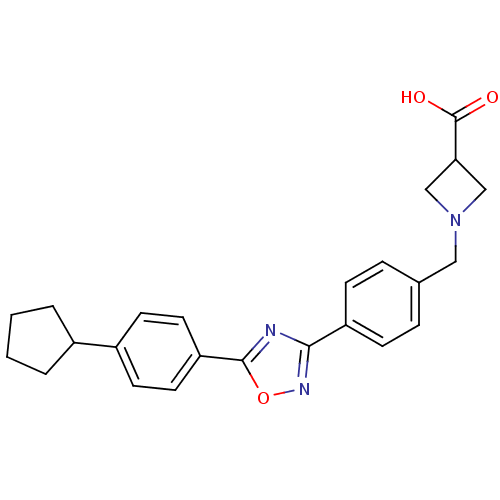 (1,2,4-oxadiazole based compound, 32 | 1-({4-[5-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories | Assay Description The assay measures the displacement of [33P]-labeled sphingosine-1-phosphate (S1P) by test compounds from human S1P receptors expressed on CHO cell m... | J Med Chem 48: 6169-73 (2005) Article DOI: 10.1021/jm0503244 BindingDB Entry DOI: 10.7270/Q26971W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM22218 (1,2,4-oxadiazole based compound, 35 | 1-[(4-{5-[4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories | Assay Description The assay measures the displacement of [33P]-labeled sphingosine-1-phosphate (S1P) by test compounds from human S1P receptors expressed on CHO cell m... | J Med Chem 48: 6169-73 (2005) Article DOI: 10.1021/jm0503244 BindingDB Entry DOI: 10.7270/Q26971W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM22220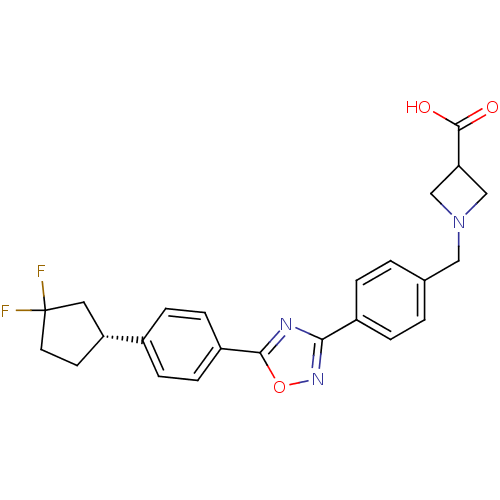 (1,2,4-oxadiazole based compound, 37 | 1-{[4-(5-{4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Research Laboratories | Assay Description The assay measures the displacement of [33P]-labeled sphingosine-1-phosphate (S1P) by test compounds from human S1P receptors expressed on CHO cell m... | J Med Chem 48: 6169-73 (2005) Article DOI: 10.1021/jm0503244 BindingDB Entry DOI: 10.7270/Q26971W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50410168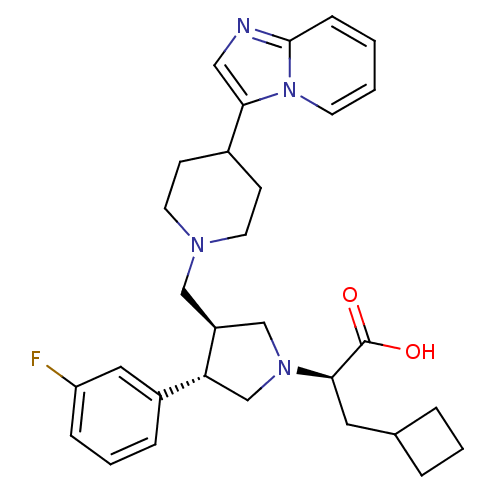 (CHEMBL2113086) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-MIP-1 alpha from human C-C chemokine receptor type 5 expressed on CHO cell membranes | Bioorg Med Chem Lett 15: 2129-34 (2005) Article DOI: 10.1016/j.bmcl.2005.02.030 BindingDB Entry DOI: 10.7270/Q21C1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM22219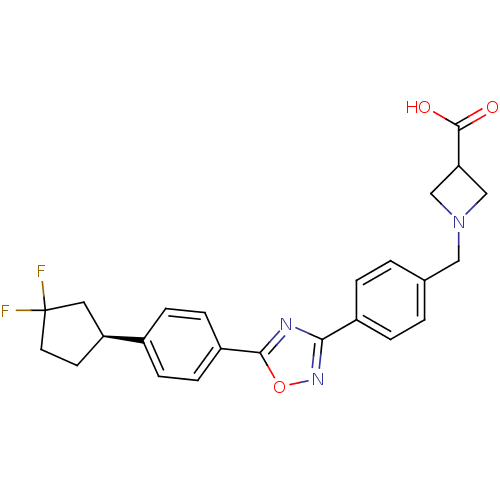 (1,2,4-oxadiazole based compound, 36 | 1-{[4-(5-{4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Research Laboratories | Assay Description The assay measures the displacement of [33P]-labeled sphingosine-1-phosphate (S1P) by test compounds from human S1P receptors expressed on CHO cell m... | J Med Chem 48: 6169-73 (2005) Article DOI: 10.1021/jm0503244 BindingDB Entry DOI: 10.7270/Q26971W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119343 (2-cyclohexyl-2-{3-[4-hydroxy-4-(3-phenylpropyl)hex...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. | Bioorg Med Chem Lett 12: 3001-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6C71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119343 (2-cyclohexyl-2-{3-[4-hydroxy-4-(3-phenylpropyl)hex...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. | Bioorg Med Chem Lett 12: 3001-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6C71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM22215 (1,2,4-oxadiazole based compound, 32 | 1-({4-[5-(4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Research Laboratories | Assay Description The assay measures the displacement of [33P]-labeled sphingosine-1-phosphate (S1P) by test compounds from human S1P receptors expressed on CHO cell m... | J Med Chem 48: 6169-73 (2005) Article DOI: 10.1021/jm0503244 BindingDB Entry DOI: 10.7270/Q26971W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121831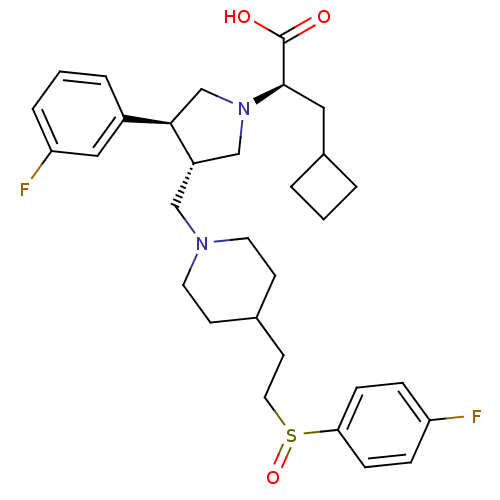 ((R)-3-Cyclobutyl-2-[(S)-3-{4-[2-(4-fluoro-benzenes...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121835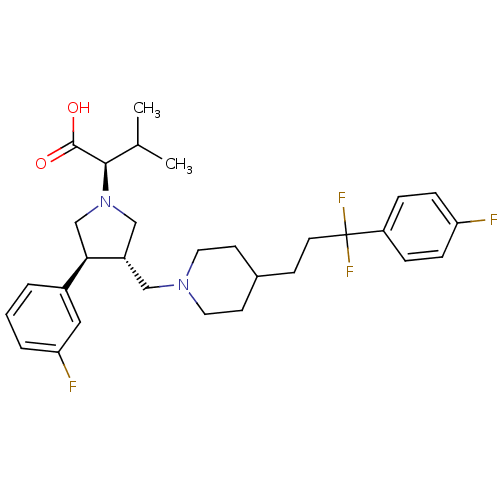 (2-[(S)-3-{4-[3,3-Difluoro-3-(4-fluoro-phenyl)-prop...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121832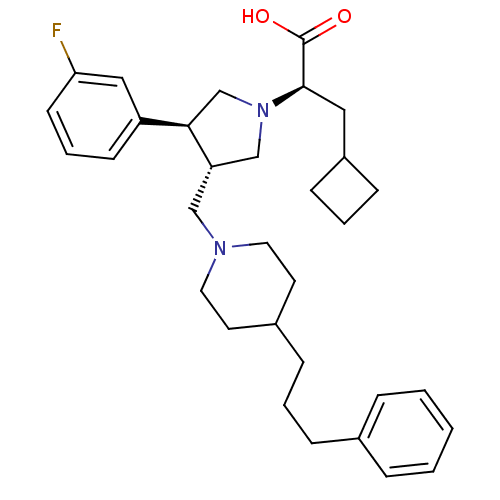 ((R)-3-Cyclobutyl-2-{(S)-3-(3-fluoro-phenyl)-4-[4-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50165085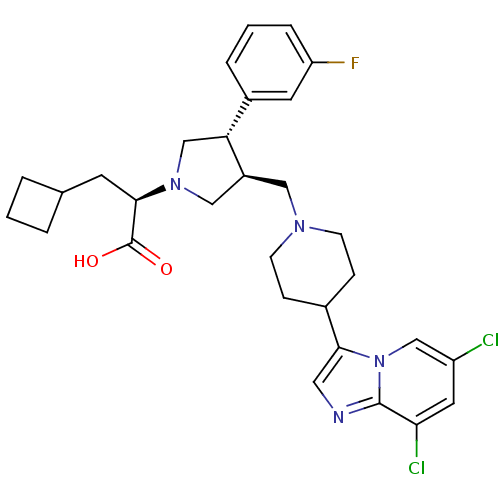 ((R)-3-Cyclobutyl-2-[(3S,4S)-3-[4-(6,8-dichloro-imi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-MIP-1 alpha from human C-C chemokine receptor type 5 expressed on CHO cell membranes | Bioorg Med Chem Lett 15: 2129-34 (2005) Article DOI: 10.1016/j.bmcl.2005.02.030 BindingDB Entry DOI: 10.7270/Q21C1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121831 ((R)-3-Cyclobutyl-2-[(S)-3-{4-[2-(4-fluoro-benzenes...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50158348 ((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine 1 phosphate from human S1P5 receptor expressed in CHO cells | J Med Chem 47: 6662-5 (2004) Article DOI: 10.1021/jm0492507 BindingDB Entry DOI: 10.7270/Q2TQ611F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM22209 (1,2,4-oxadiazole based compound, 26 | 1-[(4-{5-[4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 3564-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.090 BindingDB Entry DOI: 10.7270/Q2WS8SWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50165073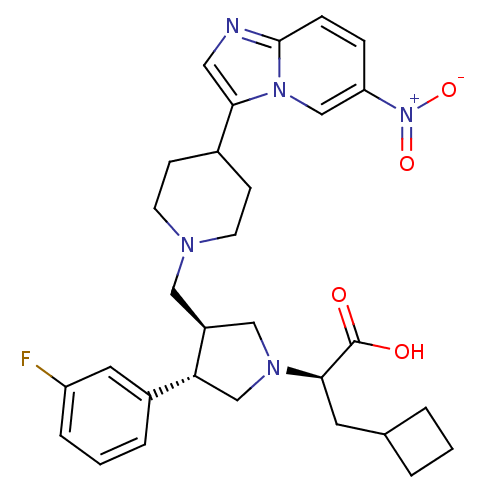 ((R)-3-Cyclobutyl-2-{(3S,4S)-3-(3-fluoro-phenyl)-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-MIP-1 alpha from human C-C chemokine receptor type 5 expressed on CHO cell membranes | Bioorg Med Chem Lett 15: 2129-34 (2005) Article DOI: 10.1016/j.bmcl.2005.02.030 BindingDB Entry DOI: 10.7270/Q21C1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 493 total ) | Next | Last >> |